Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.4020
Peer-review started: October 22, 2015
First decision: November 27, 2015
Revised: December 2, 2015
Accepted: January 17, 2016
Article in press: January 18, 2016
Published online: April 21, 2016
Processing time: 166 Days and 4 Hours
AIM: To predict the rate of lymph node (LN) metastasis in diffuse- and mixed-type early gastric cancers (EGC) for guidelines of the treatment.
METHODS: We reviewed 550 cases of EGC with diffuse- and mixed-type histology. We investigated the clinicopathological factors and histopathological components that influence the probability of LN metastasis, including sex, age, site, gross type, presence of ulceration, tumour size, depth of invasion, perineural invasion, lymphovascular invasion, and LN metastasis status. We reviewed all slides and estimated the proportions of each tumour component; pure diffuse type, mixed-predominantly diffuse type (diffuse > intestinal type), mixed-predominantly intestinal type (intestinal > diffuse type), and mixed diffuse = intestinal type. We calculated the extents of the respective components.
RESULTS: LN metastasis was observed in 12.9% (71/550) of early gastric cancers cases [15/288 mucosal EGCs (5.2%) and 56/262 submucosal EGCs (21.4%)]. Of 550 cases, 302 were diffuse-type and 248 were mixed-type EGCs. Of 248 mixed-type EGCs, 163 were mixed-predominantly diffuse type, 82 were mixed-predominantly intestinal type, and 3 were mixed diffuse = intestinal type. Mixed-type cases with predominantly diffuse type histology showed a higher frequency of LN metastasis (20.2%) than cases of pure diffuse type (9.3%) and predominantly intestinal type (12.2%) histology. We measured the dimensions of each component (intestinal and diffuse type) to determine the association of the extent of each component with LN metastasis in mixed-type gastric carcinoma. The total tumour size and the extent of poorly differentiated components was associated with LN metastasis, while that of signet ring cell components was not.
CONCLUSION: We recommend careful identification and quantitative evaluation of mixed-type early gastric cancer components after endoscopic resection to determine the intensity of the treatment.
Core tip: This study consolidates correlations between clinicopathological characteristics of early gastric cancer and the risk of lymph node (LN) metastasis, which is important to select patients that will benefit from endoscopic submucosal dissection. This paper also shows that the amount of poorly differentiated tumor cells within mixed type early gastric cancer is correlated with the highest risk of LN metastasis, thus highlighting the need for diligent histological characterization of patient specimens.
- Citation: Hwang CS, Ahn S, Lee BE, Lee SJ, Kim A, Choi CI, Kim DH, Jeon TY, Kim GH, Song GA, Park DY. Risk of lymph node metastasis in mixed-type early gastric cancer determined by the extent of the poorly differentiated component. World J Gastroenterol 2016; 22(15): 4020-4026
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/4020.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.4020
Endoscopic submucosal dissection (ESD), a recently developed technique, is a widely accepted treatment modality for early gastric cancer (EGC), including for diffuse (undifferentiated) type EGC within the expanded criteria (mucosal cancer measuring less than 2 cm without ulceration and lymphovascular tumour emboli). Large datasets indicate almost no risk of lymph node (LN) metastasis in diffuse type EGCs[1-3]. A considerable proportion of gastric adenocarcinomas show a mixed composition with both intestinal and diffuse histological components. No consensus has been established regarding the management of mixed-type histology, i.e., varying proportions of diffuse and intestinal components in the tumour.
Generally, gastric adenocarcinoma can be subclassified into intestinal (differentiated) and diffuse (undifferentiated) types based on gland formation[4,5]. According to Japanese guidelines, diffuse components are further subdivided into signet ring cell carcinoma and poorly differentiated carcinoma[6]. These guidelines refer to mixed-type EGCs by their predominant histological component, whether intestinal or diffuse. There are several reports on the management and outcomes of mixed-type EGCs[7-11]. Takizawa et al[7] reported that mixed-predominantly diffuse type showed significant LN metastasis and suggested that their treatment be similar to that of diffuse-type EGCs. Hanaoka et al[8] reported that the predominantly undifferentiated mixed-type tumours showed considerably higher LN metastasis than other types of submucosal invasive EGCs. Furthermore, Min et al[9] reported that mixed intestinal- and diffuse-type EGCs could be managed with ESD under the expanded criteria.
We investigated 550 cases of diffuse- and mixed-type EGCs to determine which components of the diffuse subtypes (signet ring cell or poorly differentiated carcinoma) are more closely associated with LN metastasis, providing evidence to establish a consensus for the management of diffuse- and mixed-type EGCs with ESD.
We retrieved early gastric cancer cases that involved gastrectomies with LN dissection at the Pusan National University Hospital treated between 2008 and 2013, and selected 550 cases of EGC with diffuse- and mixed-type histology based on the Japanese classification of gastric cancer[6]. Cases involving preoperative chemotherapy or radiotherapy or multiple gastric cancers were excluded. Pathological reports and electronic medical records were reviewed, for attributes, including sex, age, site, gross type, presence of ulceration, tumour size, depth of invasion, perineural invasion, lymphovascular invasion, and LN metastasis status. Lesions were classified into the following three groups according to gross type: elevated type (I, IIa, IIa + IIb, and IIa + IIc), flat type (IIb, IIb + IIa, and IIb + IIc), and depressed type (IIc, IIc + IIb, IIc + III, IIc + IIa, and III). Ulceration was defined as a deformity of the muscularis propria or fibrosis in the submucosal layer. Intramucosal carcinoma was categorized as M2 (lamina propria invasion) or M3 (muscularis mucosae invasion without penetration). Submucosal invasion was graded as SM1 (upper third), SM2 (middle third), or SM3 (lower third). The depth of submucosal invasion was measured directly using a micrometre and was defined as the distance from the lowest point of the muscularis mucosa (or surface of ulceration) to the point of deepest tumour penetration. The Institutional Review Board at the Hospital approved this study.
We reviewed all slides and estimated the proportions of each tumour component. Diffuse components (undifferentiated) were further subdivided into signet ring cell (sig) or poorly differentiated (por) types. Subclassification was according to the most prevalent component, applying the 50% criterion, as follows: pure diffuse type, mixed-predominantly diffuse type (diffuse > intestinal type), mixed-predominantly intestinal type (intestinal type > diffuse), and mixed diffuse = intestinal type[7]. We calculated the extents of the respective components using the following formula: extent of respective component (cm) = dimension of tumour (cm) × estimated percentage of the respective component/100.
Statistical calculations were performed using SPSS for Windows (version 12K, Chicago, IL, United States). To identify the predictive risk factors for LN metastasis, the data were statistically analysed using the chi-squared test and an unpaired Student’s t test. The independent factors for LN metastasis in diffuse- and mixed-type EGCs were analysed by binary logistic regression analysis (backward, stepwise). The ability of histological component extent to predict LN metastasis was assessed using receiver operating characteristic (ROC) curve analysis. The total areas under the ROC curves ranged from 0.5 to 1.0, with 1.0 indicating perfect predictive value and 0.5 indicating random chance. A pairwise comparison of the areas under the ROC curve was subsequently performed to assess the predictive value of each histological component. A P < 0.05 was deemed statistically significant.
Clinicopathological characteristics as a status of LN metastasis are listed in Table 1. Of 550 cases, 71 (12.9%) showed LN metastasis [15/288 mucosal EGCs (5.2%) and 56/262 submucosal EGCs (21.4%)]. Increased tumour size, gross type (elevated), depth of invasion, and perineural and lymphovascular invasion were associated with LN metastasis. Furthermore, we investigated the independent predictive risk factors for LN metastasis using binary logistic regression analysis (Supplemental Table 1). Tumour size (P = 0.001), depth of invasion (P < 0.0001), and lymphovascular invasion (P = 0.001) were independent predictive risk factors for LN metastasis in diffuse- and mixed-type EGCs. In our dataset, there were 80 cases of mucosal EGCs less than 2.0 cm in size and without ulceration; among them, only 1 case showed LN metastasis (Table 2). Hence, only 1 case within ESD eligibility criteria showed LN metastasis in this study.
