Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.3969
Peer-review started: October 25, 2015
First decision: November 27, 2015
Revised: December 6, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: April 21, 2016
Processing time: 161 Days and 22.4 Hours
AIM: To investigate the effect of integrin-linked kinase (ILK) on proliferation, metastasis, and invasion of the colorectal cancer cell line SW480.
METHODS: In this study, the colorectal cancer cell line SW480 was stably transfected with ILK plasmids, and small interfering RNA (siRNA) was used to knockdown expression of nuclear factor (NF)-κB/p65. Methylthiazole tetrazolium (MTT) assay was performed to measure proliferation, and the wound healing migration assay and matrigel invasion assay were used to test the metastasis and invasion ability of SW480 cells. To explore the epithelial-mesenchymal transition (EMT) process, embryonic development, and the invasion and metastasis of tumors, the protein level of E-cadherin, vimentin, snail, and slug was detected by western blot. Immunofluorescence was also used to detect E-cadherin expression. Western blot was used to determine the level of phosphorylated-inhibitor of kappa B (IκB)a, inhibitor of gamma B (IγB)a, and nuclear factor kappa B (NF-κB) expressions and to explore the ILK signaling pathway.
RESULTS: Western blot results revealed that ILK expression significantly increased when ILK was overexpressed in SW480 cells (P < 0.05). Proliferation, metastasis, and invasion ability were improved in the vector-ILK group compared to the vector group (P < 0.05). Immunofluorescence results revealed that E-cadherin fluorescence intensity decreased after ILK was overexpressed (P < 0.05). Western blot results revealed that the protein expression of E-cadherin was reduced, while vimentin, snail, and slug were upregulated when ILK was overexpressed in SW480 cells (P < 0.05). In order to determine the role of the NF-κB signaling pathway in ILK overexpression promoted EMT occurrence, we overexpressed ILK in SW480 cells and found that levels of NF-κB/p65 and cytoplasmic phosphorylated-IκBa were increased and that cytoplasmic IкBa levels were decreased compared to the control group (P < 0.05). Furthermore, NF-κB/p65 knockout revealed that E-cadherin was increased in the overexpressed ILK group.
CONCLUSION: ILK overexpression improved the proliferation, metastasis, and invasion ability of SW480 cells, and this effect may be mediated by the NF-κB signaling pathway.
Core tip: In this study, the colorectal cancer cell line SW480 was stably transfected with integrin-linked kinase (ILK) plasmids, and the proliferation, metastasis, and invasion ability of the cells were tested. The results demonstrated that ILK overexpression improved the proliferation, metastasis, and invasion ability of cell line SW4802 and promoted the occurrence of the epithelial-mesenchymal transition in colorectal cancer cells. These effects may be mediated by the nuclear factor-κB signaling pathway.
- Citation: Shen H, Ma JL, Zhang Y, Deng GL, Qu YL, Wu XL, He JX, Zhang S, Zeng S. Integrin-linked kinase overexpression promotes epithelial-mesenchymal transition via nuclear factor-κB signaling in colorectal cancer cells. World J Gastroenterol 2016; 22(15): 3969-3977
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/3969.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.3969
Integrin-linked kinase (ILK) is a multifunctional receptor protein and a protein interaction integrin. Moreover, ILK can recruit other adapter molecules and regulate a variety of cellular processes via coupled signaling pathways, including cell growth, proliferation, apoptosis, survival, differentiation, migration, and invasion. Recent studies have shown that ILK is overexpressed and excessively activated in a number of human cancers[1,2]. It has been reported that the overexpression of ILK can enhance the rate of lung cancer cell migration, and it was shown that this enhancement was regulated by nuclear factor (NF)-κB-mediated matrix metalloproteinase (MMP)-9 expression[3,4]. Researchers have found that ILK was highly expressed in colorectal cancer tissues; and that ILK promoted tumor transfer and corrosion, which is mediated through the epithelial-mesenchymal transition (EMT) process[5,6]. However, the role and mechanism of ILK in colorectal cancer cells remains unclear. Some experts have reported that ILK overexpression can induce transcription factor snail and zinc finger E-box binding homeobox 1 (ZEB1) expression, resulting in the inhibition of E-cadherin expression[7-9]. The colorectal cancer cell line SW480 was used in this study, and ILK expression levels in this cell line were found to be relatively low. The present study aims to investigate the effect of ILK in colorectal cancer cell proliferation, invasion, and metastasis and to explore its underlying mechanism.
Transfection reagent lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States), PVDF film (Millipore, Bedford, MA, United States), anti-ILA antibody (Cell Signaling Technology, Danvers, MA, United States), anti-E-cadherin antibody and anti-Vimentin antibody (Santa Cruz Biotechnology, Dallas, TX, United States), anti-Slug antibody (Abcam, Cambridge, MA, United States), and anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO, United States).
The human colorectal cancer cell line SW480 was obtained from the Cell Bank, Chinese Academy of Medical Sciences (Shanghai, China) and cultured in Leibovitz L-15 medium (Gibco, Grand Island, NY, United States) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, United States) and antibodies. Cells were cultured in an incubator containing 5% CO2 at 37 °C and were passaged for 2-3 d until 85% confluence was achieved.
Human ILK gene coding sequence was obtained by polymerase chain reaction (PCR) amplification and connected the target gene to the pcDNA3.1 vector. Sequencing detection revealed no mutation in the target gene. In a six-well plate, 2 × 105 cells were seeded into each well. After 1 d of culture, cells were transfected, and transfection reagent lipofectamine 2000 was applied to transfect 2 μg/mL of overexpressed ILK plasmids (pcDNA3.1-ILK) or empty vector. After 48 h of transfection, cells were placed into a selective medium (G418, 800 mg/mL) for 3-4 wk. G418-resistant clones were filtered and amplified after reverse transcriptase (RT)-PCR and western blot confirmation.
Cells were cultured in 96-well plates (2 × 103 cells/well) for 24, 48, and 72 h. Then, 20 μL of MTT was added into each well and cultured in an incubator for another 2 h. At the end of the culture period, the liquid in the well was discarded, 200 μL of dimethylsulfoxide (DMSO) was added, and optical density (OD) was measured with a microplate reader at 450 nm.
Cells were cultured in six-well plates, and the culture medium was discarded when it reached approximately 80% confluence. Then, the monolayer was scraped using the tip of a 200-μL pipette, cells were washed three times with phosphate buffered saline (PBS), and a photograph was taken under a microscope. Subsequently, cells were maintained in serum-free medium for another 6 h. Then, a photograph was taken and the migration rate was computed.
The culture medium was discarded when cells reached 60% confluence. Cells were washed three times with PBS and cultured in an incubator for another 24 h. After trypsinization, cells were collected in a tube, centrifuged, and resuspended in FBS free culture medium to a final concentration of 1 × 105 cells/mL. The transwell chamber was placed onto a 24-well plate containing 800 μL of culture medium with 20% FBS. Then, cell suspensions were injected into the devices through the inlet channels. The plate was incubated for 24 h in 5% CO2 at 37 °C. After incubation, the transwell chamber was taken out, washed with PBS, and the upper layer of the chamber was cleaned with a cotton swab. The chamber was fixed with formalin for 20 min, dyed with hematoxylin for 5 min, and washed with PBS. Then, the number of cells were observed and counted with a microscope.
NF-κBp65 small interfering RNA (siRNA) 5’-CCUCCUU
UCAGGAGAUGAATT-3’; scramble control 5’-UUCUCCGA
ACGUGUCACGUTT-3’. After transfection with lipofectamine 2000 for 48 h, cells were obtained and analyzed.
Cells were lysed for cytosol-nuclear isolation; and nuclear and cytoplasmic lysates were collected. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed, and proteins were transferred onto a polyvinylidene (PVDF) membrane. Primary antibodies used for incubation were as follows: membrane anti-ILK antibody (diluted 1:1000), anti-E-cadherin antibody (diluted 1:100), anti-NF-KBp65 antibody (diluted 1:1000), anti-inhibitor of kappa B (IkB)a antibody (diluted 1:1000), anti-phosphorylated IkBa antibody (diluted 1:1000), anti-vimentin antibody (diluted 1:1000), anti-E-cadherin antibody (diluted 1:500), anti-snail antibody (diluted 1:500), anti-slug antibody (diluted 1:1000), and anti-β-actin antibody (diluted 1:5000). Then, membranes were treated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Specific protein bands were detected using an enhanced chemiluminescence assay kit (Santa Cruz Biotechnology).
Cells were cultured on round coverslips and fixed with 4% paraformaldehyde for 30 min. Then, 0.1% Triton X-100 was used for permeabilization. Cells were incubated in fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibodies for 90 min, washed with secondary antibodies, and fixed by 4’,6-diamidino-2-phenylindole (DAPI). A laser scanning confocal microscope (FV1000S-SIM/IX81; Olympus, Tokyo, Japan) was used to observe staining.
All data are presented as mean ± SD. Using SPSS 13.0 software (SPSS, Chicago, IL), data were compared by one-way analysis of variance (ANOVA), and P < 0.05 was considered statistically significant.
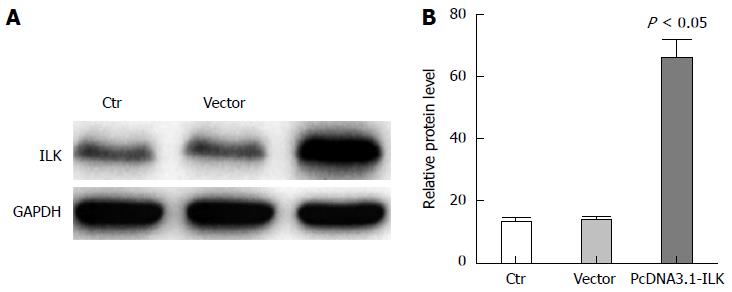
To verify the low expression levels of ILK in stably transfected SW480 cells, there were three transfection treatment groups: SW480, pcDNA3.1, and pcDNA3.1-ILK. After 24 h of transfection, ILK expression level was detected by western blot. Results revealed that ILK expression was significantly increased in the pcDNA3.1-ILK group compared with the vector group (Figure 1A). In addition, grayscale analysis revealed that ILK expression increased in the pcDNA3.1-ILK group, compared with the vector group (Figure 1B), and this difference was statistically significant (P < 0.05).
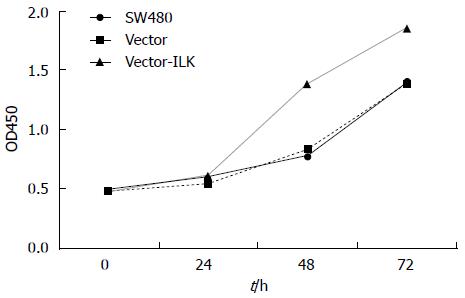
MTT assay was used to detect cell proliferation and validate whether the increase in ILK expression affected the proliferation of SW480 cells. The results (Figure 2) revealed that there was no statistical difference between the vector and control groups. However, the proliferation rate was much higher at 48 and 72 h in the vector-ILK group than in the vector group; and the difference was statistically significant (P < 0.05).
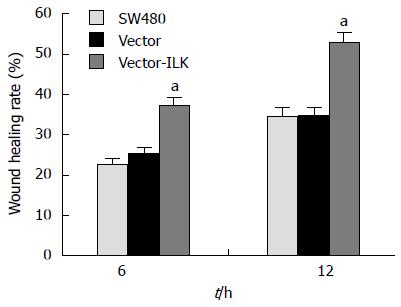
Wound healing assay is an easily performed experiment that can detect migration ability, invasiveness, and metastasis of cells (Figures 3 and 4). Six and 12 h of culture after the scrape was made, the cells migrated much faster in the vector-ILK group than in the control group (P < 0.05).
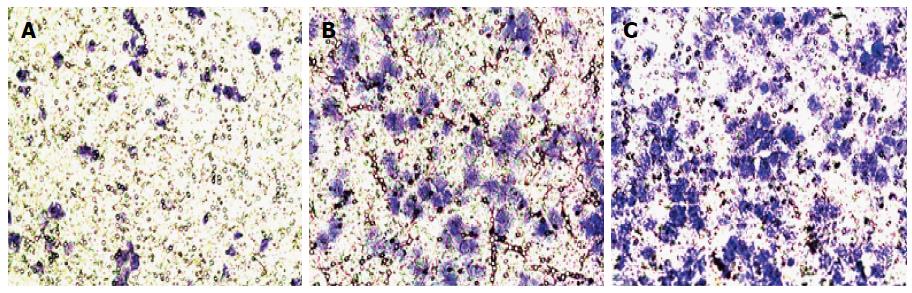
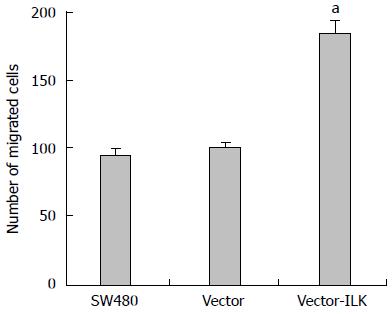
In the transwell method, cells can be induced to migrate from low nutrient culture medium to the high nutrient culture medium, allowing for the detection of the invasion ability of cells (Figures 5 and 6). The number of cells that migrated to the other side of the chamber was slightly higher in the vector group than in the control group, but the difference was not statistically significant (P < 0.05). More cells migrated to the other side of the chamber in the vector-ILK group (P < 0.05).
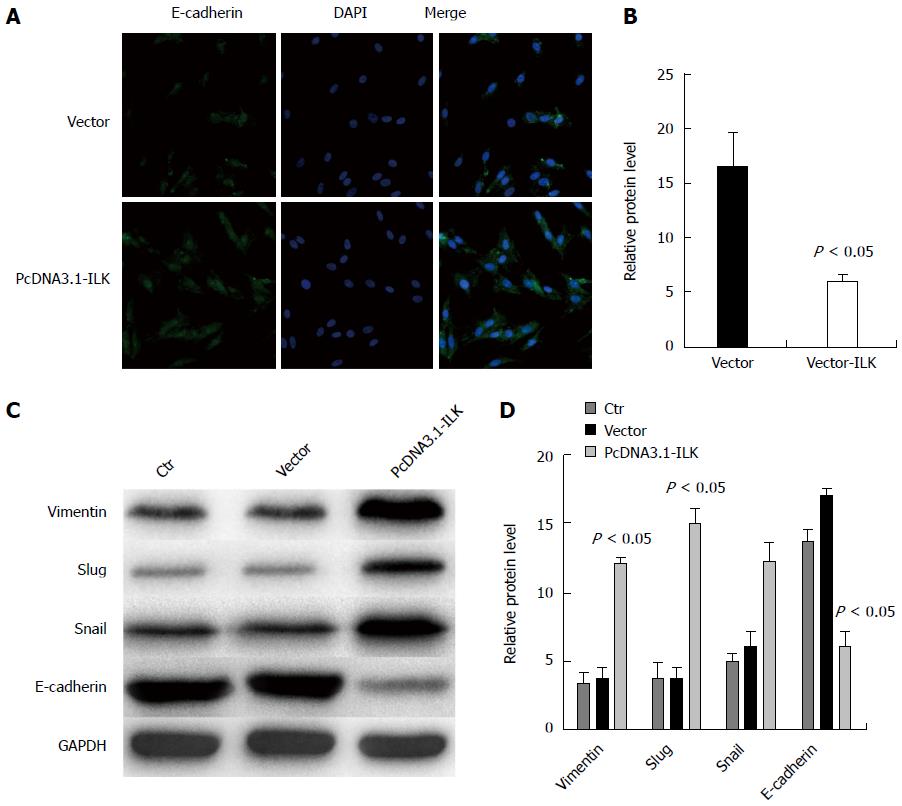
We over-expressed ILK in SW480 cells to investigate whether EMT occurrence and ILK expression in colorectal cancer are correlated. Using immunofluorescence staining, we found that E-cadherin fluorescence intensity was significantly reduced in the overexpressed ILK group (Figure 7B) compared with the no-load group (Figure 7A, P < 0.05). As explained, mutual adhesion between cells decreased, and EMT may have occurred. Therefore, EMT occurrence in colorectal cancer may be mediated by ILK. The transcription factors vimentin, snail, and slug have regulatory roles in cell EMT occurrence. To further validate our results, we overexpressed ILK in SW480 cells and used western blot for protein detection. We found that the expression of vimentin, snail, and slug was increased, while expression of E-cadherin was decreased (Figure 7C). In grayscale analysis, vimentin, snail, slug, and E-cadherin expression differences were statistically significant (P < 0.05, Figure 7D). Experimental results revealed that EMT occurrence in SW480 cells was promoted by ILK.
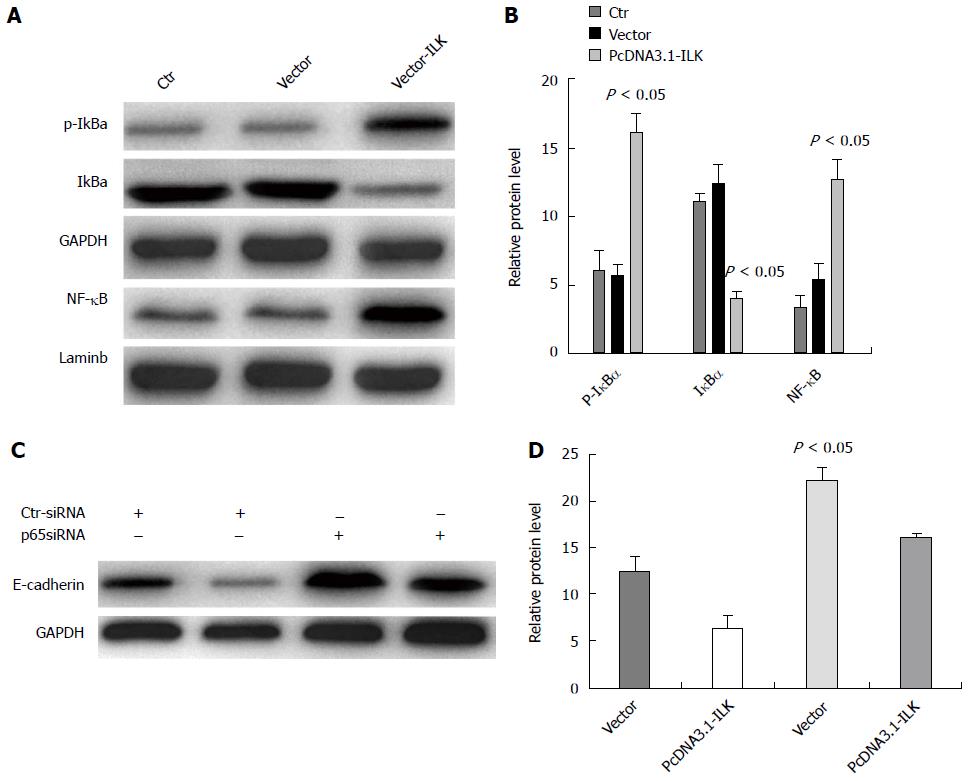
In order to investigate whether ILK overexpression-induced EMT occurrence was directly or indirectly regulated via the NF-κB signaling pathway, we overexpressed ILK in SW480 cells and found using western blot that NF-κBp65 and cytoplasmic phosphorylated p-IκBa expression levels were significantly higher and that cytoplasmic IγBa expression was reduced in the overexpressed ILK group compared with the control group (P < 0.05, Figure 8A and B). In addition, we carried out siRNAp65 interference treatments on SW480 cells and simultaneously overexpressed ILK. By western blot, we found that E-cadherin expression in the vector-ILK cell line increased in the siRNAp65 treated group compared with that in the ILK overexpression group and the empty vector transfection group; and the difference was statistically significant (P < 0.05, Figure 8C and D). Taken together, these results demonstrate that the NF-κB signaling pathway mediated ILK-induced EMT occurrence.
ILK is a vinculin serine/threonine kinase that is highly expressed in malignant tumors[10]. Some researchers have found that the role of ILK in different tumor types is not the same. In progressive pediatric tumors, breast cancer, and rhabdomyosarcoma tumors, ILK has been linked with tumor suppression[3,11,12], whereas in colon cancer, pancreatic cancer, melanoma, prostate cancer, and glioblastoma, ILK plays a role in the promotion of tumor metastasis and erosion[13-15]. Currently, many experts have reported that ILK has carcinogenic effects in colorectal cancer. Furthermore, pathological results have shown that high ILK expression levels are related to colorectal cancer staging, lymph node metastasis, and survival of patients[16,17]. For example, Li et al[6] reported that patients with colorectal cancer who have high ILK expression levels have a shorter survival time compared to patients with colorectal cancer who have low ILK expression levels. However, how ILK influences the migration of colorectal cancer cells and its mechanism remains unclear[6,18,19]. In this study, ILK was overexpressed in the cell line SW480 to detect the proliferation, migration, and invasion ability of cells. Then, immunofluorescence was used to detect E-cadherin levels (a biomarker of EMT) and to explore the possible mechanisms underlying the effects of ILK.
Tumor metastasis and recurrence caused by tumor cell invasion or metastasis are the leading causes of death among cancer patients[20,21]. A metastatic tumor manifests when cancer cells leave the tumor lesion, invade the adjacent tissue, grow, and proliferate in that area[22,23]. A portion of the tumor cells invades the lymphatic system, blood vessels, and body cavity and enters tissues far from the tumor lesion; resulting in the formation of a secondary tumor. In this study, we found that enhancing ILK expression levels in SW480 enhanced the proliferation ability of cells. Wound healing and matrigel invasion assays revealed that enhanced ILK expression levels improved metastasis and invasion of colorectal cancer cells. These results were consistent with some clinical reports in which patients with colorectal cancer who had high ILK expression levels were with poor prognosis.
Many theories can be used to explain the invasion and metastasis of tumors. Among these theories, EMT is a very classical theory[24,25]. EMT is a molecular program in which an epithelial cell loses its intercellular adhesion and acquires a migratory mesenchymal phenotype. E-cadherin expression is affected by this change, and the shape of cells are also converted from an epithelial state to a mesenchyma state; changing the motility of cells[26,27]. EMT plays an important role in the early stage of embryonic development, the invasion and metastasis of advanced tumors, and fibrosis after chronic inflammation[28]. Studies have found that cancer cells overexpress EMT-related genes and that these cells also have an initial cancer metastasis function. After EMT is induced in cells, epithelial cells lose their polarity. At the same time, cell adhesion ability also declines, cells relatively disperse, and migration ability is enhanced[29]. These changes become the main form of local tumor cell invasion and distant metastasis. In colorectal cancer experiments in vitro, ILK was overexpressed in SW480 cells. By western blot, we found that E-cadherin expression was significantly reduced, while vimentin expression was significantly increased; and by immunofluorescence staining, we found that E-cadherin in the overexpressed ILK group was significantly reduced (P < 0.05). It was further demonstrated in colorectal cancer that the increase in ILK expression levels and decrease in E-cadherin expression were linked. Our results showed that ILK can decrease the expression of E-cadherin; thereby promoting EMT, which leads to tumor metastasis and invasion. However, the expression of snail, slug, and other E-cadherin inhibitors was increased. Our results also confirmed that ILK can promote the EMT process in colorectal cancer via upregulation of the expression of snail and slug.
This study found that activation of the NF-κB signaling pathway may directly or indirectly regulate target proteins. Furthermore, activation of NF-κB is related to an aggressive phenotype, and its target proteins include snail and slug. Moreover, the NF-κB pathway plays a crucial role in EMT. Some experts have reported that ILK regulated melanoma angiogenesis via the NF-κB/interleukin (IL)-6 pathway[21]. Our results revealed that ILK overexpression activated the NF-κB signaling pathway, increased NF-κB/p65 levels, increased cytoplasmic levels of phosphorylated p-IκBa, and reduced cytosolic IγBa (P < 0.05). In colorectal cancer, we confirmed that the NF-κB signaling pathway participated in the overexpression of ILK in vitro, which induced EMT occurrence. In addition, we used siRNAp65 knockout experiments to successfully demonstrate that ILK inhibited the expression of E-cadherin partly by activation of the NF-κB signaling pathway. In conclusion, our results confirmed that the overexpression of ILK induces EMT occurrence, which promotes the invasion and metastasis of colorectal cancer in vitro. Moreover, this was partly mediated by the NF-κB signaling pathway; which also shows that the NF-κB pathway plays an important role in EMT in colorectal cancer.
In conclusion, this in vitro colorectal cancer study confirmed that overexpression of ILK can promote EMT occurrence in colorectal cancer cells, which was partly regulated via the NF-κB signaling pathway. As previously described, ILK plays an important role in promoting EMT occurrence in colorectal cancer cells and provides a new therapeutic target for the treatment of colorectal cancer. However, the mechanism of EMT regulatory factor expressions remains unclear and further research is needed.
Integrin-linked kinase (ILK) is a multifunctional receptor protein and a protein interaction integrin. Moreover, ILK can recruit other adapter molecules and regulate a variety of cellular processes via coupled signaling pathways, including cell growth, proliferation, apoptosis, survival, differentiation, migration, and invasion. Recent studies have shown that ILK is overexpressed and excessively activated in a number of human cancers.
Some researchers have found that the role of ILK in different tumors is not the same. In progressive pediatric tumors, breast cancer, and rhabdomyosarcoma tumors, ILK can play a role in tumor suppression. However, in colon cancer, pancreatic cancer, melanoma, prostate cancer, and glioblastoma, ILK plays a role in promoting tumor metastasis and erosion. Currently, many experts have reported that ILK has carcinogenic effects in colorectal cancer. Furthermore, pathological results have shown that high ILK expression levels are related to colorectal cancer staging, lymph node metastasis, and survival of patients.
Overexpression of ILK promotes epithelial-mesenchymal transition (EMT) occurrence in colorectal cancer cells, which was partly regulated via the nuclear factor (NF)-κB signaling pathway.
ILK plays an important factor in the process of promoting EMT occurrence in colorectal cancer cells and provides a new therapeutic target for the treatment of colorectal cancer.
This in vitro colorectal cancer experiment confirms that overexpression of ILK can promote EMT occurrence in colorectal cancer cells, which was partly regulated via the NF-κB signaling pathway. ILK plays an important factor in the process of promoting EMT occurrence in colorectal cancer cells and may provide a new therapeutic target for the treatment of colorectal cancer.
P- Reviewer: Isomoto H, Kim ES S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Li J, Zhang H, Wu J, Guan H, Yuan J, Huang Z, Li M. Prognostic significance of integrin-linked kinase1 overexpression in astrocytoma. Int J Cancer. 2010;126:1436-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Li J, Yang ZL, Ren X, Zou Q, Yuan Y, Liang L, Chen M, Chen S. ILK and PRDX1 are prognostic markers in squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Tumour Biol. 2013;34:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Becker-Santos DD, Guo Y, Ghaffari M, Vickers ED, Lehman M, Altamirano-Dimas M, Oloumi A, Furukawa J, Sharma M, Wang Y. Integrin-linked kinase as a target for ERG-mediated invasive properties in prostate cancer models. Carcinogenesis. 2012;33:2558-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Chen D, Zhang Y, Zhang X, Li J, Han B, Liu S, Wang L, Ling Y, Mao S, Wang X. Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochem. 2013;115:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Zhao D, Tang XF, Yang K, Liu JY, Ma XR. Over-expression of integrin-linked kinase correlates with aberrant expression of Snail, E-cadherin and N-cadherin in oral squamous cell carcinoma: implications in tumor progression and metastasis. Clin Exp Metastasis. 2012;29:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Li R, Liu B, Yin H, Sun W, Yin J, Su Q. Overexpression of integrin-linked kinase (ILK) is associated with tumor progression and an unfavorable prognosis in patients with colorectal cancer. J Mol Histol. 2013;44:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Liu P, Lu J, Cardoso WV, Vaziri C. The SPARC-related factor SMOC-2 promotes growth factor-induced cyclin D1 expression and DNA synthesis via integrin-linked kinase. Mol Biol Cell. 2008;19:248-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | McPhee TR, McDonald PC, Oloumi A, Dedhar S. Integrin-linked kinase regulates E-cadherin expression through PARP-1. Dev Dyn. 2008;237:2737-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Watzka SB, Posch F, Pass HI, Flores RM, Hannigan GE, Bernhard D, Weber M, Mueller MR. Serum concentration of integrin-linked kinase in malignant pleural mesothelioma and after asbestos exposure. Eur J Cardiothorac Surg. 2013;43:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yu YP, Luo JH. Phosphorylation and interaction of myopodin by integrin-link kinase lead to suppression of cell growth and motility in prostate cancer cells. Oncogene. 2011;30:4855-4863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Durbin AD, Somers GR, Forrester M, Pienkowska M, Hannigan GE, Malkin D. JNK1 determines the oncogenic or tumor-suppressive activity of the integrin-linked kinase in human rhabdomyosarcoma. J Clin Invest. 2009;119:1558-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Morgner J, Ghatak S, Jakobi T, Dieterich C, Aumailley M, Wickström SA. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat Commun. 2015;6:8198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Woo JA, Zhao X, Khan H, Penn C, Wang X, Joly-Amado A, Weeber E, Morgan D, Kang DE. Slingshot-Cofilin activation mediates mitochondrial and synaptic dysfunction via Aβ ligation to β1-integrin conformers. Cell Death Differ. 2015;22:1069-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Maydan M, McDonald PC, Sanghera J, Yan J, Rallis C, Pinchin S, Hannigan GE, Foster LJ, Ish-Horowicz D, Walsh MP. Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3beta) phosphorylation. PLoS One. 2010;5:e12356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Albasri A, Al-Ghamdi S, Fadhil W, Aleskandarany M, Liao YC, Jackson D, Lobo DN, Lo SH, Kumari R, Durrant L. Cten signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011;30:2997-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Xiao L, Yue X, Ming X, Xu L, Ding M, Xu J, Liu Q. The integrin-linked kinase gene up-regulated by hypoxia plays its pro-survival role in colorectal cancer cells. J Recept Signal Transduct Res. 2014;34:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Matsui Y, Assi K, Ogawa O, Raven PA, Dedhar S, Gleave ME, Salh B, So AI. The importance of integrin-linked kinase in the regulation of bladder cancer invasion. Int J Cancer. 2012;130:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2388] [Cited by in RCA: 2962] [Article Influence: 211.6] [Reference Citation Analysis (0)] |
| 20. | Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 604] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 21. | Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013;15:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 22. | Waters L, Si Q, Caraway N, Mody D, Staerkel G, Sneige N. Secondary tumors of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: a 10-year experience. Diagn Cytopathol. 2014;42:738-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Kong D, Li Y, Wang Z, Sarkar FH. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins? Cancers (Basel). 2011;3:716-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 24. | Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 743] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 25. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6575] [Cited by in RCA: 7914] [Article Influence: 494.6] [Reference Citation Analysis (0)] |
| 26. | Haraguchi M, Sato M, Ozawa M. CRISPR/Cas9n-Mediated Deletion of the Snail 1Gene (SNAI1) Reveals Its Role in Regulating Cell Morphology, Cell-Cell Interactions, and Gene Expression in Ovarian Cancer (RMG-1) Cells. PLoS One. 2015;10:e0132260. [PubMed] |
| 27. | Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 569] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 28. | Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 7749] [Article Influence: 484.3] [Reference Citation Analysis (0)] |
| 29. | Wani AA, Jafarnejad SM, Zhou J, Li G. Integrin-linked kinase regulates melanoma angiogenesis by activating NF-κB/interleukin-6 signaling pathway. Oncogene. 2011;30:2778-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |