Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.4034
Peer-review started: July 4, 2015
First decision: August 26, 2015
Revised: October 27, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: April 21, 2016
Processing time: 277 Days and 2.7 Hours
AIM: To evaluate whether sorafenib use after resection impacts tumor relapse and survival in Barcelona Clinic Liver Cancer (BCLC) stage C hepatocellular carcinoma (HCC).
METHODS: This retrospective study enrolled 36 male BCLC stage C HCC patients with portal vein thrombus and Child-Pugh class A liver function. Twenty-four patients received only surgical resection (SR), and 12 patients received oral sorafenib within 30 d after surgery. The primary outcomes were time to progression (TTP) (the time from surgical resection until HCC recurrence or extrahepatic metastases) and overall survival (OS). The secondary outcome was the rate of postoperative recurrence or metastasis. TTP and OS were analyzed using Kaplan Meier curves.
RESULTS: There were no significant differences between the two groups in the serum levels of alpha-fetoprotein, copies of hepatitis B virus-DNA, preoperative laboratory results, degree of hepatic fibrosis, types of portal vein tumor thrombus, number of satellite lesions, tumor diameter, pathological results, volume of blood loss, volume of blood transfusion, or surgery time (all P > 0.05). Patients in the SR + sorafenib group had a significantly longer TTP (29 mo vs 22 mo, P = 0.041) and a significantly longer median OS (37 mo vs 30 mo, P = 0.01) compared to patients in the SR group. The SR group had 18 cases (75%) of recurrence/metastasis while the SR + sorafenib group had six cases (50%) of recurrence/metastasis. A total of 19 patients died after surgery (five in the SR + sorafenib group and 14 in the SR group). The most common sorafenib-related adverse events were skin reactions, diarrhea, and hypertension, all of which were resolved with treatment.
CONCLUSION: Sorafenib after SR was well-tolerated. Patients who received sorafenib after SR had better outcomes compared to patients who received only SR.
Core tip: Barcelona Clinic Liver Cancer stage C patients with portal vein thrombus and Child-Pugh class A liver function who received sorafenib after surgical resection had significantly longer overall survival (37 mo vs 20 mo, P = 0.01) and significantly longer time to progression compared to patients who received only resection (29 mo vs 22 mo, P = 0.041). Our data suggested that better outcomes can be achieved with sorafenib after surgical resection, rather than sorafenib monotherapy.
- Citation: Li J, Hou Y, Cai XB, Liu B. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol 2016; 22(15): 4034-4040
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/4034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.4034
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide[1]. The most important risk factors for HCC include hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, and the presence of liver cirrhosis[2-4]. The Barcelona Clinic Liver Cancer (BCLC) classification system recommended a standard classification system for the treatment of HCC that has been accepted by the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of Liver[5]. Based on these guidelines, patients diagnosed with HCC at an early stage (BCLC Stage 0, A) currently undergo surgical resection (SR), liver transplantation, or percutaneous ablation and have a survival rate of 60%-70%[3,6,7]. However, HCC recurrence after these therapies is still common. Patients at an intermediate stage (BCLC Stage B) undergo transarterial chemoembolization (TACE)[8,9], where abnormal neovascularization is identified and injected with an emulsion of chemotherapeutic drug and lipidol and then embolized with gelfoam to induce tumor necrosis[10]. Although repeated treatment with TACE was associated with improved survival in patients with intermediate HCC[9], its use is limited to patients with well-compensated cirrhosis[11]. SR was shown to result in high hepatic functional reserve in HCC patients with portal vein tumor thrombus (PVTT), while postoperative TACE delayed recurrence and prolonged overall survival (OS) in patients who could tolerate the treatment[12]. Interestingly, TACE has been shown to induce neoangiogenesis and upregulation of vascular endothelial growth factor (VEGF), which is an independent negative predictor of survival[13].
Patients with advanced HCC have a poor prognosis[14]. The multi-kinase inhibitor sorafenib, which is the only approved agent recommended by the AASLD for HCC BCLC Stage C, is currently recommended as the first-line therapy in these patients[14-16]. Two randomized controlled clinical trials recently showed that sorafenib prolonged OS and delayed the time to progression (TTP) in patients with advanced HCC[17,18], likely by inhibiting a number of growth factor pathways, including VEGFR-1,-2,-3, platelet derived growth factor receptor (PDGFR)-β, Raf, rearranged during transfection (RET), and FMS-like tyrosine kinase (FLT)-3[19]. However, certain BCLC stage C patients with Child-Pugh class A liver function have been shown to have better outcomes with SR than with sorafenib monotherapy[20,21]. Additionally, recent reports, which showed that (1) advanced HCC patients at BCLC stage C had favorable outcomes with SR, and (2) the presence of multinodular tumors, macrovascular invasion, and portal hypertension were not contraindications for SR, suggested that the guidelines for the use of sorafenib monotherapy for advanced HCC should be re-evaluated[22,23].
The main purpose of this retrospective study was to evaluate whether sorafenib use after liver resection had an impact on tumor relapse and OS in BCLC stage C HCC patients. We also evaluated the safety and tolerability of oral sorafenib after surgical resection in these patients.
This retrospective study evaluated the medical records of 36 male HCC patients who underwent surgical resection and were treated at the First Affiliated Hospital of Kunming Medical University between January 2009 and December 2013. All patients were HBV positive and had cirrhosis.
Inclusion criteria were: (1) age 18-70 years old; (2) newly diagnosed liver cancer with no treatment received prior to surgical resection; (3) BCLC stage C [tumor thrombus in left/right branches or main portal vein; Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2], Child-Pugh liver function class A; (4) tumor confined to left/right lobe (single or multiple); maximum tumor diameter ≥ 5 cm; all patients underwent anatomic left/right lobe resection + thrombus dissection, with negative surgery margin (R0). All operative procedures were classified according to the Brisbane terminology[24]. Anatomic left/right lobe resection was defined as resection of the tumor along with the related portal vein branches and corresponding hepatic artery territory; (5) patients treated with oral sorafenib received a dose of 200-800 mg/d within 30 d after surgery. The dose was reduced to 200 mg twice daily in the event of drug-related adverse effects; and (6) time of follow up ≥ 6 mo (before June 2014); patients underwent at least once B-type ultrasound or computed tomography (CT)/magnetic resonance (MR) + chest X-ray every 2 mo during the follow-up period (if B-type ultrasound or X- ray found a new lesion, a confirmatory CT/MR scan was performed).
Exclusion criteria were: (1) presence of acute or chronic lesions in other organs outside the liver; (2) presence of metastases or suspected metastatic lesions outside the liver; (3) presence of other tumors at the time of diagnosis or during the follow-up period; (4) patient received any treatments (including TACE or RF) other than sorafenib after SR and before tumor recurrence or metastasis; and (5) tumor recurrence or metastasis within 3 mo after surgical resection or unnatural death during follow-up.
The primary outcome was TTP (the time from SR until HCC recurrence or extrahepatic metastases discovered by CT/MR) and OS. The secondary outcome was the rate of postoperative recurrence or metastasis.
The demographic data and clinical characteristics of the patients were summaized as mean ± SD for continuous data, n (%) for categorical data, and median (range: min to max) for time-related data. Differences between groups were compared using two-sample t-test for continuous data, Pearson χ2 test or Fisher exact test for categorical data, and log-rank test for time-related data. Additionally, a Mann-Whitney U test was considered for continuous data if data did not follow normal distribution. Time-related data (TTP and OS times) are represented using Kaplan-Meier curves, and differences between groups were analyzed by the log-rank test. The TTP and OS times were also summarized as median with 95%CI for both groups, separately. All statistical assessments were two-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences 17.0 for Windows (SPSS Inc., Chicago, IL, United States).
Of a total of 36 study patients, 12 patients received oral sorafenib after SR (SR + sorafenib group) and 24 received only SR (SR group). None of the patients exhibited any serious complications, including liver dysfunction, bleeding, or infection, within 30 d of surgical resection.
Table 1 summarizes the demographics and baseline clinical characteristics in the SR + sorafenib and the SR groups. All study patients were male. The average ages of patients in the SR + sorafenib and the SR groups were 49.8 ± 6.5 years and 52.8 ± 6.9 years, respectively (P = 0.226). There were no significant differences in the serum levels of alpha fetoprotein (AFP), copies of HBV-DNA, preoperative laboratory results, degree of hepatic fibrosis, types of PVTT, number of satellite lesions, tumor diameter, pathological results, volume of blood loss, volume of blood transfusion, or surgery time between the two groups (all P > 0.05).
| Variables | SR + sorafenib | SR | P value |
| (n = 12) | (n = 24) | ||
| Age1, yr | 49.8 ± 6.5 | 52.8 ± 6.9 | 0.226 |
| ECOG PS score2 | 1.000 | ||
| 0 | 10 (83.3) | 19 (79.2) | |
| 1 | 2 (16.7) | 5 (20.8) | |
| Largest tumor diameters1, cm | 9.8 ± 2.1 | 11.2 ± 2.5 | 0.103 |
| Pathologic results3 | 0.404 | ||
| Well differentiated (grade 1) | 3 (25.0) | 2 (8.3) | |
| Moderately differentiated (grade 2) | 7 (58.3) | 18 (75) | |
| Poorly differentiated (grade 3) | 2 (16.7) | 4 (16.7) | |
| Satellite lesion4, n | 4 (33.3) | 9 (37.5) | 1.000 |
| AFP | 0.700 | ||
| < 400 ng/mL | 4 (33.3) | 6 (25) | |
| ≥ 400 ng/mL | 8 (66.7) | 18 (75) | |
| HBV-DNA | 0.664 | ||
| < 1000 cps/mL | 9 (75) | 20 (83.3) | |
| ≥ 1000 cps/mL | 3 (25) | 4 (16.7) | |
| Degree of fibrosis5 | 1.000 | ||
| 0-2 | 2 (16.7) | 3 (12.5) | |
| 3-4 | 10 (83.3) | 21 (87.5) | |
| Types of PVTT6 | 0.764 | ||
| First-order branch (VP3) | 10 (83.3) | 19 (79.2) | |
| Main trunk (VP4) | 2 (16.7) | 5 (20.8) | |
| Preoperative laboratory results | |||
| ALT1, μmol/L | 57.3 ± 19.9 | 50.8 ± 22.1 | 0.397 |
| Albumin1, g/L | 39.5 ± 3.5 | 39.9 ± 4.5 | 0.781 |
| Bilirubin1, μmol/L | 17.4 ± 4.5 | 19.8 ± 6.0 | 0.236 |
| Hemoglobin1, g/L | 137.3 ± 10.9 | 132.0 ± 7.8 | 0.103 |
| Platelet count1, 109/L | 185.4 ± 46.2 | 164.3 ± 48.6 | 0.220 |
| Prothrombin time1, s | 12.1 ± 1.0 | 12.0 ± 1.0 | 0.785 |
| INR7 | 1.4 ± 0.5 | 1.3 ± 0.4 | 0.436 |
| Blood loss7, mL | 304.2 ± 151.4 | 343.8 ± 143.2 | 0.458 |
| Blood transfusion7, mL | 37.5 ± 93.2 | 62.5 ± 124.5 | 0.679 |
| Surgery time7, min | 218.3 ± 33.3 | 232.5 ± 47.8 | 0.265 |
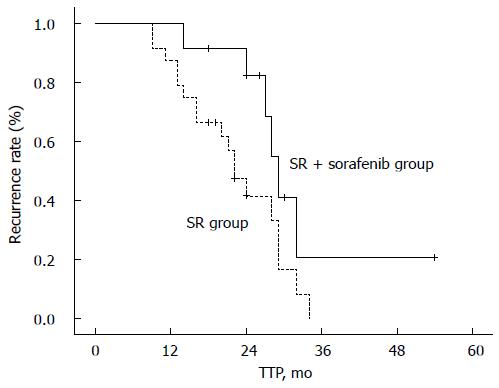
| TTP times8, mo | 26.5 (14-54) | 21.5 (9-34) | 0.0419 |
| Patients with recurrence after SR2 | 6 (50) | 18 (75) | 0.157 |
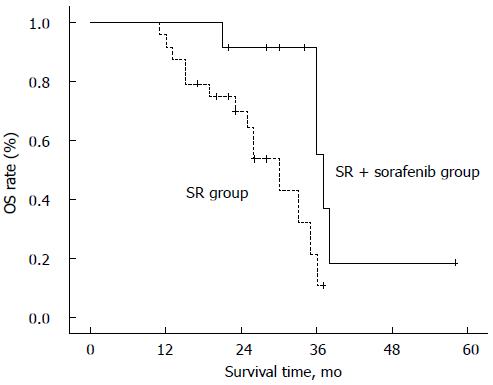
| Survival times8, mo | 32 (21-58) | 25.5 (11-37) | 0.0109 |
| Patients died after SR2 | 5 (41.7) | 14 (58.3) | 0.483 |
The median follow-up time after SR for all patients was 23 mo (range of 9-54 mo). During the follow-up period, a total of 19 patients experienced residual liver relapse (n = 5 in the SR + sorafenib group, and n = 14 in the SR group), three patients developed lung metastasis (n = 1 in the SR + sorafenib group, and n = 2 in the SR group), one patient developed right adrenal gland metastasis (n = 1 in the SR group), and one patient developed thoracic vertebral metastasis (n = 1 in the SR group) (data not shown). The rate of patients with at least once recurrence (including relapse or metastasis) was, therefore, derived as 50% (6/12) for the SR + sorafenib group and 75% (18/24) for the SR group (P = 0.157) (Table 1).
Patients in the SR + sorafenib and SR groups had a median TTP of 29 mo (95%CI: 26.5-31.5 mo) and 22 mo (95%CI: 18.0-26.0 mo), respectively. The log-rank test showed that this difference was significant (P = 0.041), suggesting that sorafenib therapy after SR might prolong the time until recurrence compared to SR alone (Figure 1).
A total of 19 patients died after surgery (five in the SR + sorafenib group and 14 in the SR group) (Table 1). The SR + sorafenib and SR groups had median OS times of 37 mo (95%CI: 34.8-39.2 mo) and 30 mo (95%CI: 24.1-36.0 mo), respectively. The log-rank test showed that this difference was significant (P = 0.010), suggesting that patients who received sorafenib therapy after SR had longer survival times compared to patients who received only SR (Figure 2)
The most common sorafenib-related adverse events during the follow-up period included hand-foot-skin reaction (11 patients; 91.67%), diarrhea (10 patients; 83.3%), and hypertension (10 patients; 83.3%). All the sorafenib-related adverse events were resolved with treatment, and no treatment-related deaths occurred (data not shown).
In this study, we showed that BCLC stage C patients who received sorafenib after SR had significantly longer OS and significantly longer TTP compared to patients who received only SR. There were fewer cases of recurrence/metastasis in the SR + sorafenib group compared to the SR only group. Sorafenib after SR was generally safe and well-tolerated.
Based on the current standard of care, patients with advanced HCC (stage B or C) who cannot undergo radical resection, receive local palliative treatment, including TACE, hepatic arterial infusion chemotherapy, and systemic chemotherapy[10,25,26]. Patients with stage C (defined as portal vein aggressiveness, lymph node or distant metastasis, ECOG PS ≤ 2, Child-Pugh liver function class A or B) are treated with sorafenib, which is an oral multi-kinase inhibitor with anti-tumor activity[27-29]. The multi-center European Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial, which evaluated the efficacy of sorafenib monotherapy (400 mg twice daily) in 602 HCC BCLC stage C patients with Child-Pugh class A liver function, showed a significantly lower OS in the placebo group compared to the sorafenib group (7.9 mo vs 10.7 mo, HR = 0.69; 95%CI: 0.55-0.87)[18]. These data were similar to a study in the Asia-Pacific region that demonstrated an improvement in OS from 4.2 mo in the placebo group to 6.5 mo in the sorafenib group (HR = 0.68; 95% CI: 0.50-0.93)[17]. The shorter OS in the latter trial compared to the SHARP study could be because of the higher number of cases with extrahepatic metastases, larger tumor diameters, higher ECOG PS scores, and higher AFP levels compared to the SHARP trial. Data from both trials formed the basis for the widespread recommendation of sorafenib for the treatment of advanced liver cancer. It was recently suggested that the relatively low response rates in sorafenib-treated patients could be because sorafenib induces tumor dormancy and a prolonged duration of stable disease (SD). Interestingly, patients with a longer duration of SD had a better OS compared to patients with a shorter duration of SD[30].
In contrast, data from two HCC clinical institutes in Asia showed that selected HCC patients at BCLC stage C had better outcomes with SR compared to other treatment modalities[20,21]. Similarly, a recent study that used the same inclusion criteria as the SHARP trial (BCLC stage C, Child-Pugh class A, ECOG PS ≤ 2) showed that patients who received SR (n = 68), locoregional ablation therapy (n = 8); transarterial embolization (n = 140); systemic chemotherapy or radiotherapy (CT/RT, n = 96) and sorafenib (n = 11) had a median OS of 33.4 mo, 9.5 mo, 9.2 mo, 6.6 mo, and 15.7 mo, respectively[31]. These data suggested that some advanced HCC patients who had tumors in a single lobe without extrahepatic spread had a better prognosis with SR compared to other methods.
There are currently no studies that have investigated the use of sorafenib after SR for advanced HCC. In our present study, we used the same inclusion criteria as the SHARP study. We included only newly diagnosed, naïve HCC patients in order to avoid the confounding effects of previous therapy. Additionally, all our study patients who underwent SR received no other treatment except for sorafenib prior to tumor recurrence. We used a follow-up time period of > 6 mo because of the difficulty in differentiating early recurrence from a residual tumor. A longer term follow-up is necessary to evaluate therapeutic efficacy. In this study, all the patients in the SR + sorafenib group received sorafenib within 30 d after surgery. This was because (1) comparisons between the two groups would be more reliable if all the patients were at a similar stage of recovery after SR, and (2) sorafenib would inhibit any VEGF-mediated promotion of tumor growth in patients who had a sub-optimal response to SR. It is important to note that sorafenib treatment is usually initiated 2 wk after SR, since sorafenib is known to delay healing.
Our data showed that BCLC stage C HCC patients who received oral sorafenib treatment after SR had a significantly longer TTP and a significantly longer median OS than patients who received only SR. Patients in the SR + sorafenib group also had a lower rate of tumor recurrence and extrahepatic metastasis than the SR only group. Interestingly, our data showed that patients in the SR + sorafenib as well as the SR groups had a longer TTP than the OS of patients in the sorafenib group from the SHARP study (29 mo and 22 mo vs 11 mo). These data suggested that it may be advantageous to use sorafenib after SR, rather than sorafenib monotherapy, in order to have better outcomes. Our data also showed that sorafenib was safe and well-tolerated. All sorafenib-related adverse events were resolved with dose reduction treatment, as previously reported[32]. Our data were consistent with a recent study that reported that adjuvant sorafenib therapy after hepatic resection in HCC patients resulted in reduced mortality and prolonged OS, possibly via inhibition of tumor growth after tumor recurrence[33].
Sorafenib has been shown to have anti-angiogenic as well as antitumor activities, possibly mediated via a number of tyrosine kinases, including VEGF, PDGFRs, Raf, and the phosphatidylinositol 3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathways[29,34-37]. Since vascular invasion was shown to be a critical predictor of HCC recurrence[38], it will be important to define further the mechanism of action of sorafenib in order to optimize the clinical management of patients with advanced HCC.
Since the cost of sorafenib therapy in China is almost three times the cost of SR, a few patients with advanced HCC are sometimes recommended SR by experienced physicians after a careful case-by-case assessment. The criteria for allowing SR in this group of patients are very restricted, making it a challenge to study large sample sizes. Although it is important to investigate whether the two groups were comparable for characteristics, such as presence of satellite tumors, and etiology of cirrhosis with HBV/HCV, the small sample size of our study precluded such an analysis. Indeed, the major limitation of this study was the small sample size. Other limitations were (1) its retrospective nature; and (2) factors such as surgeon preference and the socio-economic heterogeneity of our study population. The decision to be treated with sorafenib was largely based on the condition of the patients, and this could result in an overestimation of the effect of sorafenib after SR. Additionally, our study patients received different HCC treatment modalities (TACE, RAF, Chinese traditional medicines, or experimental immunotherapy) after tumor recurrence or metastasis. OS comparison may be impacted by these confounding factors.
In conclusion, our data showed that BCLC stage C HCC patients with Child-Pugh class A liver function who received oral sorafenib after SR had better postoperative TTP. It is important to validate our data via large multicenter randomized controlled trials.
Patients with advanced hepatocellular carcinoma (HCC) have a poor prognosis. The multi-kinase inhibitor sorafenib is the currently recommended first-line therapy for patients with HCC Barcelona Clinic Liver Cancer (BCLC) Stage C based on data from two recent randomized controlled clinical trials that showed that sorafenib prolonged overall survival (OS) and delayed time to progression (TTP). However, certain BCLC stage C patients with Child-Pugh class A liver function had better outcomes with surgical resection than with sorafenib monotherapy, suggesting a need to re-evaluate the guidelines for the use of sorafenib monotherapy for advanced HCC. This retrospective study evaluated whether sorafenib use after liver resection had an impact on tumor relapse and OS in BCLC stage C HCC patients.
Although sorafenib is currently the standard of care for advanced HCC, certain patients have better outcomes with surgical resection rather than with sorafenib monotherapy. This study investigated the use of sorafenib after surgical resection in specific groups of patients with advanced HCC and contributes to our ability to improve the clinical management of these patients.
There are currently no studies that have investigated the use of sorafenib after surgical resection (SR) for advanced HCC. The authors’ data indicated that sorafenib after SR was safe and well-tolerated. BCLC stage C patients who received sorafenib after SR had significantly longer OS, significantly longer TTP, and a lower rate of recurrence/metastasis compared to patients who received only SR.
Sorafenib therapy after surgical resection may result in better postoperative TTP in certain populations of HCC BCLC stage C patients with Child-Pugh class A liver function who may not benefit from sorafenib monotherapy. This study calls for re-evaluation of current guidelines for treating advanced HCC.
The manuscript is an interesting study showing the benefit of using adjuvant sorafenib in patients in BCLC C after liver resection. The article is well-organized, and the study objectives are clearly stated in the introduction, pointing out the relevance of this study. The study is built stepwise, and the description of the results is well-written.
P- Reviewer: Geller DA, Giovannetti E, Muntane J S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294-S308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 338] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 4. | Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132-2137. [PubMed] |
| 5. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2874] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 6. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 7. | Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, Chen CH, Changchien CS, Hsu HC, Hu TH. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer. 2010;46:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 10. | Lencioni R. Chemoembolization in patients with hepatocellular carcinoma. Liver Cancer. 2012;1:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Colombo M, Sangiovanni A. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver Int. 2015;35 Suppl 1:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Ye JZ, Zhang YQ, Ye HH, Bai T, Ma L, Xiang BD, Li LQ. Appropriate treatment strategies improve survival of hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol. 2014;20:17141-17147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 14. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3243] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 16. | Jeng WJ, Lin CC, Chen WT, Sheen IS, Lin CY, Lin SM. Adjuvant therapy for hepatocellular carcinoma after curative treatment. Dig Dis. 2014;32:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4649] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10265] [Article Influence: 603.8] [Reference Citation Analysis (2)] |
| 19. | Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, Chen PJ, Chen KF. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol. 2011;55:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Wang JH, Changchien CS, Hu TH, Lee CM, Kee KM, Lin CY, Chen CL, Chen TY, Huang YJ, Lu SN. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma - Survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Changchien CS, Chen CL, Yen YH, Wang JH, Hu TH, Lee CM, Wang CC, Cheng YF, Huang YJ, Lin CY. Analysis of 6381 hepatocellular carcinoma patients in southern Taiwan: prognostic features, treatment outcome, and survival. J Gastroenterol. 2008;43:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, Lu JH, Yang GS, Wu MC. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. 2012;138:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 24. | Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer. 2012;1:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 28. | Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1205] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 30. | Arizumi T, Ueshima K, Chishina H, Kono M, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y. Duration of stable disease is associated with overall survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Dis. 2014;32:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Wang JH, Kuo YH, Wang CC, Chen CL, Cheng YF, Hsu HC, Lu SN. Surgical resection improves the survival of selected hepatocellular carcinoma patients in Barcelona clinic liver cancer stage C. Dig Liver Dis. 2013;45:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Bolondi L, Craxi A, Trevisani F, Daniele B, Di Costanzo GG, Fagiuoli S, Cammà C, Bruzzi P, Danesi R, Spandonaro F. Refining sorafenib therapy: lessons from clinical practice. Future Oncol. 2015;11:449-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Zhang W, Zhao G, Wei K, Zhang Q, Ma W, Song T, Wu Q, Zhang T, Kong D, Li Q. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends. 2014;8:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Kudo M. Signaling pathway and molecular-targeted therapy for hepatocellular carcinoma. Dig Dis. 2011;29:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Zhu AX. Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer. 2008;112:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Nagai T, Arao T, Furuta K, Sakai K, Kudo K, Kaneda H, Tamura D, Aomatsu K, Kimura H, Fujita Y. Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2011;10:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Zhang CZ, Wang XD, Wang HW, Cai Y, Chao LQ. Sorafenib inhibits liver cancer growth by decreasing mTOR, AKT, and PI3K expression. J BUON. 2015;20:218-222. [PubMed] |
| 38. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 39. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3782] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 40. | Japan Liver Cancer Study Group. Classification of primary liver cancer. 1st ed. Tokyo: Kanehara & Co 1997; . |