| Total | Lymph node status | P value | ||
| Negative(n = 479) | Positive(n = 71) | |||
| Sex | 0.950 | |||
| Male | 304 | 265 (87.2) | 39 (12.8) | |
| Female | 246 | 214 (87.0) | 32 (13.0) | |
| Age (yr) | 550 | 54.5 ± 0.7 | 55.5 ± 2.5 | 0.686 |
| Site1 | 0.059 | |||
| Upper | 129 | 120 (93.0) | 9 (7.0) | |
| Middle | 252 | 217 (86.1) | 35 (13.9) | |
| Lower | 169 | 142 (84.0) | 27 (16.0) | |
| Gross type | 0.030 | |||
| Elevated | 59 | 45 (76.3) | 14 (23.7) | |
| Flat | 51 | 46 (90.2) | 6 (9.8) | |
| Depressed | 440 | 388 (88.2) | 52 (11.8) | |
| Ulceration | 0.640 | |||
| Absent | 445 | 389 (87.4) | 56 (12.6) | |
| Present | 105 | 90 (85.7) | 15 (14.3) | |
| Tumour size (cm)2 | < 0.0001 | |||
| ≤ 1 | 40 | 39 (97.5) | 1 (2.5) | |
| ≤ 2 | 126 | 120 (95.2) | 6 (4.8) | |
| ≤ 3 | 141 | 125 (88.7) | 16 (11.3) | |
| ≤ 3 | 243 | 195 (80.2) | 48 (19.8) | |
| Depth of invasion3 | < 0.0001 | |||
| Mucosa | 288 | 273 (94.8) | 15 (5.2) | |
| M2 | 50 | 49 (98.0) | 1 (2.0) | |
| M3 | 238 | 224 (94.1) | 14 (5.9) | |
| Submucosa | 262 | 206 (78.6) | 56 (21.4) | |
| SM1 | 33 | 30 (90.9) | 3 (9.1) | |
| SM2 | 67 | 55 (82.1) | 12 (17.9) | |
| SM3 | 162 | 121 (74.7) | 41 (25.3) | |
| Depth (μm) | 262 | 1754 ± 89.4 | 2017 ± 130.3 | 0.153 |
| Perineural invasion | < 0.0001 | |||
| Absent | 495 | 442 (89.3) | 53 (10.7) | |
| Present | 55 | 37 (67.3) | 18 (32.7) | |
| LV invasion | < 0.0001 | |||
| Absent | 518 | 464 (89.6) | 54 (10.4) | |
| Present | 32 | 15 (46.9) | 17 (53.1) | |
| Mucosal carcinoma | Submucosal carcinoma | |||||||
| No ulcer | Ulcer | SM1 | SM2 | SM3 | ||||
| ≤2 cm | > 2 cm | ≤3 cm | > 3 cm | ≤3 cm | > 3 cm | Any size | ||
| Diffuse (n = 302) | 1/61 (1.6) | 5/86 (5.8) | 2/22 (9.0) | 1/19 (5.3) | 0/12 (0) | 2/7 (28.6) | 6/32 (18.8) | 11/63 (17.5) |
| Mixed (n = 248) | 0/19 (0) | 5/64 (7.8) | 0/4 (0) | 1/10 (10) | 0/6 (0) | 1/8 (12.5) | 6/35 (17.1) | 30/99 (30.3) |
| Total (n = 550) | 1/80 (1.3) | 10/150 (6.7) | 2/26 (7.7) | 2/29 (6.9) | 0/18 (0) | 3/15 (20.0) | 12/67 (17.9) | 41/162 (25.3) |
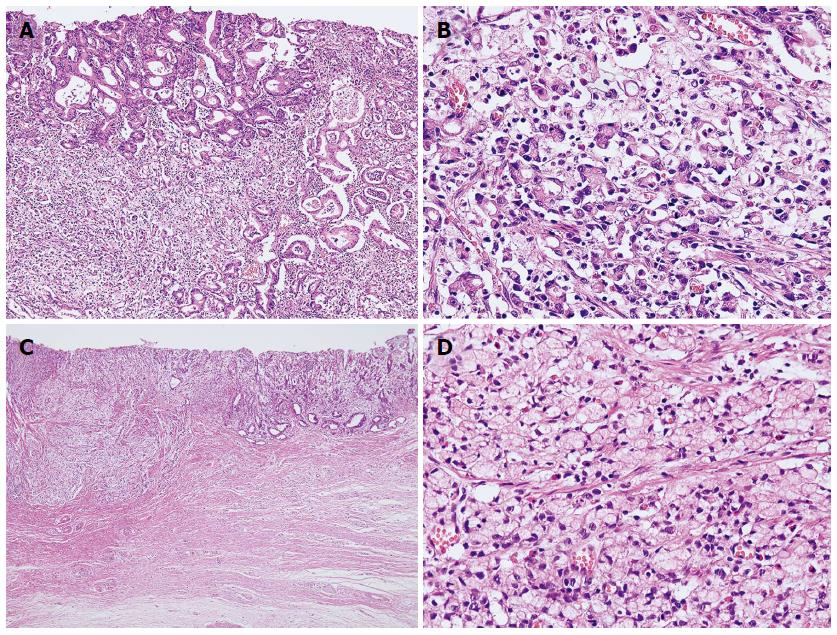
Of 550 cases, 302 were diffuse-type and 248 were mixed-type EGCs (Figure 1; Supplemental Tables 2 and 3). Furthermore, 28 of the 302 diffuse-type (9.3%) and 43 of the 248 mixed-type EGCs (21.0%) showed LN metastasis. The clinicopathological features associated with LN metastasis in each type were similar to those associated with LN metastasis when diffuse and mixed types were considered together, e.g., tumour size, depth of invasion, and perineural and lymphovascular tumour invasion. Depth of submucosal invasion was significantly associated with LN metastasis in mixed-type (P = 0.044), but not in diffuse-type EGCs (P = 0.905)
| Status of lymph node metastasis | |||
| Total | Negative | Positive | |
| Pure diffuse type | 302 | 274 (90.7) | 28 (9.3)a |
| Sig | 233 | 210 (90.1) | 23 (9.9) |
| Por | 69 | 64 (92.8) | 5 (7.2) |
| Mixed type | 248 | 205 (83.7) | 43 (16.3) |
| Predominantly diffuse | 163 | 130 (79.8) | 33 (20.2)a |
| Sig predominant | 107 | 86 (80.4) | 21 (19.6) |
| Por predominant | 56 | 44 (78.6) | 12 (21.4) |
| Predominantly intestinal | 82 | 72 (87.8) | 10 (12.2)a |
| Diffuse = intestinal | 3 | 3 (100.0) | 0 (0.0) |
Of 248 mixed-type EGCs, 163 were mixed-predominantly diffuse type, 82 were mixed-predominantly intestinal type, and 3 were mixed diffuse = intestinal type (Table 3). Interestingly, LN metastasis was more frequent in the mixed-predominantly diffuse type (33/163, 20.2%) than in either the pure diffuse type (28/302, 9.3%) or the mixed-predominantly intestinal histological type (10/82, 12.2%; P = 0.003).
We measured the extent of the individual components to determine the quantitative power of each to predict LN metastasis. The total tumour size and the extent of the poorly differentiated component were significantly larger in the metastatic LN group (Table 4). Interestingly, the extent of the poorly differentiated component was associated with LN metastasis (P = 0.012), whereas that of the signet ring cell component was not (P = 0.161).
| Lymph node status | |||
| Negative(n = 479) | Positive(n = 71) | P value | |
| Total (cm) | 3.30 ± 0.09 | 4.46 ± 0.29 | < 0.001 |
| Diffuse type (cm) | 2.73 ± 0.11 | 3.61 ± 0.24 | 0.005 |
| Signet ring cell | 2.07 ± 0.12 | 2.54 ± 0.29 | 0.161 |
| Poorly differentiated | 0.66 ± 0.05 | 1.07 ± 0.20 | 0.012 |
| Intestinal type (cm) | 0.65 ± 0.05 | 0.87 ± 0.17 | 0.172 |
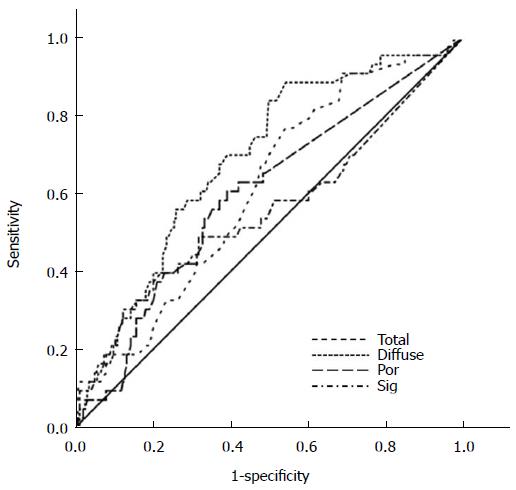
In the 248 mixed-type EGCs, we assessed the ability of the histological components to predict LN metastasis using ROC curve analysis (Figure 2). The areas under the ROC curve for each component were as follows: diffuse component (0.687, 95%CI: 0.604-0.701, P < 0.0001), total size (0.610, 95%CI: 0.523-0.698, P = 0.023), poorly differentiated component (0.598, 95%CI: 0.505-0.691, P = 0.043), and signet ring cell component (0.556, 95%CI: 0.450-0.661, P = 0.249). These results confirm that poorly differentiated component was significantly associated with LN metastasis (P = 0.043) while signet ring cell component was not (P = 0.249).
With the development of ESD, the criteria for diffuse-type EGCs eligible for endoscopic resection expanded to include mucosal lesions measuring less than 2.0 cm without ulceration or lymphovascular tumour emboli; this was based on the risk of LN metastasis[1-3]. Hirasawa et al[12] reported that 310 diffuse-type EGCs within the expanded criteria showed no LN metastasis, in contrast to an earlier report by Gotoda et al[13]. Shim and Lee summarized previously published datasets describing the risks of LN metastasis for diffuse-type EGCs that meet the proposed criteria[3], concluding that these expanded criteria for ESD of diffuse type EGC are feasible with regard to efficacy and safety.
Consistent with other reports that reported low or no LN metastasis rates, we noted only one such metastasis among cases within the established expanded ESD criteria (1/80, 1.3%). We also identified various clinicopathological factors associated with LN metastasis, including increased size, gross type (elevated), depth of invasion, and perineural and lymphovascular invasion; this was consistent with previously published data[14,15]. Taken together, the expanded criteria for diffuse-type EGC can be used to determine the primary option for treatment. Mixed- predominantly diffuse EGCs showed a higher frequency of LN metastasis (20.2%) than either the pure diffuse type (9.3%) or the mixed-predominantly intestinal type (12.2%), in accordance with previous reports[7,8], suggesting that mixed-predominantly diffuse EGC should be more stringently managed than pure diffuse-type EGC.
We further sub-categorized diffuse histological components into signet ring cell and poorly differentiated carcinoma, and evaluated the impact of each component on LN metastasis. Interestingly, we identified an increase in the extent of the total diffuse component - especially the poorly differentiated component - was associated with LN metastasis. There was no such association between the extents of signet ring cell components or intestinal components with LN metastasis. Furthermore, ROC curve analysis of mixed-type EGCs showed that the total tumour size along with the extents of the diffuse and poorly differentiated components affected LN metastasis with statistical significance, while the extent of the signet ring cell component did not (Figure 2).
Many studies reported favourable outcomes of early signet ring cell carcinoma and recommended endoscopic resection as the primary treatment option, which is not the case for poorly differentiated carcinoma[16-18]. Ha et al[17] demonstrated that signet ring cell carcinoma within the expanded criteria showed no LN metastasis and favourable outcomes compared to undifferentiated carcinoma. Kim et al[18] reported that signet ring cell carcinoma had a lower rate of LN metastasis compared to poorly differentiated or tubular adenocarcinoma of the submucosal type. However, none of aforementioned studies demonstrated the association of signet ring cell carcinoma in mixed-type EGCs with LN metastasis. This study demonstrates that a larger, poorly differentiated histological component was associated with LN metastasis, whereas a larger signet ring cell component was not.
There are many limitations and possible errors in our method of measuring the exact size of the lesions. We measured the extent of each component by multiplying the percentage of each component with the largest diameter of the lesion. Although this method is not perfect, we believed that it would be sufficiently indicative of the role of each component.
In conclusion, our results support the use of the extended criteria for the therapeutic use of ESD in cases of diffuse-type EGCs, and we recommend careful identification and quantitative evaluation of each tumour component in mixed-type EGC specimens obtained by endoscopic resection.
This study was presented in part at the 2015 USCAP meeting (Boston, MA, United States).
A considerable proportion of gastric adenocarcinomas show a mixed composition with both intestinal and diffuse histological components. No consensus has been established regarding the management of mixed-type histology.
The authors investigated which components of the diffuse subtypes (signet ring cell or poorly differentiated carcinoma) are more closely associated with lymph node (LN) metastasis, providing evidence to establish a consensus for the management of diffuse- and mixed-type early gastric cancers (EGCs) with endoscopic submucosal dissection (ESD).
This study consolidates correlations between clinicopathological characteristics of early gastric cancer and the risk of LN metastasis, which is important to select patients that will benefit from endoscopic submucosal dissection. This paper also shows that the amount of poorly differentiated tumor cells within mixed type early gastric cancer is correlated with the highest risk of LN metastasis.
The results of the our present study support the use of the extended criteria for the therapeutic use of ESD in cases of diffuse-type EGCs, recommending careful identification and quantitative evaluation of each tumour component in mixed-type EGC specimens obtained by endoscopic resection.
Gastric adenocarcinoma can be subclassified into intestinal (differentiated) and diffuse (undifferentiated) types based on gland formation. Diffuse components are further subdivided into signet ring cell carcinoma and poorly differentiated carcinoma. In practice, mixed-type EGCs, consisted of intestinal and diffuse type, are frequently encountered. The expanded criteria for ESD include diffuse (undifferentiated) mucosal EGC measuring less than 2 cm without ulceration and lymphovascular tumour emboli.
This paper shows that the amount of poorly differentiated tumor cells within mixed type early gastric cancer is correlated with the risk of LN metastasis. They emphasize the need for diligent histological characterization of patient specimens as conclusion. Their findings are interesting and useful for the future treatment of early gastric cancer.
P- Reviewer: Ando T, Caboclo JL, Wang YH S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Shim CN, Lee SK. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: do we have enough data to support this? World J Gastroenterol. 2014;20:3938-3949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 5. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251-258. [PubMed] |
| 6. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 7. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, Kim JJ. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2015;18:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Komatsu S, Ichikawa D, Miyamae M, Shimizu H, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Kishimoto M, Otsuji E. Histological mixed-type as an independent prognostic factor in stage I gastric carcinoma. World J Gastroenterol. 2015;21:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Miyamae M, Komatsu S, Ichikawa D, Kosuga T, Kubota T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Kishimoto M. Histological mixed-type as an independent risk factor for nodal metastasis in submucosal gastric cancer. Tumour Biol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 13. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] |
| 14. | Son HJ, Song SY, Kim S, Noh JH, Sohn TS, Kim DS, Rhee JC. Characteristics of submucosal gastric carcinoma with lymph node metastatic disease. Histopathology. 2005;46:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Son HJ, Myung W, Yoo HS, Park SH, Song SY, Kwon YD, Rhee JC. Prognostic indicators of gastric carcinoma confined to the muscularis propria. Histopathology. 2007;51:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, Min JS. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78-83. [PubMed] |
| 17. | Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Kim BS, Oh ST, Yook JH, Kim BS. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014;155:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |