Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3879
Peer-review started: August 27, 2015
First decision: September 29, 2015
Revised: October 14, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: April 14, 2016
Processing time: 215 Days and 16.4 Hours
Rarely has a solitary, metachronous bilateral adrenal metastasis of colorectal cancer been reported. We depict a 41-year-old man who underwent sigmoid colon cancer radical surgery followed by adjuvant chemotherapy for a locally ulcerative sigmoid adenocarcinoma with metachronous bilateral adrenal metastasis revealed by a computed tomography scan. Histopathological examination showed adenocarcinoma, compatible with metastasis from the rectal cancer. The level of serum carcinoembryonic antigen had indicative significance for the presence of adrenal metastasis in the reported series. We performed a literature analysis related to this pathological characteristic and attach importance to consistent, vigilant radiological surveillance of the adrenal glands in the patients’ follow up for colorectal cancer with or without subsequent adrenal metastasis.
Core tip: Very rarely are patients with a solitary adrenal metastasis from colorectal cancer, especially coupled with metachronous contralateral adrenal metastasis, identified. The case report not only covers a rare case of metachronous bilateral isolated adrenal metastasis from colorectal adenocarcinoma but also summarizes and discusses relevant mechanisms and therapies of this type of disease reported previously in the literature.
- Citation: Liu YY, Chen ZH, Zhai ET, Yang J, Xu JB, Cai SR, Song W. Case of metachronous bilateral isolated adrenal metastasis from colorectal adenocarcinoma and review of the literature. World J Gastroenterol 2016; 22(14): 3879-3884
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3879.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3879
The most common primary sites of adrenal metastatic tumors are lung, breast, and kidney, while adrenal metastases from colorectal cancer are not rare findings based on autopsy reports. However, the presence of isolated adrenal metastases from colorectal cancer was quite rare, especially when they are bilateral. We herein report a case of solitary bilateral adrenal metastasis from rectal carcinoma and review the relevant literature.
A 41-year-old man had a sigmoid tumor, as demonstrated by sigmoidoscopy, that was confirmed as an adenocarcinoma by subsequent biopsies. Computed tomography (CT) of the abdomen and pelvis showed no evidence of metastatic disease. A radical resection of the sigmoid colon carcinoma was performed. Histology identified a moderately differentiated ulcerative adenocarcinoma with negative surgical resection margins. The stage was pT4N2M0 (LN: 5/12) according to Tumor, Node, Metastases 2009. His postoperative serum carcinoembryonic antigen (CEA) was reduced to 2.05 μg/L (within the normal limit).
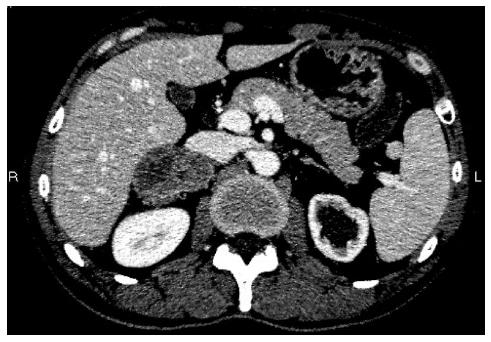
Systemic chemotherapy was initiated after surgery. The patient underwent seven cycles (XELOX) with good clinical and biological tolerance. Eleven months after the completion of the adjuvant chemotherapy, thoracoabdominopelvic CT scan revealed a heterogeneous mass in the right adrenal gland, with no evidence of metastasis elsewhere (Figure 1). A total colonoscopy showed no sign of local recurrence. General blood tests, including adrenal hormones, were normal, but his serum CEA was increased markedly up to 72.02 μg/L.
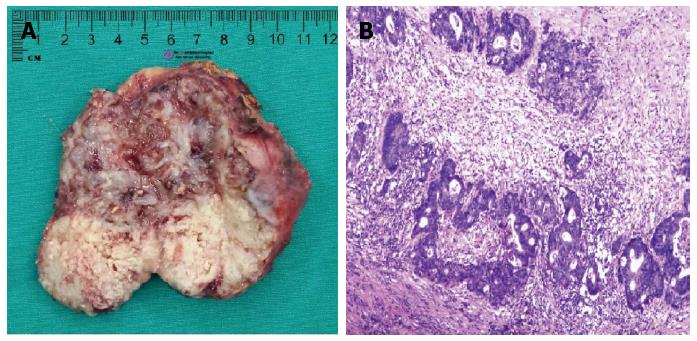
Since chest and abdominal CT scans showed no sign of other metastatic diseases, a resection of the right adrenal neoplasm was performed without complication 18 mo following initial surgery. On macroscopic examination, the tumor, which was 80 mm × 65 mm × 45 mm in size, consisted of a white, solid mass with central necrosis (Figure 2A). Histopathological findings showed an atypical duct that was comprised of high columnar epithelium with necrosis within it (Figure 2B). The adrenal tumor was considered a metastasis from the sigmoid colon cancer based on comparative analysis of histopathological findings of previous sigmoid colon cancer and those of the adrenal tumor.
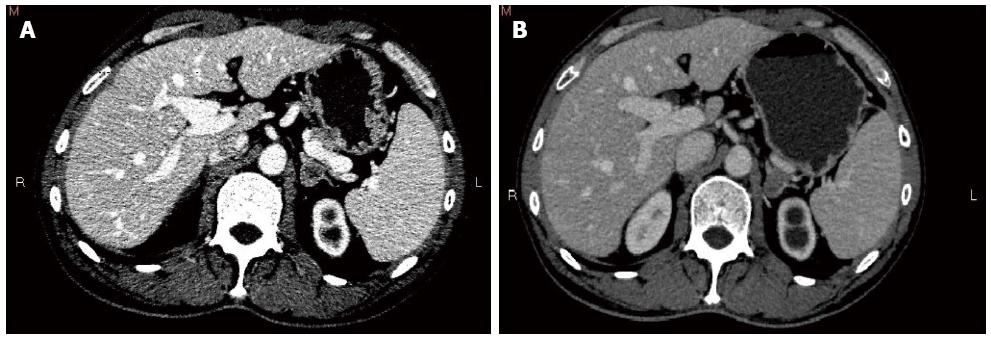
Chemotherapy (XELOX) was taken back. The patient received one cycle, and his general health was good when his serum CEA was 10.33 μg/L. Four months later, surveillance CT identified a left adrenal nodule (Figure 3A) and absence of the previous right adrenal mass, coupled with an expansion of the left kidney and proximal ureter. Left ureteral stent implantation was performed followed by a FOLFIRI chemotherapy regimen that was adopted for the first time. Serum CEA at this time was elevated to 15.84 μg/L.
The patient received four cycles of FOLFIRI with good clinical and biological tolerance. Three months after the completion of the adjuvant chemotherapy, suspicious nodules were noted in the right lung by chest radiography. Meanwhile, thoracoabdominopelvic CT scan revealed that the size of the left adrenal nodule was almost the same as previously measured (Figure 3B). In addition, imaging studies showed local recurrence at the site of the resected sigmoid and expansion to the left kidney and proximal ureter combined with mild atrophy of the left kidney. Serum CEA of the patient was 13.66 μg/L.
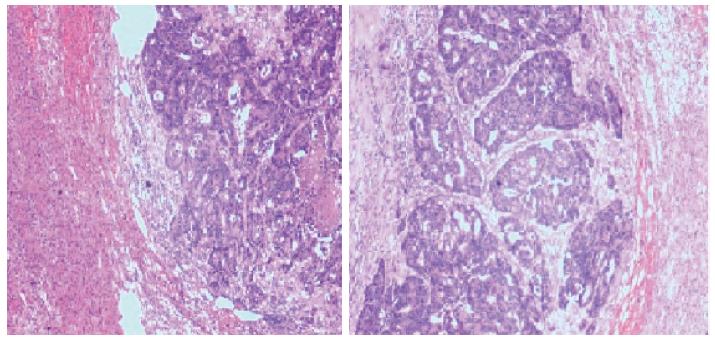
Seven months following removal of the right adrenal mass, an exploratory laparotomy, including neoplasm resection of the left adrenal, was performed. A 50 mm × 35 mm × 30 mm adrenal mass containing several white nodules was confirmed by histology as a moderately differentiated adenocarcinoma, compatible with the previous resected mass specimen of the right adrenal (Figure 4).
The incidence of adrenal metastases of primary malignancies is not rare. The most common primary sites of adrenal metastatic tumors are lung, breast, and kidney. However, adrenal metastases from colorectal carcinomas are less frequent (Table 1). The incidence of adrenal metastases from any primary carcinoma ranges from 8.6% to 27.0%, while that from a colorectal malignant lesion ranges between 1.9% and 17.4%[1]. However, this incidence may be underestimated because of the adrenal mass being mistaken for a lombo-aortic node[1].
A previous study reported that of 47 patients (17%) who received an adrenalectomy for metastatic cancers, eight patients’ adrenal metastases were derived from colorectal cancer[2]. Moreover, another study addressing fine needle aspiration of adrenal masses showed that five of 39 adrenal metastatic malignancies cases (12.8%) originated from colorectal cancer[3]. Lam et al[4] described the prevalence of colorectal cancer in adrenal metastases in both autopsy and biopsy series. Around 5% of metastases were from large intestinal primary sites in an Asian series[4]. In spite of a discrepancy within the reported literature, it is conceivable that the incidence of adrenal metastases from colorectal carcinomas is not rare. Several studies have demonstrated that the vast majority of autopsies with adrenal metastases also involved metastases of multiple other sites.
It is acknowledged that the spread of adrenal metastases occurs via the arterial, portal venous, or lymphatic routes. Generally, hematogenous spread is considered the major route of primary carcinoma metastasis to the adrenal. Katayama et al[5] described a plausible route of hematogenous metastasis from the primary site via the lungs to the adrenals. Although the lung metastasis in our patient was not detected until after the left adrenal metastasis, it is possible that a silent lung metastasis had gradually occurred when the adrenal metastasis was detected. In general, adrenal metastasis is thought to indicate widespread disease; and, hence, patients with a solitary adrenal metastasis from colorectal carcinoma are rare, especially coupled with metachronous contralateral adrenal metastasis, according to our review of the literature[5-10].
Serum CEA is thought to be essential in predicting the presence of adrenal metastasis in the postoperative period of colorectal cancer[5,9,10]. As in our patient, serum CEA levels indicated the presence of adrenal metastases in the reported series, which was confirmed by the imaging findings. Therefore, early detection and resection of the adrenal metastasis based on regular follow-up could improve the prognosis to some extent despite systemic diseases. In addition, attention should be paid to the occurrence of adrenal metastasis series after a relatively long-term course. However, there have been multiple cases in which serum CEA level remains within the normal range in spite of an occurrence of adrenal metastasis[11-13]. Therefore, a variety of modalities combined with serum CEA levels should be applied to accurately detect the status of colorectal cancer.
The past several years have witnessed a substantial improvement in the detection of clinically quiescent adrenal masses owing to the common use of imaging modalities such as CT, positron emission tomography (PET) CT, and magnetic resonance imaging (MRI).
Among various radiological studies used to assess adrenal metastases, CT and ultrasonography are prevalently preferred due to their wide applicability and non-invasive nature[14]. On CT, our patient displayed low density of the adrenal gland, consistent with adrenal masses in other reports[3,15,16]. CT scan allows for diagnosis of the adrenal masses with accuracy to sub-centimetric lesions. However, it fails to distinguish between primary lesions, incidentalomas, or secondary lesions. Percutaneous fine needle-biopsy was, therefore, proposed for the differential diagnosis by attaining histological confirmation[17]. This procedure was not performed on our patient since the CT characteristics and the story of the disease were sufficient to support the diagnosis of adrenal metastases.
MRI is also an effective procedure for appraising the tumors in adrenal glands. It plays a significant role in distinguishing between adenoma, carcinoma, and metastasis in cases of “adrenal incidentaloma”. On the other hand, it allows for identification of the functional nature of the adrenal mass when an adrenal incidentaloma is found[18,19].
The emergence of PET-CT for detection and surveillance has allowed for more accurate specifics of adrenal metastases, with better characterizations of the metabolic activities. This approach permits choosing an adapted treatment and avoiding a useless laparotomy in cases of generalized metastases[20,21].
Adrenal metastasis from colorectal malignancy is not uncommon in autopsy series. Therefore, adrenal metastasis should be tested in the follow-up of colorectal cancer patients who undergo an initial surgery, even though the lung and liver are the primary metastatic sites. For early detection of adrenal metastasis, measuring of serum CEA and imaging examination, such as ultrasonography and CT, should be done routinely.
Although it is generally thought that an adrenal metastasis is part of a widespread metastasis[8,22,23], the secondary adrenal gland lesions may be involved in the lung metastases and even neoplastic invasion of other organs without negative effect on the prognosis[24]. A resection of the involved adrenal gland is supposed to contribute to a good prognosis when a separate adrenal metastasis is found[8-10]. Therefore, it is essential to make allowance for the possibility of adrenal metastasis from colorectal cancer, especially a delayed contralateral adrenal metastasis when unilateral adrenal was implicated, during follow-up after the primary operation. Our patient is currently in stable condition, and further surveillance is needed because this case has been observed for only half a year.
In conclusion, it is suggested in this case that separate, metachronous metastases in bilateral adrenal glands may not achieve desired results through chemotherapy alone but surgical resection and continual follow-up as well. A number of cases of similar adrenal metastasis are needed to elucidate this issue.
A 41-year-old man presented with metachronous bilateral adrenal metastases with a history of sigmoid tumor resection and chemotherapy.
Bilateral adrenal metastases from sigmoid tumor.
Pheochromocytoma and adrenocortical carcinoma
All of the laboratory results were within the normal ranges, with the exception of a fluctuant carcinoembryonic antigen level.
Abdominal computed tomography demonstrated heterogeneous masses in bilateral adrenal glands heterochronously.
Bilateral adrenal tumors were confirmed as a metastasis from the sigmoid colon cancer through comparative analysis of histopathological findings of previous sigmoid colon cancers and those of the adrenal tumors.
Resection of bilateral adrenal tumor with chemotherapy.
This report presents a rare case of metachronous bilateral adrenal metastases from colorectal cancer and improves our understanding of this disease process.
This rare case indicates that resection of the involved adrenal gland improves prognosis when a separate adrenal metastasis is found. Additionally, it is essential to consider the possibility of adrenal metastasis from colorectal cancer during follow-up after the primary operation.
P- Reviewer: Lam AK S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma S
| 1. | Murakami S, Terakado M, Hashimoto T, Tsuji Y, Okubo K, Hirayama R. Adrenal metastasis from rectal cancer: report of a case. Surg Today. 2003;33:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Wade TP, Longo WE, Virgo KS, Johnson FE. A comparison of adrenalectomy with other resections for metastatic cancers. Am J Surg. 1998;175:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Candel AG, Gattuso P, Reyes CV, Prinz RA, Castelli MJ. Fine-needle aspiration biopsy of adrenal masses in patients with extraadrenal malignancy. Surgery. 1993;114:1132-1136; discussion 1136-1137. [PubMed] |
| 4. | Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf). 2002;56:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 299] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Katayama A, Mafune K, Makuuchi M. Adrenalectomy for solitary adrenal metastasis from colorectal carcinoma. Jpn J Clin Oncol. 2000;30:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Omoigui NA, Cave WT, Chang AY. Adrenal insufficiency. A rare initial sign of metastatic colon carcinoma. J Clin Gastroenterol. 1987;9:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lo CY, van Heerden JA, Soreide JA, Grant CS, Thompson GB, Lloyd RV, Harmsen WS. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Moreno-Elola A, Moreno-Gonzalez E, Alonso-Casado O, Meneu-Diaz JC, García-García I, Abradelo-Usera M. Exceptional bilateral adrenalectomy after secondaries from colorectal cancer. Hepatogastroenterology. 2004;51:103-105. [PubMed] |
| 9. | Watatani M, Ooshima M, Wada T, Terashita H, Matsuda T, Shindo K, Yasutomi M. Adrenal metastasis from carcinoma of the colon and rectum: a report of three cases. Surg Today. 1993;23:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Fujita K, Kameyama S, Kawamura M. Surgically removed adrenal metastasis from cancer of the rectum. Report of a case. Dis Colon Rectum. 1988;31:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Thrumurthy SG, Jadav AM, Pitt M, Dobson M, Hearn A, Scott NA, Susnerwala SS. Metachronous bilateral adrenal metastases following curative treatment for colorectal carcinoma. Ann R Coll Surg Engl. 2011;93:e96-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Jabir H, Tawfiq N, Moukhlissi M, Akssim M, Guensi A, Kadiri B, Bouchbika Z, Taleb A, Benchekroun N, Jouhadi H. Metachronous bilateral isolated adrenal metastasis from rectal adenocarcinoma: a case report. Case Rep Gastrointest Med. 2014;2014:516403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Capaldi M, Ricci G, Bertolini R, Alessandroni L, Di Castro A, Saraco E, Guiducci A, Tersigni R. Colon cancer adrenal metastasis: case report and review of the literature. G Chir. 2011;32:361-363. [PubMed] |
| 14. | Francis IR, Smid A, Gross MD, Shapiro B, Naylor B, Glazer GM. Adrenal masses in oncologic patients: functional and morphologic evaluation. Radiology. 1988;166:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Chezmar JL, Robbins SM, Nelson RC, Steinberg HV, Torres WE, Bernardino ME. Adrenal masses: characterization with T1-weighted MR imaging. Radiology. 1988;166:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Brunt LM, Moley JF. Adrenal incidentaloma. World J Surg. 2001;25:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Terzolo M, Ali A, Osella G, Mazza E. Prevalence of adrenal carcinoma among incidentally discovered adrenal masses. A retrospective study from 1989 to 1994. Gruppo Piemontese Incidentalomi Surrenalici. Arch Surg. 1997;132:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Sheeler LR, Myers JH, Eversman JJ, Taylor HC. Adrenal insufficiency secondary to carcinoma metastatic to the adrenal gland. Cancer. 1983;52:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Chong S, Lee KS, Kim HY, Kim YK, Kim BT, Chung MJ, Yi CA, Kwon GY. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics. 2006;26:1811-1824; discussion 1824-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, Sedlackova E, Lipska L, Stahalova V. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Wade TP, Virgo KS, Li MJ, Callander PW, Longo WE, Johnson FE. Outcomes after detection of metastatic carcinoma of the colon and rectum in a national hospital system. J Am Coll Surg. 1996;182:353-361. [PubMed] |
| 22. | Berge T, Ekelund G, Mellner C, Pihl B, Wenckert A. Carcinoma of the colon and rectum in a defined population. An epidemiological, clinical and postmortem investigation of colorectal carcinoma and coexisting benign polyps in Malmö, Sweden. Acta Chir Scand Suppl. 1973;438:1-86. [PubMed] |
| 23. | Cedermark BJ, Blumenson LE, Pickren JW, Holyoke DE, Elias EG. Ths significance of metastases to the adrenal glands in adenocarcinoma of the colon and rectum. Surg Gynecol Obstet. 1977;144:537-546. [PubMed] |
| 24. | Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998;82:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |