Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3852
Peer-review started: December 7, 2015
First decision: December 31, 2015
Revised: January 11, 2016
Accepted: February 20, 2016
Article in press: February 22, 2016
Published online: April 14, 2016
Processing time: 113 Days and 18.4 Hours
AIM: To investigate the expression of integrin αvβ6 and matrix metalloproteinase 9 (MMP-9), their association with prognostic factors and to assess their predictive role in gastric cancer patients.
METHODS: Immunohistochemistry was used to determine the expressions of integrin αvβ6 and MMP-9 in 126 specimens from patients with primary gastric carcinoma. Associations between immunohistochemical staining and various clinic pathologic variables of tissue specimens were evaluated by the χ2 test and Fisher’s exact test. Expression correlation of αvβ6 and MMP-9 was assessed using bivariate correlation analysis. The patients were followed-up every 3 mo in the first two years and at least every 6 mo afterwards, with a median follow-up of 56 mo (ranging from 2 mo to 94 mo). Four different combinations of αvβ6 and MMP-9 levels (that is, both markers positive, both markers negative, αvβ6 positive with MMP-9 negative, and αvβ6 negative with MMP-9 positive) were evaluated for their relative effect on survival. The difference in survival curves was evaluated with a log-rank test. Survival analysis was conducted using the Kaplan-Meier survival and Cox proportional hazards model analysis.
RESULTS: The expressions of integrin αvβ6 and MMP-9 were investigated in 126 cases, among which 34.92% were positive for αvβ6 expression, and 42.06% for MMP-9 expression. The expression of αvβ6 was associated with Lauren type, differentiation, N stage, and TNM stage (the P values were 0.006, 0.038, 0.016, and 0.002, respectively). While MMP-9 expression was associated with differentiation, T stage, N stage, and TNM stage (the P values were 0.039, 0.014, 0.033, and 0.008, respectively). The positive correlation between αvβ6 and MMP-9 in gastric cancer was confirmed by a correlation analysis. The Kaplan-Meier survival analysis showed that patients with expression of αvβ6 or MMP-9 alone died earlier than those with negative expression and that patients who were both αvβ6 and MMP-9 positive had a shorter overall survival than those with the opposite pattern (both αvβ6 and MMP-9 negative) (P = 0.000). A Cox model indicated that positive expression of αvβ6 and MMP-9, diffuse Lauren type, as well as a senior grade of N stage, M stage, and TNM stage were predictors of a poor prognosis in univariate analysis. Only αvβ6 and MMP-9 retained their significance when adjustments were made for other known prognostic factors in multivariate analysis (RR = 2.632, P = 0.003 and RR = 1.813, P = 0.007).
CONCLUSION: The expression of αvβ6 and MMP-9 are closely correlated, and the combinational pattern of αvβ6 and MMP-9 can serve as a more effective prognostic index for gastric cancer patients.
Core tip: Both integrin αvβ6 and matrix metalloproteinase 9 (MMP-9) play an important role in the development and progression of cancer. However, the combined effect of the two proteins in gastric cancer is not yet clear. We would like to draw attention to this aspect. In the present study, we demonstrated that the expression of αvβ6 and MMP-9 are closely correlated, and the combinational pattern of αvβ6 and MMP-9 can serve as a more effective prognostic index for gastric cancer patients.
- Citation: Lian PL, Liu Z, Yang GY, Zhao R, Zhang ZY, Chen YG, Zhuang ZN, Xu KS. Integrin αvβ6 and matrix metalloproteinase 9 correlate with survival in gastric cancer. World J Gastroenterol 2016; 22(14): 3852-3859
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3852
Gastric cancer is the fourth most common cancer world-wide and accounts for approximately 989600 cases annually, with more than half of these cases occurring in East Asia. Gastric cancer also ranks as the second leading cause of cancer-related death[1]. Clinical management of gastric cancer is, to some extent, based on the availability of markers that are predictive of prognosis and alterations commonly present in gastric cancer have the potential to serve such a purpose.
Integrin αvβ6 is a member of the integrin family and is expressed in epithelial cells only, with fibronectin (FN) as its major ligand. The expression of αvβ6 is rare and can hardly be detected in normal epithelial cells[2], but increases substantially in response to injury and/or inflammation and in epithelial tumors[3]. Previous studies have shown that the de novo expression of integrin αvβ6 is involved in the pathogenic processes of gastrointestinal malignancies, including cell adhesion, proliferation, apoptosis, and matrix metalloproteinase (MMPs) secretion[4-6].
Similar to the integrin family, members of the MMP family, including MMP-9, have been implicated in tumor invasion and metastasis, which are characterized by a zinc atom at the active site and classified according to homologies in sequence and substrate affinity[7]. MMP-9 has been confirmed to be associated with the malignant behavior of tumors or the eventual clinical outcome of patients afflicted with the disease and has a unique ability to degrade type IV collagen, which is a major component of the basement membrane[8].
Our prior research has suggested that αvβ6 integrin is an independent prognostic indicator of gastric cancer[9]. Since that initial report, limited work has been conducted to highlight the predictive value of αvβ6 in gastrointestinal cancers, until the research of Peng et al[10] on colorectal cancer. The fact that no index alone can be simply explicable in terms of its effect on cancer prognosis prompted us to focus on the combined analysis of both αvβ6 and MMP-9. In the present study, we undertook an immunohistochemical analysis of αvβ6 and MMP-9 expression in gastric cancer. Their correlation with clinical characteristics and prognosis was assessed to determine whether the combinational pattern of αvβ6 and MMP-9 expression could be used as a more effective prognostic index for gastric cancer patients after curative surgery.
The mouse monoclonal integrin αvβ6 antibody 6.2A1 (1:500, Biogen, Cambridge, MA, United States)[9] and rabbit polyclonal MMP-9 antibody ab38898 (1:100, Abcam, Cambridge, MA, United States)[11] were used for integrin αvβ6 and MMP-9 immunohistochemical staining, respectively. Specificity of the two staining patterns was confirmed by the use of irrelevant control antibodies.
Immunohistochemistry was performed on 5 micrometer sections of the formalin-fixed, paraffin-embedded routine sections, and tissue microarray (TMA) analysis using antibodies against αvβ6 (6.2A1) and MMP-9 (ab38898) was applied. The sections were deparaffinized and hydrated, and the heat induced epitope retrieval was performed with Borg decloaking high pH buffer in the Biocare decloaking chamber. Subsequently, endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and then the slides were incubated first with an avidin-biotin kit, followed by incubations with the αvβ6 primary antibody (1:500) and the MMP-9 primary antibody (1:100) overnight at 4 °C, streptavidin-horseradish peroxidase, and betazoid diaminobenzidine for color development. Negative controls were prepared by using the identical concentration of mouse immunoglobulin IgG1 (Dako). The next day, biotinylated anti-mouse IgG and anti-rabbit IgG (1:200) were applied to the slides, which were subsequently treated using horseradish peroxidase (HRP)-labelled streptoantibiotin (Dako) for 15 min. Finally, all the slides were counterstained with Dako hematoxylin, rinsed with water, and then dehydrated with alcohol and xylene, and cover-slipped. Appropriate controls were included. All incubations were conducted at room temperature.
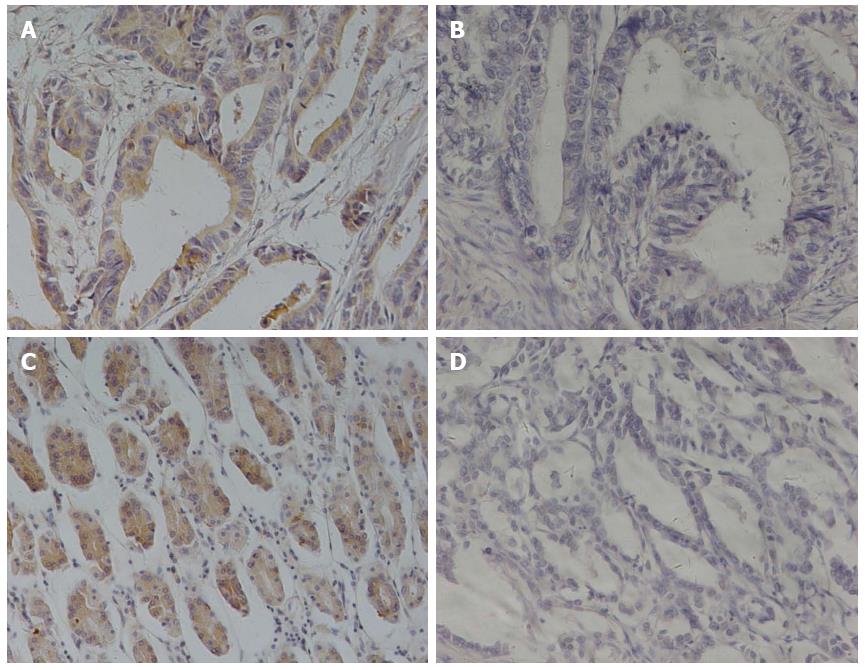
Assessment of the two proteins’ staining was evaluated independently by two pathologists who were blinded to the patients’ clinical outcome. Disagreements were resolved by discussion in a meeting to obtain the final results. The staining stratification was established based on two scores: (1) the proportion score representing the fraction of positive staining cells (0, 0%; 1, < 20%; 2, 20%-50%; 3, 51%-75%; and 4, > 75%, respectively); and (2) the intensity of the staining (0, no staining; 1, weak staining, pale brown; 2, moderate staining, brown; and 3, strong staining, dark brown, respectively). Such assessment allows for a semi-quantitative estimate of the expression levels of protein in the tissue section. The two scores were added, and the final definition of every section was obtained. Finally, tumors with a final score of < 2 were designated as negative, and > 2 as positive, in both αvβ6 and MMP-9 expression.
A series of consecutive specimens from tumors of 126 primary gastric cancer patients, with curative resection, were obtained from the Department of Pathology, Qilu Hospital, Shandong University (Jinan, China), from January 2007 to June 2010 and were formalin-fixed and paraffin-embedded. All of the specimens were procured before any adjuvant treatment and after the informed consent from the patients, with the approval of the Ethics Committee of Qilu Hospital.
According to our protocol, all patients were followed up every 3 mo in the first two years and at least every 6 mo afterwards, until June 2015, with a median follow-up of 56 mo (ranging from 2 mo to 94 mo). They were examined by regular abdomen ultrasonography or computed tomography (CT), monitoring the relapse of the disease. Observations were censored at either the last follow-up date or the terminal date of the follow-up if death had not occurred.
Statistical analyses were performed with SPSS 13.0. Associations between immunohistochemical staining and clinicopathologic variables of tissue specimens were evaluated by the χ2 test and Fisher’s exact test. Expression correlation of αvβ6 and MMP-9 was assessed using bivariate correlation analysis. The difference in survival curves was evaluated with a log-rank test. Survival analysis was conducted using the Kaplan-Meier survival and Cox proportional hazards model analysis. A value of P < 0.05 was considered to be statistically significant.
We characterized the expression of αvβ6 and MMP-9 by immunohistochemical staining in gastric cancer from 126 patients (Figure 1), aged from 27 to 81. Of these, 44 (34.92%) were positive for αvβ6 expression, with 82 (65.08%) being negative. For MMP-9, 53 (42.06%) patients were positive for expression, and 73 (57.93%) were negative.
As in our clinical correlation studies, the immunohistochemical analysis was conducted without knowledge of any clinical information regarding prognostic outcome. Levels of the two proteins’ expression were compared with tumor characteristics and risk factors (Table 1). The following analysis showed that Lauren type, differentiation, N stage, and TNM stage were associated with αvβ6 expression (the P values were 0.006, 0.038, 0.016, and 0.002, respectively). Regarding MMP-9 expression, statistically significant differences existed in differentiation, T stage, N stage, and TNM stage (the P values were 0.039, 0.014, 0.033, and 0.008, respectively). No evidence of a significant association was observed between alteration of αvβ6 or MMP-9 expression and other characteristics or risk factors.
| Characteristic | No. | αvβ6 | P value | MMP-9 | P value | ||
| Negative | Positive | Negative | Positive | ||||
| Age (yr) | |||||||
| < 60 | 51 | 33 | 18 | 31 | 20 | ||
| > 60 | 75 | 49 | 26 | 0.942 | 42 | 33 | 0.593 |
| Gender | |||||||
| Male | 78 | 50 | 28 | 43 | 35 | ||
| Female | 48 | 32 | 16 | 0.769 | 30 | 18 | 0.416 |
| Location | |||||||
| Upper | 27 | 20 | 7 | 16 | 11 | ||
| Middle | 51 | 28 | 23 | 28 | 23 | ||
| Lower | 48 | 34 | 14 | 0.136 | 29 | 19 | 0.847 |
| Lauren type | |||||||
| Intestinal | 83 | 47 | 36 | 46 | 37 | ||
| Diffuse | 43 | 35 | 8 | 0.006 | 27 | 16 | 0.427 |
| Differentiation | |||||||
| Well | 19 | 15 | 4 | 6 | 13 | ||
| Moderate | 30 | 14 | 16 | 18 | 12 | ||
| Poor | 77 | 53 | 24 | 0.038 | 49 | 28 | 0.039 |
| T stage | |||||||
| T1 | 14 | 10 | 4 | 3 | 11 | ||
| T2 | 13 | 7 | 6 | 8 | 5 | ||
| T3 | 18 | 10 | 8 | 14 | 4 | ||
| T4 | 81 | 55 | 26 | 0.578 | 48 | 33 | 0.014 |
| N stage | |||||||
| N0 | 37 | 26 | 11 | 18 | 19 | ||
| N1 | 17 | 12 | 5 | 10 | 7 | ||
| N2 | 26 | 10 | 16 | 11 | 15 | ||
| N3 | 46 | 34 | 12 | 0.016 | 34 | 12 | 0.033 |
| M stage | |||||||
| M0 | 124 | 81 | 43 | 73 | 51 | ||
| M1 | 2 | 1 | 1 | 0.652 | 0 | 2 | 0.094 |
| TNM stage | |||||||
| I | 12 | 4 | 8 | 3 | 9 | ||
| II | 42 | 36 | 6 | 22 | 20 | ||
| III | 70 | 41 | 29 | 48 | 22 | ||
| IV | 2 | 1 | 1 | 0.002 | 0 | 2 | 0.008 |
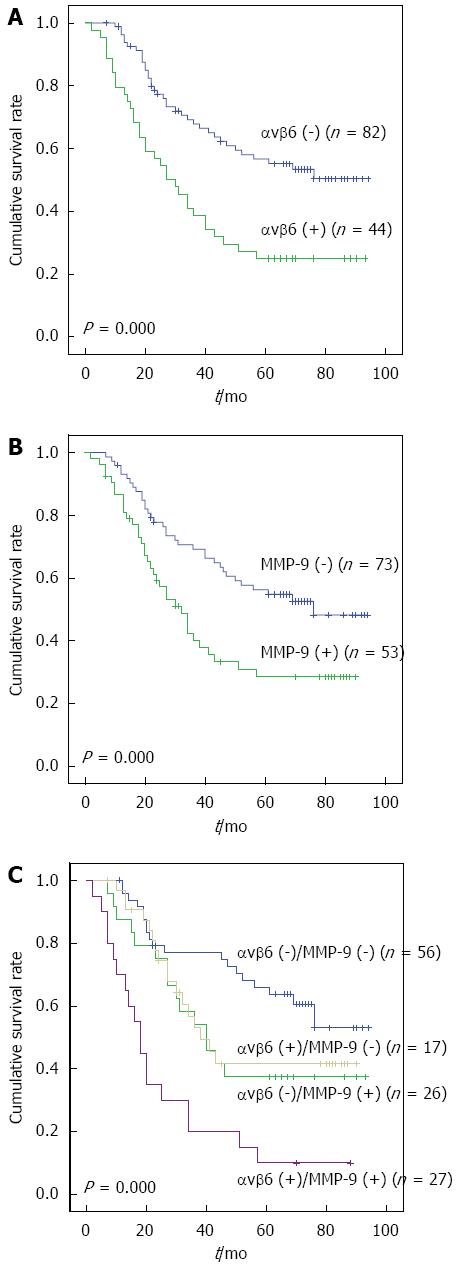
The Kaplan-Meier plot (Figure 2A and B) showed that patients with positive αvβ6 expression had a much shorter survival time than the negative cases (P = 0.000), consistent with our previous study[9]. The same tendency was present in MMP-9 positive expression cases (P = 0.000).
The expression of αvβ6 and MMP-9 were closely correlated in gastric cancers (r = 0.286, P = 0.001) (Table 2), which indicated that the combined analysis of the two proteins may be more helpful in predicting the survival for this disease. A striking stratification of mortality risk was observed when four different combinations of αvβ6 and MMP-9 levels (that is, both markers positive, both markers negative, αvβ6 positive with MMP-9 negative, and αvβ6 negative with MMP-9 positive) were evaluated for their relative effect on survival. Kaplan-Meier plots showed that patients who were both αvβ6 and MMP-9 positive died earlier (26.45 ± 5.54) than the other three groups (53.40 ± 5.90, 51.54 ± 6.86, and 68.86 ± 4.87, respectively; P = 0.000). The greatest difference in survival was found between patients with both markers being positive and those with the opposite pattern (both markers negative) (Figure 2C).
| αvβ6 expression | MMP-9 expression | Total | |
| Negative | Positive | ||
| Negative | 56 | 26 | 82 |
| Positive | 17 | 27 | 44 |
| Total | 73 | 53 | 126 |
Sufficient clinical follow-up data were obtained from all 126 patients, allowing for evaluation of the association between protein alteration and prognostic outcome. Of these, 69 (54.8%) cases were confirmed as cancer-related deaths within 5 years of prognosis and 57 (45.2%) were censored, as the follow-up survey was discontinued or patients were alive beyond 60 mo or died of reasons other than this disease.
We estimated the relative risks (RR) (both univariate and multivariate) of dying and 95%CI, using the Cox proportional hazard model (Table 3). The Cox model indicated that positive expressions of αvβ6 and MMP-9, diffuse Lauren as well as high grades of N, M, and TNM stages were predictors of a poor prognosis in the univariate analysis. Then, the variables with statistical values of P < 0.05 were chosen for multivariate analysis, and only αvβ6 and MMP-9 retained their significance after adjustments for other known prognostic factors, which revealed that positive expression of αvβ6 and MMP-9 were unfavorable independent prognostic factors (P = 0.003 and P = 0.007, respectively) and superior to all other known prognostic factors.
| Factors | Univariate analysis | Multivariate analysis | ||
| Relative risk (95%CI) | P value | Relative risk (95%CI) | P value | |
| Age (yr) | ||||
| < 60 | 1 | |||
| > 60 | 1.800 (1.090-2.973) | 0.258 | ||
| Gender | ||||
| Female | 1 | |||
| Male | 1.450 (0.882-2.381) | 0.143 | ||
| Location | ||||
| Upper | 1 | |||
| Middle/Lower | 1.171 (0.856-1.602) | 0.323 | ||
| Lauren type | ||||
| Intestinal | 1 | |||
| Diffuse | 1.836 (1.061-3.177) | 0.03 | 1.431 (0.805-2.545) | 0.222 |
| Differentiation | ||||
| Well | 1 | |||
| Moderate/Poor | 1.045 (0.764-1.429) | 0.784 | ||
| T stage | ||||
| T1 | 1 | 1 | ||
| T2-4 | 1.295 (1.006-1.668) | 0.050 | 1.248 (0.813-1.916) | 0.311 |
| N stage | ||||
| N0 | 1 | 1 | ||
| N1-3 | 1.343 (1.105-1.633) | 0.003 | 1.249 (0.818-1.909) | 0.303 |
| M stage | ||||
| M0 | 1 | 1 | ||
| M1 | 26.25 (2.887-238.81) | 0.004 | 8.555 (0.735-99.62) | 0.087 |
| TNM stage | ||||
| I | 1 | 1 | ||
| II-IV | 2.314 (1.502-3.565) | < 0.001 | 1.654 (0.643-4.257) | 0.296 |
| αvβ6 | ||||
| Negative | 1 | 1 | ||
| Positive | 2.862 (1.452-3.747) | < 0.001 | 2.632 (1.569-4.416) | 0.003 |
| MMP-9 | ||||
| Negative | 1 | 1 | ||
| Positive | 2.193 (1.110-2.787) | 0.005 | 1.813 (1.398-2.351) | 0.007 |
Molecular markers associated with distinct clinical outcomes have been identified in gastric cancer, laying the groundwork for improved clinical management through more personalized medicine. Despite improvements during the past years, the prognostic outcome of gastric cancer remains unsatisfactory. Molecular investigation of cancerous pathogenesis would contribute to the treatment of patients with gastric cancer[12], which suggests that the current molecular research is far from sufficient.
Considerable evidence now exists that alteration in the expression of αvβ6 or MMP-9 may serve as an important pathogenic event for some human malignancies. We have previously shown that αvβ6 is an independent prognostic indicator in gastric cancer[9] and simultaneously extended its pathogenic role to mediating the potential for colon cancer cells to colonize in and metastasize to the liver[13]. When it came to MMP-9, a number of studies have been published on its prognostic value in human cancers. High expression of MMP-9 was found to be associated with lymph node metastasis and a higher tumor stage in breast cancer[14,15]. Moreover, MMP-9 is also implicated in the progression of esophageal squamous cell carcinoma[16]. Meanwhile, MMP-9 status represents a novel prognostic factor in evaluation of colorectal cancer[17], especially in combination with the membrane-anchored MMP regulator RECK[18]. As to the prognostic role of MMP-9 in gastric cancer, a consensus has not been reached in previous reports[19-23]. Given the variable natural history and heterogeneity of gastric cancer, conflicting studies published to date suffer from a similar problem: the interference from neoadjuvant chemotherapy, which was recently confirmed[24,25]. To exclude the possibility of chemotherapy interfering with the two proteins’ predictive role in gastric cancer, only patients who had not received neoadjuvant chemotherapy were enrolled.
In the present study, the expression of integrin αvβ6 and MMP-9 in 126 gastric cancer specimens was investigated, which showed a 34.92% incidence of αvβ6 expression, concordant with previously published positive rates of 36.7% in gastric cancer[9], as well as a 42.06% incidence of MMP-9 expression. Subsequently, a positive correlation between the two proteins was confirmed, and each of them was associated with a poor prognosis in gastric cancer. When comparing different combinational expression patterns in the Kaplan-Meier survival analysis, patients who were both αvβ6 and MMP-9 positive suffered a higher mortality rate than the other three expression states, especially in comparison to the opposite pattern. Furthermore, a Cox model indicated that positive expression of αvβ6 and MMP-9, diffuse Lauren type, as well as a higher grade of N, M, and TNM stage were predictors of a poor prognosis in univariate analysis. Only αvβ6 and MMP-9 retained their significance when adjustments were made for other known prognostic factors in multivariate analysis. Based on these data, we proposed that the concomitant expression of αvβ6 and MMP-9 in gastric cancer could serve as a more effective independent predictor to determine early relapse, metastases and prognosis in gastric cancer patients.
As an exploratory attempt, this paper intends to provide some enlightenment for the clinical management of gastric cancer patients in the future. We have previously confirmed a direct linkage between ERK2 and the cytoplasmic domain of β6, as well as the binding domain for ERK2 within the cytoplasmic tail of the β6-integrin subunit[26,27], through which integrin αvβ6 transmitted a growth enhancing signal to cancer cells. Previous studies have shown that the expression of MMP-9 has a direct correlation to integrin αvβ6[5,28]. Thus, a signaling pathway mediated by ERK2 between αvβ6 and MMP-9 was theoretically supposed to exist to play a key role in the biological behavior and/or clinical outcome of human gastric cancer.
In summary, we concur with Zhang et al[22] that αvβ6 should be a focus of attention for diagnosis and therapy in human gastric cancers; our current findings extend this idea to the combination of αvβ6 and MMP-9. The expression of αvβ6 and MMP-9 are closely correlated, and the combinational pattern of αvβ6 and MMP-9 can serve as a more effective prognostic index for gastric cancer patients. However, further research is necessary to identify the signaling pathway between αvβ6 and MMP-9 as an important target for gastric cancer therapy.
Both integrin αvβ6 and matrix metalloproteinase 9 (MMP-9) play an important role in the development and progression of cancer. However, the combined effect of the two proteins in gastric cancer is not yet clear. The fact that no index alone can be simply explicable in terms of its effect on cancer prognosis prompted us to focus on the combined analysis of both αvβ6 and MMP-9.
The prior research by the authors suggested that αvβ6 integrin is an independent prognostic indicator of gastric cancer. Since that initial report, limited work has been conducted to highlight the predictive value of αvβ6 in gastrointestinal cancers, until the research of Peng et al on colorectal cancer.
The combinational pattern of αvβ6 and MMP-9 expression was demonstrated to be more effective than αvβ6 alone in prognosis of gastric cancer.
The combinational detection of αvβ6 and MMP-9 expression could be used as a more effective prognostic index for gastric cancer patients after curative surgery.
Integrin αvβ6 is a member of the integrin family and is expressed in epithelial cells only, but increases substantially in response to injury and/or inflammation and in epithelial tumors.
In the present study, the authors undertook an immunohistochemical analysis of αvβ6 and MMP-9 expression in gastric cancer. The correlation with clinical characteristics and prognosis was assessed to determine whether the combinational pattern of αvβ6 and MMP-9 expression could be used as a more effective prognostic index for gastric cancer patients after curative surgery. Over all, this is an interesting manuscript about the expression of integrin αvβ6 and MMP-9 in gastric cancer patients.
P- Reviewer: Azoulay L, Sharma P, Zea N S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25545] [Article Influence: 1824.6] [Reference Citation Analysis (7)] |
| 2. | Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241-2251. [PubMed] |
| 4. | Niu J, Gu X, Turton J, Meldrum C, Howard EW, Agrez M. Integrin-mediated signalling of gelatinase B secretion in colon cancer cells. Biochem Biophys Res Commun. 1998;249:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Agrez M, Gu X, Turton J, Meldrum C, Niu J, Antalis T, Howard EW. The alpha v beta 6 integrin induces gelatinase B secretion in colon cancer cells. Int J Cancer. 1999;81:90-97. [PubMed] |
| 6. | Zhao R, Liu XQ, Wu XP, Liu YF, Zhang ZY, Yang GY, Guo S, Niu J, Wang JY, Xu KS. Vascular endothelial growth factor (VEGF) enhances gastric carcinoma invasiveness via integrin alpha(v)beta6. Cancer Lett. 2010;287:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491-21494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3230] [Cited by in RCA: 3153] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 8. | Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135-1149. [PubMed] |
| 9. | Zhang ZY, Xu KS, Wang JS, Yang GY, Wang W, Wang JY, Niu WB, Liu EY, Mi YT, Niu J. Integrin alphanvbeta6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol (R Coll Radiol). 2008;20:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Peng C, Gao H, Niu Z, Wang B, Tan Z, Niu W, Liu E, Wang J, Sun J, Shahbaz M. Integrin αvβ6 and transcriptional factor Ets-1 act as prognostic indicators in colorectal cancer. Cell Biosci. 2014;4:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Li XS, Xu Q, Fu XY, Luo WS. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer. 2014;14:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Niu Z, Wang J, Muhammad S, Niu W, Liu E, Peng C, Liang B, Sun Q, Obo S, He Z. Protein expression of eIF4E and integrin αvβ6 in colon cancer can predict clinical significance, reveal their correlation and imply possible mechanism of interaction. Cell Biosci. 2014;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, Wang JS, Chen R, Niu J. Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci. 2008;99:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, Xu XC. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008;122:2050-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Vizoso FJ, González LO, Corte MD, Rodríguez JC, Vázquez J, Lamelas ML, Junquera S, Merino AM, García-Muñiz JL. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer. 2007;96:903-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Yamamoto H, Vinitketkumnuen A, Adachi Y, Taniguchi H, Hirata T, Miyamoto N, Nosho K, Imsumran A, Fujita M, Hosokawa M. Association of matrilysin-2 (MMP-26) expression with tumor progression and activation of MMP-9 in esophageal squamous cell carcinoma. Carcinogenesis. 2004;25:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Cho YB, Lee WY, Song SY, Shin HJ, Yun SH, Chun HK. Matrix metalloproteinase-9 activity is associated with poor prognosis in T3-T4 node-negative colorectal cancer. Hum Pathol. 2007;38:1603-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Takeuchi T, Hisanaga M, Nagao M, Ikeda N, Fujii H, Koyama F, Mukogawa T, Matsumoto H, Kondo S, Takahashi C. The membrane-anchored matrix metalloproteinase (MMP) regulator RECK in combination with MMP-9 serves as an informative prognostic indicator for colorectal cancer. Clin Cancer Res. 2004;10:5572-5579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Fanelli MF, Chinen LT, Begnami MD, Costa WL, Fregnami JH, Soares FA, Montagnini AL. The influence of transforming growth factor-α, cyclooxygenase-2, matrix metalloproteinase (MMP)-7, MMP-9 and CXCR4 proteins involved in epithelial-mesenchymal transition on overall survival of patients with gastric cancer. Histopathology. 2012;61:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Sun WH, Sun YL, Fang RN, Shao Y, Xu HC, Xue QP, Ding GX, Cheng YL. Expression of cyclooxygenase-2 and matrix metalloproteinase-9 in gastric carcinoma and its correlation with angiogenesis. Jpn J Clin Oncol. 2005;35:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Chu D, Zhang Z, Li Y, Zheng J, Dong G, Wang W, Ji G. Matrix metalloproteinase-9 is associated with disease-free survival and overall survival in patients with gastric cancer. Int J Cancer. 2011;129:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Zhang S, Li L, Lin JY, Lin H. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. 2003;9:899-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Mrena J, Wiksten JP, Nordling S, Kokkola A, Ristimäki A, Haglund C. MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J Clin Pathol. 2006;59:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Manu KA, Shanmugam MK, Ramachandran L, Li F, Fong CW, Kumar AP, Tan P, Sethi G. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin Cancer Res. 2012;18:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Liu S, Wang J, Niu W, Liu E, Wang J, Peng C, Lin P, Wang B, Khan AQ, Gao H. The β6-integrin-ERK/MAP kinase pathway contributes to chemo resistance in colon cancer. Cancer Lett. 2013;328:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Ahmed N, Niu J, Dorahy DJ, Gu X, Andrews S, Meldrum CJ, Scott RJ, Baker MS, Macreadie IG, Agrez MV. Direct integrin alphavbeta6-ERK binding: implications for tumour growth. Oncogene. 2002;21:1370-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Wang B, Wang W, Niu W, Liu E, Liu X, Wang J, Peng C, Liu S, Xu L, Wang L. SDF-1/CXCR4 axis promotes directional migration of colorectal cancer cells through upregulation of integrin αvβ6. Carcinogenesis. 2014;35:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Scott KA, Arnott CH, Robinson SC, Moore RJ, Thompson RG, Marshall JF, Balkwill FR. TNF-alpha regulates epithelial expression of MMP-9 and integrin alphavbeta6 during tumour promotion. A role for TNF-alpha in keratinocyte migration? Oncogene. 2004;23:6954-6966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |