Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3793
Peer-review started: November 23, 2015
First decision: December 11, 2015
Revised: January 3, 2016
Accepted: January 30, 2016
Article in press: January 30, 2016
Published online: April 14, 2016
Processing time: 130 Days and 16.7 Hours
AIM: To determine the optimal method of endoscopic preoperative biliary drainage for malignant distal biliary obstruction.
METHODS: Multicenter retrospective study was conducted in patients who underwent plastic stent (PS) or nasobiliary catheter (NBC) placement for resectable malignant distal biliary obstruction followed by surgery between January 2010 and March 2012. Procedure-related adverse events, stent/catheter dysfunction (occlusion or migration of PS/NBC, development of cholangitis, or other conditions that required repeat endoscopic biliary intervention), and jaundice resolution (bilirubin level < 3.0 mg/dL) were evaluated. Cumulative incidence of jaundice resolution and dysfunction of PS/NBC were estimated using competing risk analysis. Patient characteristics and preoperative biliary drainage were also evaluated for association with the time to jaundice resolution and PS/NBC dysfunction using competing risk regression analysis.
RESULTS: In total, 419 patients were included in the study (PS, 253 and NBC, 166). Primary cancers included pancreatic cancer in 194 patients (46%), bile duct cancer in 172 (41%), gallbladder cancer in three (1%), and ampullary cancer in 50 (12%). The median serum total bilirubin was 7.8 mg/dL and 324 patients (77%) had ≥ 3.0 mg/dL. During the median time to surgery of 29 d [interquartile range (IQR), 30-39 d]. PS/NBC dysfunction rate was 35% for PS and 18% for NBC [Subdistribution hazard ratio (SHR) = 4.76; 95%CI: 2.44-10.0, P < 0.001]; the pig-tailed tip was a risk factor for PS dysfunction. Jaundice resolution was achieved in 85% of patients and did not depend on the drainage method (PS or NBC).
CONCLUSION: PS has insufficient patency for preoperative biliary drainage. Given the drawbacks of external drainage via NBC, an alternative method of internal drainage should be explored.
Core tip: To determine the optimal method of endoscopic preoperative biliary drainage for malignant distal biliary obstruction, we conducted a multicenter retrospective study in 419 patients who underwent plastic stent (PS) or nasobiliary catheter (NBC) placement for resectable malignant distal biliary obstruction followed by surgery. The dysfunction rate for PS was significantly higher than that for NBC. Since the current limitations of nasobiliary catheter may not be overcome by a plastic stent, further studies may need to focus on the use of other stents that could remain patent for a longer period of time.
- Citation: Sasahira N, Hamada T, Togawa O, Yamamoto R, Iwai T, Tamada K, Kawaguchi Y, Shimura K, Koike T, Yoshida Y, Sugimori K, Ryozawa S, Kakimoto T, Nishikawa K, Kitamura K, Imamura T, Mizuide M, Toda N, Maetani I, Sakai Y, Itoi T, Nagahama M, Nakai Y, Isayama H. Multicenter study of endoscopic preoperative biliary drainage for malignant distal biliary obstruction. World J Gastroenterol 2016; 22(14): 3793-3802
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3793
Surgical resection is the only treatment option for cure in patients with periampullary cancers, including pancreatic and biliary tract cancers. In such patients, obstructive jaundice is often complicated. The efficacy of preoperative biliary drainage on improving peri- and postoperative outcomes for jaundice due to periampullary cancers is controversial[1-6]; however, biliary drainage is widely incorporated into clinical practice in many centers as a procedure following diagnostic cholangiography combined with pathological confirmation of malignancy or for the prevention of jaundice progression when the waiting time to a major surgery is prolonged, especially in tertiary centers[7,8].
Endoscopic biliary drainage (EBD) is preferred over percutaneous transhepatic biliary drainage (PTBD) for preoperative management of malignant distal biliary obstruction, because PTBD is more invasive, imposed considerable patient discomfort[9,10], and more importantly, is frequently susceptible to catheter tract recurrence after surgery[11]. Although EBD is usually performed via plastic stent (PS) or nasobiliary catheter (NBC), NBC is unacceptable, especially in western countries, because of patient intolerance. However, NBC is considered advantageous in preventing reflux cholangitis and for early resolution of jaundice and is often employed in Japan.
In this multicenter retrospective study entitled endoscopic preoperative biliary drainage (E-POD) study, we evaluated the outcomes of preoperative EBD using both PS and NBC and analyzed the risk factors for PS/NBC dysfunction.
In this study, data from 33 referral centers in Japan were consecutively collected. Patients who were diagnosed with distal biliary obstruction due to periampullary cancer and who underwent EBD via PS or NBC followed by surgical resection with curative intent between January 2010 and March 2012 were included. Patients who underwent initial drainage by PTBD or underwent surgery more than 100 d after initial EBD were excluded. This study was approved by the ethics committee at each hospital and was registered with UMIN-CTR (clinical trial registration number: UMIN000008492).
Data on baseline patient characteristics, procedures of EBD, procedure-related adverse events, outcomes of PS/NBC, types of surgery, and survival were collected retrospectively. All data were made anonymous and were collected from a collaborative web-based database.
Distal biliary obstruction was defined as biliary stricture located ≥ 2 cm downstream from the hilar bifurcation. Performance status was determined using the World Health Organization classification. Tumor invasion to the duodenum[12] was diagnosed on the basis of pathological findings of resected specimens. Dysfunction of PS/NBC was defined as occlusion or migration of PS/NBC, development of cholangitis, or suspected insufficient drainage that required repeat endoscopic biliary intervention. Occlusion or cholangitis included jaundice or re-elevation of liver enzyme with or without fever-up, and fever-up without other causes even if no elevation of liver enzyme. Insufficient drainage included persistent liver dysfunction or limited improvement of the elevated liver enzyme even though it is sometimes difficult to distinguish from other causes of liver dysfunction. Designed PS replacement was defined as NBC replacement with PS in patients without NBC dysfunction. Diagnostic re-endoscopic retrograde cholangiopancreatography (re-ERCP) was defined as further endoscopic examination (e.g., confirmation of pathological diagnosis and re-evaluation of biliary system) that was required without PS/NBC dysfunction. The jaundice was defined as 3.0 mg/dL by using the application with modifications of Child-Pugh classification for cirrhotic patients, which was clinically used as one of the indications for surgery. Jaundice resolution was defined as reduction of serum total bilirubin from a pre-drainage level of ≥ 3.0 mg/dL to < 3.0 mg/dL. The time to jaundice resolution and dysfunction of PS/NBC was defined as the interval between the initial EBD and each outcome. Procedure-related adverse events were diagnosed and graded according to the American Society of Gastrointestinal Endoscopy lexicon’s severity grading system[13].
Results are expressed as numbers and percentages of patients or as medians and interquartile ranges (IQRs). Continuous variables were compared using Student’s t-test or Wilcoxon’s rank-sum test, as appropriate. Categorical variables were compared using the χ2 or Fisher exact test, as appropriate. Cumulative incidence of jaundice resolution and dysfunction of PS/NBC were estimated using competing risk analysis[14] and were compared using the Gray’s test[15]. During the analysis of the cumulative incidence of jaundice resolution, surgery prior to jaundice resolution was considered as a competing risk event. On the other hand, during the analysis of the cumulative incidence of PS/NBC dysfunction, surgery without PS/NBC dysfunction, PS/NBC removal at the time of diagnostic re-ERCP, or designed PS replacement was considered as a competing risk event. Patient characteristics and preoperative biliary drainage were evaluated for association with the time to jaundice resolution and PS/NBC dysfunction using competing risk regression analysis[16]. Factors with a P-value of < 0.20 in the univariate analysis were further analyzed in multivariate models. Subdistribution hazard ratios (SHRs) and 95%CIs were calculated for each factor. A P-value of < 0.05 was considered statistically significant. All analyses were performed using R software version 2.12.0 (R Development Core Team) and its cmprsk package.
In total, 425 consecutive patients who underwent preoperative EBD for resectable periampullary cancers were identified. Six patients with placement of metal stent in this setting were excluded from the analyses. The characteristics of 419 patients are summarized in Table 1 (Those in patients with pancreatic and biliary tract cancer were shown separately in Supplementary Table 1A and B). Primary cancers included pancreatic cancer in 194 patients (46%), bile duct cancer in 172 (41%), gallbladder cancer in three (1%), and ampullary cancer in 50 (12%). Eighty-four patients (20%) had diabetes, three (1%) had liver cirrhosis, and three (1%) had dementia. Sixty-four patients (15%) had acute cholangitis at the initial drainage. The median serum total bilirubin was 7.8 mg/dL and 324 patients (77%) had ≥ 3.0 mg/dL. Two patients underwent neoadjuvant chemotherapy. The following surgeries were performed: pancreatoduodenectomy in 166 patients (39.6%), pylorus-preserving pancreatoduodenectomy in 142 (33.9%), subtotal stomach-preserving pancreatoduodenectomy in 90 (21.5%), choledochectomy in 10 (2.4%), total pancreatectomy in four (1.0%), and other surgeries in seven (1.7%). Cancer invasion of the duodenum was confirmed in the resected specimens obtained from 213 patients (51%).
| All patients (n = 419) | PS (n = 253) | NBC (n = 166) | P value | |
| Sex, male | 269 (64) | 163 (64) | 106 (64) | 0.905 |
| Age (yr) | 69 (63-75) | 69 (62-75) | 70 (63-76) | 0.274 |
| Performance status, 0/1/2 | 331/81/7 (79/19/2) | 201/50/2 (79/20/1) | 130/31/5 (78/19/3) | 0.781 |
| Primary cancer, pancreas/biliary tract/ampullary | 194/175/50 (46/42/12) | 133/91/29 (53/36/11) | 61/84/21 (37/51/13) | 0.003 |
| Primary tumor size (mm) | 20 (15-30) | 20 (14-28) | 22 (18-30) | 0.008 |
| Length of biliary stricture (mm) | 16 (11-22) | 15 (11-22) | 17 (12-23) | 0.154 |
| Diameter of proximal bile duct (mm) | 15 (12-18) | 15 (12-18) | 14 (11-17) | 0.050 |
| Involvement of intrapancreatic bile duct | 354 (84) | 221 (87) | 133 (80) | 0.048 |
| Tumor invasion into duodenum | 213 (51)1 | 132 (53) | 81 (51) | 0.823 |
| Cholangitis on admission | 64 (15) | 34 (13) | 30 (18) | 0.200 |
| Total bilirubin (mg/dL) | 7.8 (3.3-13.2) | 7.5 (3.3-12.6) | 8.6 (3.4-13.5) | 0.536 |
PS and NBC were placed in 253 (60%) and 166 (40%) patients, respectively. NBC was placed in 30 of 64 patients (47%) with acute cholangitis and in 136 of 355 patients (38%) without acute cholangitis. The procedure for initial EBD is shown in Table 2 (Those in patients with pancreatic and biliary tract cancer was shown separately in Supplementary Table 2A and B). The diameters of PSs were 5 Fr in 18 patients (7%), 7 Fr in 150 (59%), and 8.5 Fr or 10 Fr in 85 (34%), whereas those of NBCs were 5 Fr or 6 Fr in 80 patients (48%), 7 Fr in 80 (48%), and 7.5 Fr in 6 (4%). An NBC with a diameter larger than ≥ 8.5 Fr was not commercially available; thus, the mean diameter of NBCs were significantly smaller than that of PSs (P < 0.001). Approximately two-thirds of PSs were straight tipped, whereas almost the same proportion of NBCs was pig-tail tipped. Endoscopic sphincterotomy (39%) or endoscopic papillary balloon dilation (3%) was performed before placement of PS/NBC. During the initial EBD procedure, biliary biopsy or brushing cytology examination was performed in 362 patients (86.4%), and 250 of them (69.1%) were found to be positive for cancer. There were no significant differences in the initial EBD procedures between pancreatic cancer and biliary tract cancer.
| All patients (n = 419) | PS (n = 253) | NBC (n = 166) | P value | |
| Procedures of the initial ERCP | ||||
| EST or EPBD | 178 (43) | 101 (40) | 77 (46) | 0.202 |
| Biopsy or brushing of the bile duct | 362 (86) | 214 (85) | 148 (89) | 0.234 |
| Positive for cancer | 250 (60) | 150 (59) | 100 (60) | |
| Biliary stents/catheters placed | ||||
| Diameter 5-6/7-7.5/8.5-10 Fr | 98/236/85 (23/56/20) | 18/150/85 (7/59/34) | 80/86/0 (48/52/0) | < 0.001 |
| Pig-tailed tip/straight tip | 152/2241 | 74/177 (29/71) | 78/47 (62/38) | < 0.001 |
| Placement of pancreatic stent | 41 (10) | 25 (10) | 16 (10) | 0.935 |
| Adverse events (other than cholangitis) | ||||
| Pancreatitis | 40 (10) | 26 (10) | 14 (8) | 0.612 |
| Mild/moderate/severe | 30/10/0 | |||
| Cholecystitis | 4 (1) | 2 (1) | 2 (1) | 0.650 |
| Others | 4 (1) | 2 (1) | 2 (1) | 0.650 |
| Stent/catheter dysfunction | 118 (36) | 88 (35) | 30 (18) | < 0.001 |
| Causes of PS/NBC dysfunction | ||||
| Occlusion or cholangitis | 85 | 69 (27) | 16 (10) | 0.013 |
| Insufficient drainage | 19 | 14 (6) | 5 (3) | 0.330 |
| Migration | 10 | 5 (2) | 5 (3) | 0.526 |
| Inadvertent removal | 4 | NA | 4 (2) | NA |
| Number of ERCPs | 2 (1-2) | 1 (1-1) | 2 (1-2) | < 0.0001 |
PEP was noted in 40 patients (9.5%). The PEP rate was higher in patients with bile duct cancer than in those with pancreatic or ampullary cancer (12.0% vs 7.8%, P = 0.15), but it was not related to PS/NBC (10.3% vs 8.4%, P = 0.53), the PS/NBC diameter (9.7% in < 7.5 Fr vs 9.4% in > 7.5 Fr, P = 0.91), and necessity for sphincterotomy (7.3% vs 11.3%, P = 0.17).
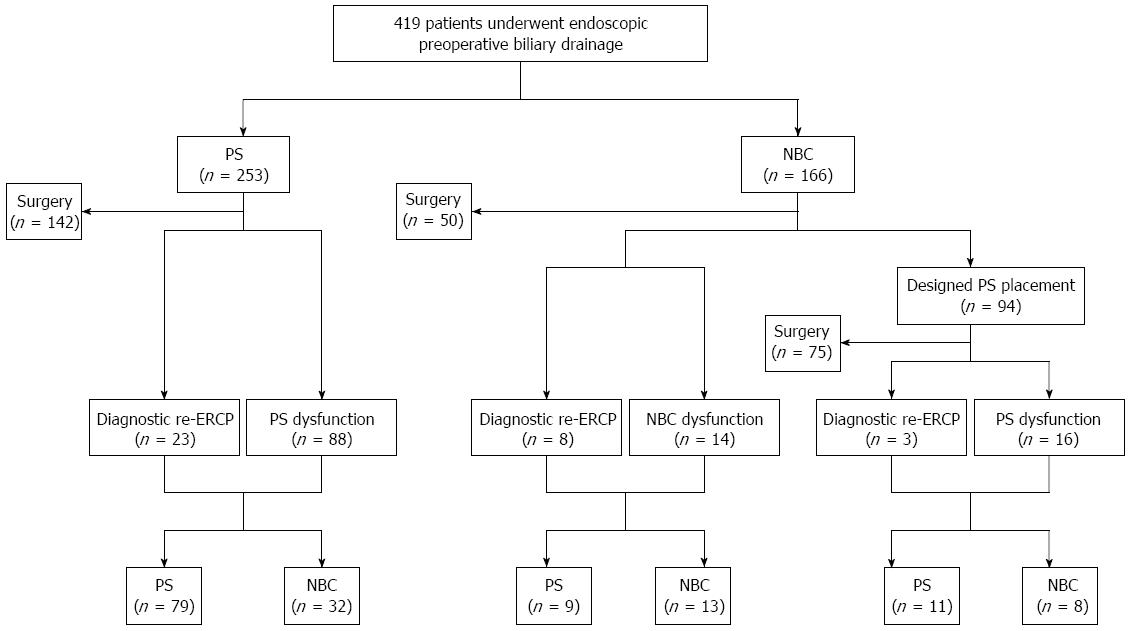
The median time to surgery was 29 d (IQR, 30-39 d). Figure 1 demonstrates the clinical course of the patients after the initial drainage. In the PS group, 111 of 253 patients (44%) underwent additional ERCP (23 for diagnostic re-ERCP and 88 for PS dysfunction) and NBC was replaced in 32 of them. In the NBC group, 94 of 166 patients (57%) underwent designed PS replacement after a median of 8.4 d (IQR, 6-10 d). There were 41 patients (25%) who underwent additional ERCP (11 for diagnostic re-ERCP, 14 for initial NBC dysfunction, and 16 for designed PS dysfunction).
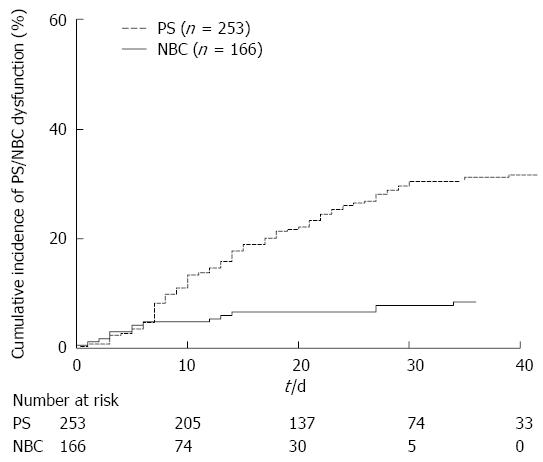
Multivariate analysis showed that PS placement had a significantly higher risk for dysfunction than NBC placement (SHR = 4.76, 95%CI: 2.44-10.0, P < 0.001) (Table 3). Figure 2 illustrates the cumulative incidences of PS/NBC dysfunction. The cumulative incidences of PS vs NBC was 13% vs 5% at 10 d, 22% vs 7% at 20 d, and 30% vs 8% at 30 d.
| Univariate analysis | Multivariate analysis | |||
| SHR (95%CI) | P value | SHR (95%CI) | P value | |
| Sex, male | 0.96 (0.64-1.44) | 0.839 | ||
| Age > 70 yr | 1.06 (0.72-1.56) | 0.773 | ||
| Performance status ≥ 1 | 0.67 (0.39-1.14) | 0.139 | 0.70 (0.39-1.24) | 0.221 |
| Primary cancer | ||||
| Biliary tract | Reference | Reference | ||
| Pancreas | 1.61 (1.06-2.44) | 0.025 | 1.30 (0.84-2.03) | 0.240 |
| Ampullary | 0.89 (0.43-1.84) | 0.758 | 0.81 (0.40-1.65) | 0.568 |
| Primary tumor size > 20 mm | 0.90 (0.61-1.33) | 0.603 | ||
| Length of biliary stricture > 20 mm | 1.04 (0.71-1.53) | 0.854 | ||
| Diameter of proximal bile duct > 15 mm | 0.94 (0.64-1.38) | 0.755 | ||
| Tumor invasion into duodenum | 1.10 (0.75-1.63) | 0.621 | ||
| Cholangitis on admission | 0.77 (0.44-1.37) | 0.378 | ||
| Total bilirubin ≥ 8 mg/dL | 1.16 (0.79-1.71) | 0.447 | ||
| Placement of PS | 4.76 (2.63-8.33) | < 0.001 | 4.76 (2.44-10.0) | < 0.001 |
| Diameter of biliary stent/catheter | ||||
| 5-6 Fr | Reference | Reference | ||
| 7-7.5 Fr | 1.92 (1.07-3.43) | 0.029 | 0.95 (0.50-1.83) | 0.885 |
| 8.5-10 Fr | 2.44 (1.28-4.66) | 0.007 | 0.93 (0.44-1.99) | 0.850 |
| Pig-tailed tip | 1.05 (0.71-1.57) | 0.796 | ||
Table 4 shows the results of the analyses for identifying risk factors for PS dysfunction. Only the pig-tailed tip stent was an independent risk factor for PS dysfunction (SHR = 1.72, 95%CI: 1.13-2.64, P = 0.012); a larger diameter of PS did not decrease the risk for PS dysfunction.
| Univariate analysis | Multivariate analysis | |||
| SHR (95%CI) | P value | SHR (95%CI) | P value | |
| Sex, male | 1.00 (0.65-1.55) | 0.993 | ||
| Age > 70 yr | 1.15 (0.76-1.74) | 0.509 | ||
| Performance status ≥ 1 | 0.84 (0.49-1.45) | 0.534 | ||
| Primary cancer | ||||
| Biliary tract | Reference | |||
| Pancreas | 1.27 (0.81-1.99) | 0.303 | ||
| Ampullary | 0.83 (0.39-1.79) | 0.637 | ||
| Primary tumor size > 20 mm | 1.10 (0.72-1.69) | 0.657 | ||
| Length of biliary stricture > 20 mm | 1.01 (0.66-1.54) | 0.957 | ||
| Diameter of proximal bile duct > 15 mm | 0.85 (0.56-1.28) | 0.432 | ||
| Tumor invasion into duodenum | 1.10 (0.72-1.67) | 0.659 | ||
| Cholangitis on admission | 0.79 (0.41-1.52) | 0.479 | ||
| Total bilirubin ≥ 8 mg/dL | 1.32 (0.87-2.00) | 0.192 | 1.43 (0.94-2.18) | 0.096 |
| Diameter of biliary stent/catheter | ||||
| 5-6 Fr | Reference | |||
| 7-7.5 Fr | 0.76 (0.37-1.56) | 0.446 | ||
| 8.5-10 Fr | 0.67 (0.31-1.46) | 0.314 | ||
| Pig-tailed tip | 1.73 (1.14-2.63) | 0.010 | 1.72 (1.13-2.64) | 0.012 |
Jaundice was resolved at the time of surgery in 275 of 324 patients (84.9%). The other 49 patients (15.1%) underwent surgery without resolution of jaundice after a median of 21 d (IQR, 14-31 d) from the initial EBD. The prognostic factors for jaundice resolution are shown in Table 5. Placement of PS or NBC did not result in earlier resolution of jaundice, and only a high total bilirubin level (SHR = 0.43, 95%CI: 0.32-0.58, P < 0.001) was identified as the significant risk factor for jaundice resolution.
| Univariate analysis | Multivariate analysis | |||
| SHR (95%CI) | P value | SHR (95%CI) | P value | |
| Sex, male | 0.90 (0.71-1.14) | 0.387 | ||
| Age ≥ 69 yr | 1.01 (0.80-1.27) | 0.932 | ||
| Performance status ≥ 1 | 1.00 (0.73-1.39) | 0.979 | ||
| Primary cancer | ||||
| Biliary tract | Reference | |||
| Pancreas | 1.07 (0.84-1.36) | 0.602 | ||
| Ampullary | 1.19 (0.80-1.77) | 0.389 | ||
| Primary tumor size > 20 mm | 0.81 (0.63-1.04) | 0.094 | 0.91 (0.69-1.20) | 0.524 |
| Length of biliary stricture > 20 mm | 0.80 (0.63-1.01) | 0.059 | 0.82 (0.63-1.06) | 0.134 |
| Diameter of proximal bile duct > 15 mm | 0.78 (0.62-0.98) | 0.033 | 0.81 (0.63-1.05) | 0.107 |
| Tumor invasion to duodenum | 0.96 (0.76-1.21) | 0.721 | ||
| Cholangitis on admission | 1.11 (0.80-1.54) | 0.528 | ||
| Total bilirubin ≥ 8 mg/dL | 0.41 (0.31-0.55) | < 0.001 | 0.43 (0.32-0.58) | < 0.001 |
| EST or EPBD | 1.02 (0.81-1.28) | 0.897 | ||
| Diameter of biliary stent/catheter | ||||
| 5-6 Fr | Reference | |||
| 7-7.5 Fr | 0.96 (0.73-1.25) | 0.738 | ||
| 8.5-10 Fr | 0.97 (0.70-1.34) | 0.859 | ||
| Pig-tailed tip | 1.05 (0.83-1.34) | 0.681 | ||
| Placement of NBC | 0.83 (0.66-1.06) | 0.129 | 0.92 (0.71-1.19) | 0.520 |
In this multicenter retrospective study of 419 patients who underwent preoperative EBD for malignant distal biliary obstruction, PS placement was a risk factor for PS/NBC dysfunction, whereas NBC placement was not, and the cumulative incidence of PS dysfunction increased almost linearly with time after the initial drainage.
Preoperative biliary drainage in patients with distal biliary obstruction has been a major matter of debate for decades. Although randomized controlled trials and meta-analyses failed to demonstrate the effectiveness of routine preoperative biliary drainage, this procedure is still performed widely, particularly in symptomatic patients with an expected long waiting time who have complication such as cholangitis or intense pruritus or because of some other reasons. In the 33 hospitals that participated in the present study, most patients with jaundice underwent preoperative biliary drainage because a major surgery that lasts for 5-10 h cannot always be scheduled immediately after a diagnosis of cancer[17] and such drainage also allows time for further staging of primary cancer, physical work-up of comorbidities, and potentially, for neoadjuvant chemotherapy, which is increasingly utilized for pancreatic cancer.
EBD is preferred over PTBD for preoperative drainage because it is less invasive and insusceptible to tumor seeding. During EBD, PSs are preferred over NBCs with regard to comfort, but the rate of PS dysfunction before surgery is reported to be as high as 34%-70%[18-20]. Although Sugiyama et al[20] demonstrated that the time to dysfunction of PS and NBC did not differ significantly in a retrospective study of 76 patients, the rate of PS dysfunction was significantly higher than that of NBC dysfunction in the present study. In the PS group, a pig-tailed PS was the only risk factor for stent dysfunction and PS with a larger diameter failed to prolong the time to stent dysfunction. Interestingly, although the cumulative incidences of PS/NBC dysfunction were quite similar within a week of the initial biliary drainage, the cumulative incidence of PS dysfunction linearly increased thereafter, whereas that of NBC dysfunction almost plateaued. At 30 d after the initial EBD, which was almost the median time to surgery in the present cohort, the cumulative incidence of dysfunction reached as high as 30% in the PS group, whereas that in the NBC group remained at 8%. In this study, the PS dysfunction rate and median time to dysfunction of 14 d were similar to those reported in a prospective study by van der Gaag et al[4] (30% and 13 d, respectively).
NBC has other advantages such as repetitions of bile cytology to confirm malignancy[21] and contrast medium injection for cholangiography to evaluate longitudinal tumor spreading, especially in patients with bile duct cancer. However, there are several obvious disadvantages. For example, external bile drainage by NBC may impair enterohepatic circulation of bile, potentially leading to deterioration of intestinal immunity and coagulopathy, and prolonged placement of NBC may impose discomfort in the pharynx. NBC followed by the replacement of PS is another option for preoperative drainage, although it requires additional ERCP.
Longer patency of self-expandable metal stent (SEMS) compared with PS has been well-established in unresectable distal biliary obstruction[22,23] and recently, SEMS in the preoperative setting has been reported to be effective because of its longer patency and lower dysfunction rate (< 20%)[24-27]. Therefore, despite some potential disadvantages of SEMS, including cholecystitis[28], pancreatitis[29], and cost, SEMS is a promising device for preoperative biliary drainage and deserves further investigation. Considering that the ultimate endpoint of endoscopic preoperative biliary drainage for malignant distal biliary obstruction is overall survival rather than outcomes, the most appropriate method should be determined by a randomized controlled trial that compares PS, NBC, and SEMS, with the survival time set as the primary endpoint.
We acknowledge some limitations of this study. First, it was based on a non-randomized retrospective design and did not evaluate surgical outcomes. A randomized controlled trial is required to draw a definite conclusion of the optimal endoscopic preoperative biliary drainage procedure for malignant distal biliary obstruction, surgical outcomes (R0 resection rate, rate of surgical adverse events, and ultimately, overall survival), length of hospitalization, and cost-effectiveness. Second, we included only patients who underwent EBD and subsequent surgery; some patients in whom surgery could not be performed because of unsuccessful biliary drainage may have been excluded. Third, various types of PSs and NBCs were used in the present study.
In conclusion, PS was associated with a higher rate of cholangitis or occlusion compared with NBC. Because internal bile drainage is not susceptible to inherent disadvantages of external bile drainage, internal bile drainage via SEMS should be evaluated in a preoperative setting.
We thank the following investigators and clinical research coordinators. Investigators of the study: Hiroshi Nitta, Department of Surgery, Fukaya Red Cross Hospital; Shigeaki Mizuno and Yuji Shiozawa, Department of Gastroenterology and Hepatology, Nihon University School of Medicine; Naminatsu Takahara, Department of Gastroenterology, Kanto Central Hospital; Saburo Matsubara, Department of Gastroenterology, Tokyo Metropolitan Police Hospital; Tadashi Ohshima, Department of Gastroenterology, Saitama Red Cross Hospital; Shigeru Iwase, Department of Gastroenterology, Fujisawa City Hospital; Satoshi Shimizu, Department of Hepatobiliary & Pancreatic Oncology, National Cancer Center Hospital East; Naotaka Maruoka, Department of Gastroenterology, Odawara Municipal Hospital; Hiroshi Yagioka, Department of Gastroenterology, JR Tokyo General Hospital; Shinichiro Sato, Department of Gastroenterology, Chiba-Nishi General Hospital; Yukiko Ito, Department of Gastroenterology, Japanese Red Cross Medical Center; Toshihiko Arizumi, Department of Gastroenterology, Mitsui Memorial Hospital; Hiroaki Shigoka, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Toho University Ohashi Medical Center; Shujiro Tsuji, Department of Gastroenterology, Tokyo Medical University; Natsuyo Yamamoto, Hirofumi Kogure and Takashi Sasaki, Department of Gastroenterology, The University of Tokyo. Clinical study coordinators: Miyuki Tsuchida, Clinical Research Support Center, The University of Tokyo Hospital. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Although the efficacy of preoperative biliary drainage on improving peri- and postoperative outcomes for jaundice due to periampullary cancers is controversial, biliary drainage is widely incorporated into clinical practice in many centers as a procedure following diagnostic cholangiography combined with pathological confirmation of malignancy or for the prevention of jaundice progression when the waiting time to a major surgery is prolonged, especially in tertiary centers. Endoscopic biliary drainage is usually performed via plastic stent (PS) or nasobiliary catheter (NBC). NBC is unacceptable, especially in western countries, because of patient intolerance. However, NBC is considered advantageous in preventing reflux cholangitis and for early resolution of jaundice and is often employed in Japan.
The results of this study contribute to clarifying the outcomes of preoperative EBD using both PS and NBC and the risk factors for PS/NBC dysfunction in the largest cohort.
At 30 d after the initial EBD, which was almost the median time to surgery in the present cohort, the cumulative incidence of dysfunction reached as high as 30% in the PS group, whereas that in the NBC group remained at 8%. The PS dysfunction rate and median time to dysfunction of 14 d were similar to those reported in a prospective randomized study by van der Gaag et al (30% and 13 d, respectively).
As the NBC has an obvious disadvantages of imposing discomfort in the pharynx, NBC followed by the replacement of PS is one of the options for preoperative drainage, although it requires additional ERCP. Preoperative placement of self-expandable metal stent should be investigated instead of plastic stent.
Endoscopic naso-biliary drainage: Naso-biliary drainage has some advantages such as repetitions of bile cytology to confirm malignancy and contrast medium injection for cholangiography to evaluate longitudinal tumor spreading, especially in patients with bile duct cancer. However, it has several obvious disadvantages; external bile drainage by NBC may impair enterohepatic circulation of bile, potentially leading to deterioration of intestinal immunity and coagulopathy, and prolonged placement of NBC may impose discomfort in the pharynx.
Authors studied superiority between PS and NBC for preoperative drainage in a retrospective setting. This attempt is clinically valuable and the study is well-organized. Their results and conclusions are simple and reasonable.
P- Reviewer: Fujisawa T, Ogura T S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
| 1. | Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery. 1985;97:641-648. [PubMed] |
| 2. | Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 252] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 697] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 5. | Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Wang Q, Gurusamy KS, Lin H, Xie X, Wang C. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2008;CD005444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Nagino M, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Kondo S, Furuse J, Saito H, Tsuyuguchi T, Yoshikawa T. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | van der Gaag NA, Kloek JJ, de Castro SM, Busch OR, van Gulik TM, Gouma DJ. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Mueller PR, van Sonnenberg E, Ferrucci JT. Percutaneous biliary drainage: technical and catheter-related problems in 200 procedures. AJR Am J Roentgenol. 1982;138:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Choi SH, Gwon DI, Ko GY, Sung KB, Yoon HK, Shin JH, Kim JH, Kim J, Oh JY, Song HY. Hepatic arterial injuries in 3110 patients following percutaneous transhepatic biliary drainage. Radiology. 2011;261:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. 2010;97:1860-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Hamada T, Isayama H, Nakai Y, Togawa O, Kogure H, Kawakubo K, Tsujino T, Sasahira N, Hirano K, Yamamoto N. Duodenal invasion is a risk factor for the early dysfunction of biliary metal stents in unresectable pancreatic cancer. Gastrointest Endosc. 2011;74:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1842] [Article Influence: 122.8] [Reference Citation Analysis (1)] |
| 14. | Tai BC, Machin D, White I, Gebski V. Competing risks analysis of patients with osteosarcoma: a comparison of four different approaches. Stat Med. 2001;20:661-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Kawakubo K, Isayama H, Sasahira N, Kogure H, Miyabayashi K, Hirano K, Tada M, Koike K. Mucosal hyperplasia in an uncovered portion of partially covered metal stent. World J Gastrointest Endosc. 2012;4:432-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Kawakubo K, Isayama H, Nakai Y, Sasahira N, Kogure H, Sasaki T, Hirano K, Tada M, Koike K. Simultaneous Duodenal Metal Stent Placement and EUS-Guided Choledochoduodenostomy for Unresectable Pancreatic Cancer. Gut Liver. 2012;6:399-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Baron TH, Kozarek RA. Preoperative biliary stents in pancreatic cancer--proceed with caution. N Engl J Med. 2010;362:170-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Mullen JT, Lee JH, Gomez HF, Ross WA, Fukami N, Wolff RA, Abdalla EK, Vauthey JN, Lee JE, Pisters PW. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg. 2005;9:1094-1104; discussion 1104-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Boulay BR, Gardner TB, Gordon SR. Occlusion rate and complications of plastic biliary stent placement in patients undergoing neoadjuvant chemoradiotherapy for pancreatic cancer with malignant biliary obstruction. J Clin Gastroenterol. 2010;44:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 20. | Sugiyama H, Tsuyuguchi T, Sakai Y, Nisikawa T, Miyazaki M, Yokosuka O. Preoperative drainage for distal biliary obstruction: endoscopic stenting or nasobiliary drainage? Hepatogastroenterology. 2013;60:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Yagioka H, Hirano K, Isayama H, Tsujino T, Sasahira N, Nagano R, Hamada T, Miyabayashi K, Ito Y, Mohri D. Clinical significance of bile cytology via an endoscopic nasobiliary drainage tube for pathological diagnosis of malignant biliary strictures. J Hepatobiliary Pancreat Sci. 2011;18:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 697] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 23. | Isayama H, Yasuda I, Ryozawa S, Maguchi H, Igarashi Y, Matsuyama Y, Katanuma A, Hasebe O, Irisawa A, Itoi T. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): Covered Wallstent versus DoubleLayer stent. Dig Endosc. 2011;23:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Wasan SM, Ross WA, Staerkel GA, Lee JH. Use of expandable metallic biliary stents in resectable pancreatic cancer. Am J Gastroenterol. 2005;100:2056-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Singal AK, Ross WA, Guturu P, Varadhachary GR, Javle M, Jaganmohan SR, Raju RP, Fleming JB, Raju GS, Kuo YF. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: single-center experience with 79 cases. Dig Dis Sci. 2011;56:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Aadam AA, Evans DB, Khan A, Oh Y, Dua K. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: a prospective study. Gastrointest Endosc. 2012;76:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Cavell LK, Allen PJ, Vinoya C, Eaton AA, Gonen M, Gerdes H, Mendelsohn RB, D’Angelica MI, Kingham TP, Fong Y. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am J Gastroenterol. 2013;108:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Isayama H, Kawabe T, Nakai Y, Tsujino T, Sasahira N, Yamamoto N, Arizumi T, Togawa O, Matsubara S, Ito Y. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006;4:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Kawakubo K, Isayama H, Nakai Y, Togawa O, Sasahira N, Kogure H, Sasaki T, Matsubara S, Yamamoto N, Hirano K. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg Endosc. 2012;26:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |