Published online Apr 7, 2016. doi: 10.3748/wjg.v22.i13.3592
Peer-review started: September 14, 2015
First decision: January 13, 2016
Revised: January 31, 2016
Accepted: March 2, 2016
Article in press: March 2, 2016
Published online: April 7, 2016
Processing time: 198 Days and 23.2 Hours
AIM: To evaluate whether serum and tumor indoleamine 2,3-dioxygenase activities can predict lymphatic invasion (LI) or lymph node metastasis in colorectal carcinoma.
METHODS: The study group consisted of 44 colorectal carcinoma patients. The patients were re-grouped according to the presence or absence of LI and lymph node metastasis. Forty-three cancer-free subjects without any metabolic disturbances were included into the control group. Serum neopterin was measured by enzyme linked immunosorbent assay. Urinary neopterin and biopterin, serum tryptophan (Trp) and kynurenine (Kyn) concentrations of all patients were determined by high performance liquid chromatography. Kyn/Trp was calculated and its correlation with serum neopterin was determined to estimate the serum indoleamine 2,3-dioxygenase activity. Tissue sections from the studied tumors were re-examined histopathologically and were stained by immunohistochemistry with indoleamine-2,3-dioxygenase antibodies.
RESULTS: Neither serum nor urinary neopterin was significantly different between the patient and control groups (both P > 0.05). However, colorectal carcinoma patients showed a significant positive correlation between the serum neopterin levels and Kyn/Trp (r = 0.450, P < 0.01). Urinary biopterin was significantly higher in cancer cases (P < 0.05). Serum Kyn/Trp was significantly higher in colorectal carcinoma patients (P < 0.01). Lymphatic invasion was present in 23 of 44 patients, of which only 12 patients had lymph node metastasis. Eleven patients with LI had no lymph node metastasis. Indoleamine-2,3-dioxygenase intensity score was significantly higher in LI positive cancer group (44.56% ± 6.11%) than negative colorectal cancer patients (24.04% ± 6.90%), (P < 0.05). Indoleamine 2,3-dioxygenase expression correlated both with the presence of LI and lymph node metastasis (P < 0.01 and P < 0.05, respectively). A significant difference between the accuracy of diagnosis by using either total indoleamine-2,3-dioxygenase immunostaining score or of lymph node metastasis was found during the evaluation of cancer patients.
CONCLUSION: Indoleamine-2,3-dioxygenase expression may predict the presence of unrecognized LI and lymph node metastasis and may be included in the histopathological evaluation of colorectal carcinoma cases.
Core tip: Colorectal cancer (CRC) is one of the major public health problems in the world. Clinicopathological findings of patients who died of recurrent CRC after resection revealed that approximately 14% of patients with lymph node negative CRC die because of the presence of unrecognizable tumor cells that are categorized as micrometastases. Tryptophan degrading enzyme, indoleamine-2,3-dioxygenase expression by tumor cells has been shown to be correlated with a poor clinical prognosis of colon cancer. Our data indicated that high total indoleamine-2,3-dioxygenase immunostaining score is a strong predictor for immune tolerance, lymphatic invasion and subsequent lymph node metastasis. Therefore, indoleamine-2,3-dioxygenase immunostaining might be recommended for histopathological evaluation of CRC cases.
- Citation: Engin A, Gonul II, Engin AB, Karamercan A, Sepici Dincel A, Dursun A. Relationship between indoleamine 2,3-dioxygenase activity and lymphatic invasion propensity of colorectal carcinoma. World J Gastroenterol 2016; 22(13): 3592-3601
- URL: https://www.wjgnet.com/1007-9327/full/v22/i13/3592.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i13.3592
Colorectal cancer (CRC) is the foremost one among the major public health problems in the world. Its incidence is approximately 7 per 100000, with approximately 5000 new cases and 3200 deaths annually[1]. Staging of CRC has prognostic value in terms of taking decisions about adjuvant therapy and follow up. American Joint Committee on Cancer (AJCC), the TNM staging system[2] places patients into one of four stages regarding tumor size and invasion depth of bowel wall, lymph node metastasis and distant metastasis. However current prognostic criteria have been debated for their precision in stratification of patients according to these rules. The character of the invasive margin and the number of metastatic lymph nodes are already accepted as more reliable prognostic criteria in CRC[3]. Recent studies demonstrated that significant risk factors for lymph node metastasis are level of submucosal spreading and the presence of lymphovascular invasion[4,5]. Clinicopathological findings of patients who died of recurrent CRC after resection revealed that the mean survival time has been significantly influenced by the histological grade of tumor, the depth of wall invasion, the presence of lymphatic or vascular invasion and the lymph node metastasis[6].
It has been asserted that despite having removed whole macroscopic disease with curative intent, one of five CRC patients with stages one or two would develop recurrence[7]. Likewise, approximately 14% of patients with lymph node negative CRC die of tumor recurrence, which can be related to the presence of unrecognizable tumor cells that are categorized as micrometastases[8]. Micrometastases could not be detected by conventional histopathologic analysis and none of the clinicopathologic parameters examined is correlated with the presence of occult cancer cells in lymph nodes[8]. On the other hand, failure to examine enough lymph nodes may result in a failure to identify patients whose lymph nodes are affected by cancer and thus may result in mislabeling[9]. The use of techniques to increase the number of lymph nodes harvested and identify all micrometastases is a subject of debate since 1998[10]. The ultra-staging of colon cancer leads to detection of occult metastases in patient’s deemed node negative by conventional techniques of pathologic staging[11]. Nevertheless, because ultra staging involves extensive nodal sectioning and immunohistochemistry, this method remains investigational rather than routine examination[11]. However, according to others, the presence of micrometastases in patients with stages one and two CRC seems not to have any impact on cancer-specific survival[7,12,13].
These evidences reflect the unusual biologic behavior of the tumor and/or host. Therefore, other cellular criteria should be included in the evaluation of patients’ outcome. The type, density, and expression of immunomodulator substances of immune and/or tumor cells in CRC have an important prognostic value that may be superior to and independent of those of the cancer-staging systems[14]. Moreover, the pathologic interactions between tumor and host immune cells within the tumor microenvironment create an immunosuppressive network that promotes tumor growth and protects the tumor from immune attack[15]. In body fluids, information about T helper cell 1 (Th1)-derived cellular immune activation can be provided by neopterin, which is mainly synthesized by the activated monocytes/macrophages in response to induction by interferon-gamma (IFN-gamma)[16]. IFN-gamma selectively stimulates the early steps of pteridine biosynthesis in macrophages, thereby leading to accumulation and excretion of dihydroneopterin and neopterin[17]. Actually neopterin is a by-product in the biopterin biosynthesis[18]. Chronic stimulation of Th1-mediated immunity may also cause enhanced indoelamine 2,3-dioxygenase (IDO) activity in malignant diseases[19]. In cancer patients, significantly accelerated degradation of tryptophan (Trp) due to higher IDO activity with lowered serum concentrations of Trp and increased kynurenine (Kyn) as well as an increased Kyn to Trp ratio has been recognized[20,21]. It appears that decreased Trp levels or increased concentrations of its degradation products may be directly involved in diminished T-cell responsiveness[19]. This phenomenon could be best explained by IDO expression within the tumors[15]. The enzyme IDO has recently attracted special attention[22] and may be expressed constitutively by tumor cells as part of the genetic changes involved in malignant transformation[23,24]. IDO-expressing cells have been considered to create a state of immunologic unresponsiveness towards tumor-derived antigens[25]. Although IDO expression by tumor cells has been shown to correlate with a poor clinical prognosis of colon cancer[15], assessment of tumor IDO activity has not took place currently in routine histopathological evaluation concept of CRCs. “Is the evaluation of IDO activity of tumor tissue an effective method to predict the invasiveness of tumor cells?” This is a rational question considering the host immune tolerance against CRC cells. In this respect, estimation of total tumor tissue IDO immunostaining score during the routine histopathological examination of CRC specimens appears to be an effective strategy to predict the suppression of anti-tumor immune response and the enhancement of tumor invasion.
Aim of this study was to evaluate whether serum and tumor IDO activities can predict the lymphatic invasion or lymph node metastasis propensity in CRC patients.
A total of 87 patients with the diagnosis of primary CRC or with uncomplicated cholelithiasis which were referred to Gazi University, Faculty of Medicine, Department of General Surgery for surgical evaluation accepted as candidates for this prospective randomized study. All participants’ rights were protected and informed consents were obtained according to the Helsinki Declaration. Local Ethic Committee also approved the study protocol.
The exclusion criteria for this study were to have immune system disorders, obstacle for surgical intervention due to cardio-pulmonary or metabolic risks, to receive neoadjuvant chemotherapy due to the late stage carcinoma, having malnutrition, chronic granulomatosis, and collagen tissue or neurodegenerative diseases.
Control group (Group 1) consisted of 43 cancer-free subjects, aged 62.06 ± 2.07 (mean ± SEM) years, body mass index (BMI): 26.1 ± 0.6 kg/m2 and was programmed for elective laparoscopic cholecystectomy for cholelithiasis without acute inflammation. These patients had undergone diagnostic endoscopic evaluation whenever their complaints suggestive for digestive diseases. Patients in whom no pathology being found in either the upper or lower gastrointestinal system were included in cancer-free control group.
Group 2 constituted of 44 CRC patients, aged 60.95 ± 2.11 years, BMI: 25.4 ± 0.5 kg/m2, and underwent elective abdominal surgery for CRC. Diagnosis was made by endoscopic survey and histological examination of tumor biopsies in all CRC patients that were stratified as stage 1, 2 or 3 according to the TNM classification of the AJCC staging[2]. The presence of distant metastases was ruled out by pre-operative upper abdominal ultrasonography, computed tomography-based evaluation, chest X-ray and intra-operative exploration. All patients underwent surgery with curative intent and with histologically confirmed disease-free resection margins were included in Group 2. During the study period, uniform surgical management protocol was carried out. In all cases, curative resection had included the main lymphovascular supply to the bowel. For proximal colon tumors, lymphadenectomy was extended to the origin of the ileocolic, right colic and middle colic arteries. For distal colon tumors and rectal tumors, lymphadenectomy was extended to the origin of the lower mesenteric artery. Total mesorectal excision was performed in all patients with tumors of the middle and lower rectum. Cases with subsequently identified distant metastases or incomplete clearance of the tumor were excluded.
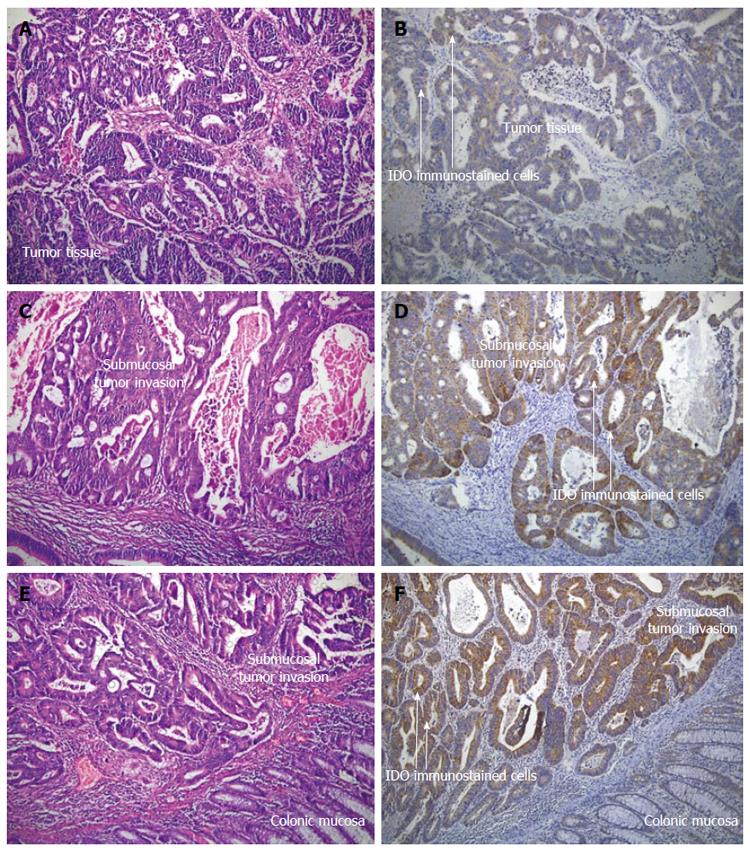
Postoperative confirmation of preoperative diagnosis in Group 2 was made by routine histopathological examination of postoperative specimens. The patients were subsequently followed-up for a mean of 28.3 ± 1.2 mo. The patients’ age and gender and localization, histological type and the differentiation of tumors were noted as standard clinical variables. It has been suggested that 12 lymph nodes could be considered as the minimum acceptable harvest from a careful specimen dissection[26]. Conventionally, lymph nodes were identified by palpation. Whenever, fewer than 12 nodes were found after careful gross examination, additional enhancement techniques were employed[27]. The same team of pathologists examined surgical specimens for tumor and lymph nodes, and the same technique for lymph node assessment was utilized during the entire period of the study. Three or more consecutive sections were examined from each lymph node[28] and routine histological examination of all specimens was performed using triple levelling techniques and the sections were stained with haematoxylin and eosin (HE) (Figure 1A, C and E).
The surgical resection materials of the primary CRC cases were re-evaluated histopathologically for the presence of lymphatic invasion, intratumoral and peritumoral lymphoid cell infiltration (lymphocytic tumor response), pathological stage and tumor grade. Lymphatic invasion was noted as present or absent. Suspicious cases for the presence of lymphatic invasion were evaluated immunohistochemically with podoplanin antibody. Lymphocytic tumor response was semi-quantitatively estimated (from point 0 to point 3) according to the intensity of infiltrating lymphocytes. Tumors were graded according to the degree of glandular differentiation: grade 1, grade 2 and grade 3. The number of metastatic and reactive lymph nodes was also noted.
Immunohistochemical staining was performed on paraffin-embedded blocks of each case. 4 μm thick sections of the representative tumor blocks were studied by indirect immunoperoxidase staining. The sections were re-hydrated in a graded series of alcohol, blocked with non-immune blocking solution. Antigen unmasking was performed by microwaving the sections in a 10mM citrate buffer, pH 6.0 for 20 min. The sections then were incubated with primary antibody of “anti-indolamine 2,3-dioxygenase” for 2 h at 37 °C (dilution; 1:300, mouse monoclonal antibody, Clone 10.1, Millipore, Billerica, MA, United States). After washing, they were incubated with secondary antibody (multi-species ultra streptavidine detection system-horse radish peroxidase, Zymed, MA, United States) and streptavidine-biotin complex (Zymed, MA, United States) for 20 min per each at room temperature, respectively. Immunoreactions were developed with diaminobenzidine (diaminobenzidinetetrachloride, Zymed, United States) as chromogen. The percentage of the tumor cells showing cytoplasmic staining and its intensity were semi-quantitatively estimated (Figure 1B, D and F).
Immunoreactivity was semi-quantitatively estimated as previously described[29]. The total IDO immunostaining score was calculated as the sum of a proportion score and an intensity score. The proportion score reflects the estimated fraction of positively stained infiltrating cells (score 0, none; score 1, < 10%; score 2, 10%-50%; score 3, 51%-80%; score 4, > 80%). The intensity score represents the estimated staining intensity (score 0, no staining; score 1, weak; score 2, moderate; score 3, strong) giving a total score ranging from 0 to 12. We defined IDO overexpression as a total score 4 or > 4. The frequency of increased total IDO immunostaining score of CRC group was estimated by comparing each patient with the cutoff value; 4 (Figure 1A-F).
After centrifugation, serum specimens were stored at -20 °C until assayed. Serum Trp and Kyn concentrations were determined by high-performance liquid chromatography as described, the ratio of Kyn to Trp was calculated to estimate the activity of IDO[30]. Serum neopterin concentrations were determined according to the manufacturer’s instructions by a commercially available enzyme immunoassay kit (ELISA, Demeditec Diagnostics, Kiel, Germany). In order to test the serum specific IDO activities of patients, correlations between the Kyn/Trp ratio and neopterin values were computed.
Neopterin, biopterin and creatinine levels in urine were analyzed by high performance liquid chromatography. Neopterin, biopterin and creatinine isocratically eluted and quantified in the same chromatographic run[31].
Data were expressed as mean ± SEM. After checking the data by the Kolmogorov-Smirnov test, normally distributed data were analyzed using independent sample t-test. Nonparametric data were compared with Mann-Whitney U test. Correlations were assessed using Spearman’s rank or Pearson tests. For two or more categorical, independent groups, Pearson’s χ2 and linear-by-linear association tests were made and P values less than 0.05 were considered to indicate statistical significance.
A pronounced rise in serum neopterin concentration above the standard cut off value (10 nmol/L) and increased urinary neopterin excretion[32] were observed in cancer patients, but they did not reach the statistical significance (P = 0.634 and P = 0.090 vs cancer-free patients, respectively) (Table 1). Both in CRC (Group 2) and cancer-free groups (Group 1) a significant correlation was observed between the serum and urinary neopterin concentrations (P = 0.00001, r =0.450 and P = 0.002, r =0.481, respectively). Although CRC patients showed a mild decrease in serum Trp levels, we could not find a statistical difference when compared with the cancer-free patients (P = 0.254). However, cancer patients had a considerable rise in serum Kyn, a toxic metabolite of Trp degradation pathway (P = 0.042 vs cancer-free patients). Therefore, a remarkable increase in Kyn/Trp which reflects the serum IDO activity of CRC group was observed when compared to controls (P = 0.006) (Table 1). CRC cases also showed a significant positive correlation between the serum neopterin levels and Kyn/Trp (r = 0.450, P = 0.003). Evaluation of relationship between serum Trp and Kyn/Trp disclosed a significant negative correlation in both groups (P = 0.012, r = -0.383 for Group 1; P = 0.0001, r = -0.670 for Group 2). Thus, significant negative correlations were calculated between the serum Trp and Kyn values of cancer cases (r = 0.332, P = 0.003). Even neopterin levels were not found statistically significant in Group 2, sum of urinary biopterin and urinary neopterin concentrations indicated significant increase in cell-mediated immune response in CRC patients compared to Group 1. While sum of urinary biopterin and urinary neopterin concentrations were calculated lower in lymph node-positive patients, they were higher in lymphatic invasion-positive CRC patients.
| Group | Group 1(n = 43) | Group 2(n = 44) | P value |
| Age (yr)1 | 62.06 ± 2.07 | 60.95 ± 2.11 | 0.708 |
| Tryptophan (μmol/L)1 | 21.95 ± 1.03 | 19.91 ± 1.33 | 0.254 |
| Kynurenine (μmol/L)2 | 1.03 ± 0.19 | 1.20 ± 0.13 | 0.042a |
| Kynurenine/tryptophan1 | 41.31 ± 3.62 | 89.47 ± 14.32 | 0.006a |
| Serum neopterin (nmol/L)2 | 13.28 ± 1.08 | 16.93 ± 2.84 | 0.634 |
| Urinary neopterin (μmol neopterin/mol creatinine | 63.54 ± 5.64 | 80.45 ± 8.09 | 0.090 |
| Urinary biopterin (μmol biopterin/mol creatinine)1 | 67.33 ± 6.22 | 91.41 ± 12.00 | 0.038a |
| Urinary neopterin (μmol neopterin/mol creatinine) + urinary biopterin (μmol biopterin/mol creatinine)1 | 129.83 ± 9.42 | 172.60 ± 17.17 | 0.048a |
We examined 1398 HE stained serial sections of 466 lymph nodes from 44 patients with CRC. The number of harvested lymph nodes per patient ranged 10 to 16 (median: 10 and mean ± SEM: 12.59 ± 1.56). The average number of serial sections from tumor tissue for each patient was 16. These were examined after staining with H-E and monoclonal antibody anti-IDO. Lymphovascular invasion was found in 23 of 44 patients (52.3%). However, we could not demonstrate lymphatic invasion in remaining 21 patients. Only 12 patients (52%) with lymphatic invasion had lymph node metastasis. Although 11 patients (25%) displayed lymphatic invasion, they had no lymph node metastasis. Taking into consideration of all patients, 32 had negative nodes whereas, 12 had evidences of nodal metastasis. We could not observe at all an association between the serum neopterin levels and presence versus absence of lymph node metastases (P = 0.368). Evaluation of serum pteridine and Trp metabolites of lymphatic invasion negative (Group 2/c) and positive (Group 2/d) CRC patients displayed serum IDO activities that were significantly higher in lymphatic invasion (Table 2). On the other hand, assessment of serum pteridine and Trp metabolites of lymph node metastasis negative (Group 2/a) and positive (Group 2/b) CRC patients displayed serum IDO activities that were not predictive for lymph node dissemination alone (Table 3).
| Group | Group 2/c(n = 21) | Group 2/d(n = 23) | P value |
| Tryptophan (μmol/L)1 | 19.14 ± 1.68 | 20.62 ± 2.06 | 0.586 |
| Kynurenine (μmol/L)2 | 1.45 ± 0.24 | 0.96 ± 0.11 | 0.080 |
| Kynurenine/tryptophan | 95.85 ± 17.40 | 83.08 ± 23.13 | 0.662 |
| Serum neopterin (nmol/L)2 | 18.43 ± 5.18 | 15.56 ± 2.78 | 0.613 |
| Urinary neopterin (μmol neopterin/mol creatinine)1 | 85.79 ± 11.76 | 75.68 ± 11.34 | 0.541 |
| Urinary biopterin (μmol biopterin/mol creatinine)1 | 76.56 ± 16.44 | 105.43 ± 17.20 | 0.234 |
| Urinary neopterin (μmol neopterin/mol creatinine) + urinary biopterin (μmol biopterin/mol creatinine)1 | 167.42 ± 21.83 | 177.50 ± 26.82 | 0.774 |
| IDO (%) | 24.04 ± 6.90 | 44.56 ± 6.11 | 0.031a |
| Group | Group 2/a(n = 32) | Group 2/b(n = 12) | P value |
| Tryptophan (μmol/L)1 | 18.04 ± 1.39 | 23.98 ± 2.98 | 0.047a |
| Kynurenine (μmol/L)1 | 1.33 ± 0.17 | 0.87 ± 0.14 | 0.139 |
| Kynurenine/tryptophan1 | 103.20 ± 18.13 | 53.27 ± 17.36 | 0.121 |
| Serum neopterin (nmol/L)2 | 17.78 ± 3.68 | 14.67 ± 3.94 | 0.368 |
| Urinary neopterin (μmol neopterin/mol creatinine)1 | 83.07 ± 9.76 | 71.29 ± 12.96 | 0.552 |
| Urinary biopterin (μmol biopterin/mol creatinine)1 | 96.87 ± 13.83 | 69.56 ± 23.13 | 0.371 |
| Urinary neopterin (μmol neopterin/mol creatinine) + Urinary biopterin (μmol biopterin/mol creatinine)1 | 179.95 ± 20.48 | 143.23 ± 24.95 | 0.400 |
| IDO (%)1 | 30.32 ± 5.74 | 48.75 ± 8.30 | 0.089 |
The tumor grade was higher in the lymph node-positive group (40%). While, IDO intensity score was 24.04% ± 6.90% in lymphatic invasion negative group, it was 44.56% ± 6.11% in lymphatic invasion positive patients (P = 0.031). The frequency of increased total IDO immunostaining score of CRC patients with lymphatic invasion was 78 % (18 of 23 patients). While 92 % of the frequency of increased total IDO immunostaining score (≥ 4) was found for patients with lymphatic invasion-lymph node metastasis positive (11 of 12 patients), 38 % was found for lymphatic invasion-lymph node metastasis negative group (8 of 21 patients). There was a significant association between the lymphatic invasion and total IDO immunostaining scores (P = 0.014; r = +0.366). Weak correlation between the lymph node metastasis and total IDO immunostaining score (P = 0.043; r = +0.310) confirmed that conventional histopathologic techniques were inefficient to reveal the lymph node invasion adequately. However, lymphatic invasion seemed the most valuable parameter indicating the lymph node metastasis (P = 0.001; r = +0.476) (Table 4). Although lymphatic invasion showed significant linear-by-linear association with total IDO immunostaining score of tumor tissue (P = 0.016) and lymph node metastasis (P = 0.002), we could not find an association neither with lymphocytic infiltration score (P = 0.211) nor tumor grade (P = 0.358) (Table 5). Regarding the evaluation of CRC cases, no statistical difference was displayed either by using total IDO immunostaining score or lymphatic invasion (P = 0.581). Positive predictive value (PPV) or the proportion of patients with the higher total IDO immunostaining scores (≥ 4) who have lymphatic invasion was 70% and negative predictive value (NPV) was calculated as 72% for the same group. The specificity and sensitivity of presence of lymphatic invasion plus lymph node metastasis in patient with higher total IDO immunostaining score was 62% and 85%, respectively, whereas, we found a significant difference between the accuracy of diagnosis by using either total IDO immunostaining score or lymph node metastasis during the evaluation of cancer patients (P = 0.0013; PPV: 38.46, NPV: 89%).
In our study, CRC patients had increased systemic immune activation. The elevated neopterin production is also associated with Trp degradation. Trp depletion as well as the accumulation of its cytotoxic metabolites results in a strong inhibitory effect on the development of immune responses[33]. Increases in both the urinary biopterin excretion and Kyn/Trp of CRC patients compared to the matched controls suggested that IFN-gamma-mediated immune response may be enhanced by the malignant growth. Actually, IFN-γ-induced guanosine triphosphate (GTP) cyclohydrolase I activity correlates with the sum of neopterin plus biopterin rather than with neopterin or biopterin alone[34]. Thus, lower values of neopterin plus biopterin in lymph node-positive patients considering the higher values of lymphatic invasion-positive cancer cases may be suggestive for insufficient immune response in these patients.
It is well known that IDO activity is characterized best by the Kyn/Trp which correlates with concentrations of immune activation marker neopterin[35]. Indeed, in our study highly significant correlation between neopterin concentrations and the increased Kyn/Trp clearly indicated that the formation of Kyn is related to IDO activity by IFN-gamma stimulation. IDO rapidly degrades Trp that is crucial to sustain proliferation of T lymphocytes[36]. Despite the phenomena of immune activation, low Trp levels or increased concentrations of its degradation products may be directly involved in diminished T-cell responsiveness to antigenic stimulation in cancer patients[37]. Actually, physiological levels of IDO may be needed for maintenance of T cells function, which is necessary for antigen presentation, but increased levels of IDO and accumulation of IDO-mediated cytotoxic metabolites from the Kyn pathway will hamper cell function in activated T cells[38]. On the other hand, IDO-induced Trp depletion from the tumor microenvironment could be the results of elevated levels of the enzyme and augmented Trp consumption by both tumor cells and antigen presenting cells of the host[22]. However, CRCs with a high density of infiltrating memory and effector T cells are less likely to disseminate to lymphovascular and perineural structures and to regional lymph nodes and, present a less advanced pathological stage, and increased survival[39].
In this study we analyzed the expression of IDO proteins in human colorectal tumor samples. Our results are also in line with previous studies, indicating that human tumors frequently express IDO[38]. 52.4% of tumor specimens showed IDO-high expression, whereas in 47.6% of tumor specimens, the staining was scored as IDO-low expression. These findings indicated that certain colon cancer subsets are different in their ability to express IDO. The finding, IDO activity significantly correlated with immunostaining scores in vitro strongly suggests that the enzyme is active and might exert its immunosuppressive activity in patients classified as IDO-high expression. However, further prospective studies by using fresh tumor tissues are required to confirm this hypothesis. In this study, we verified that lymphovascular invasion is a strong predictor of lymph node metastasis. All lymphatic invasion negative patients were also lymph node negative (21 patients; 47.7%). When compared with the lymphatic invasion-lymph node positive CRC patients (27.3%), lymphatic invasion-lymph node negative patients had significantly lower expression of IDO (4.7% ± 0.4% and 2.6% ± 0.4%, respectively, P = 0.012). The increased frequency of IDO activity could enhance immune tolerance against the cancer cells. While 23 patients with positive lymphatic invasion showed 78% increased frequency of IDO activity (IDO score ≥ 4), lymphatic invasion-lymph node positive CRC patients and lymphatic invasion-lymph node negative patients had 92% and 38%, respectively.
In this study, in order to compare with the tumor IDO activity, lymphatic invasion was demonstrated with advanced immunohistochemical techniques. However, histological evaluation of lymph nodes was made by routine conventional methods. Despite of the positive lymphatic invasion, lymph node metastases were found negative in 11 patients.
Likewise, multivariate analysis of CRC showed that lymphatic invasion is an independent predictor factor for lymph node metastasis[5]. Very recently, it has been indicated that to the patients with T1 or T2 CRC, with positive lymphovascular invasion, radical surgery should be recommended because of the greater risk for lymph node metastasis[40]. This surgical strategy was supported by our data. Additionally, in routine conventional histopathological examinations, lymphatic invasion and lymph node metastases cannot always be defined due to limited number of histological sections.
As a conclusion, these evidences coupled with our data showed that high total IDO immunostaining score is a strong predictor for immune tolerance, lymphatic invasion and subsequent lymph node metastasis. Therefore, IDO immunostaining might be recommended for histopathological evaluation of CRC cases.
Lymphatic invasion and the depth of submucosal invasion of tumor cells are risk factors for lymph node metastasis, which is the strongest prognostic determinant for colorectal cancer (CRC) patients. It was suggested that indoleamine-2,3-dioxygenase (IDO) high-expressing colorectal tumor cells enable cancer subsets to avoid immune attack. However, still a satisfactory tool does not exist for the determination of unrecognizable tumor cells that may cause recurrent CRC.
IDO high-expressing colorectal tumor cells enable cancer subsets to avoid immune attack. Although IDO expression by tumor cells has been shown to correlate with a poor clinical prognosis of colon cancer, assessment of tumor IDO activity has not took place currently in routine histopathological evaluation concept of CRCs. Thus the current research hotspot is to evaluate the adequate retrieval and assessment of colorectal mesenteric lymph nodes to ensure that the lymph nodes do not contain unrecognizable tumor cells.
In CRC patients, significantly accelerated degradation of tryptophan (Trp) with lowered serum concentrations of Trp and increased kynurenine as well as an increased Kyn/Trp has been recognized. Although IDO expression by tumor cells has been shown to correlate with a poor clinical prognosis of colon cancer, assessment of tumor IDO activity does not take place currently in routine histopathological evaluation of CRCs. This data revealed that high total IDO immunostaining score is a strong predictor for immune tolerance, lymphatic invasion and subsequent lymph node metastasis. Therefore, IDO immunostaining might be recommended for histopathological evaluation of CRC cases.
High total IDO immunostaining score is a strong predictor for immune tolerance, lymphatic invasion and subsequent lymph node metastasis. Thus, during the histopathological evaluation of CRC, IDO immunostaining might be used.
CRC is one of the major public health problems in the world. Lymphatic invasion and the depth of submucosal invasion of tumor cells are risk factors for lymph node metastasis, which are strongest prognostic determinants for colorectal cancer patients. Trp degrading enzyme, IDO expression by tumor cells has been shown to correlate with a poor clinical prognosis of colon cancer. Indicator of cellular immune response, neopterin is produced by the activated macrophages and associated with Trp degradation. Actually, IFN-gamma-induced guanosine triphosphate (GTP) cyclohydrolase I activity results in synthesis of neopterin, as well as biopterin.
This is an interesting study, a lot of work behind many thanks for authors.
P- Reviewer: Martin SG, Mayol J S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM
| 1. | Tatar M, Tatar F. Colorectal cancer in Turkey: current situation and challenges for the future. Eur J Health Econ. 2010;10 Suppl 1:S99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739-1757. [PubMed] |
| 3. | Cianchi F, Messerini L, Palomba A, Boddi V, Perigli G, Pucciani F, Bechi P, Cortesini C. Character of the invasive margin in colorectal cancer: does it improve prognostic information of Dukes staging? Dis Colon Rectum. 1997;40:1170-1175; discussion 1175-1176. [PubMed] |
| 4. | Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998-1000. [PubMed] |
| 5. | Tominaga K, Nakanishi Y, Nimura S, Yoshimura K, Sakai Y, Shimoda T. Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum. 2005;48:92-100. [PubMed] |
| 6. | Cho YB, Chun HK, Yun HR, Kim HC, Yun SH, Lee WY. Histological grade predicts survival time associated with recurrence after resection for colorectal cancer. Hepatogastroenterology. 2009;56:1335-1340. [PubMed] |
| 7. | Kronberg U, López-Kostner F, Soto G, Zúñiga A, Wistuba I, Miranda V, Pinto E, Viviani P, Marshall G. Detection of lymphatic micrometastases in patients with stages I and II colorectal cancer: impact on five-year survival. Dis Colon Rectum. 2004;47:1151-1157. [PubMed] |
| 8. | Messerini L, Cianchi F, Cortesini C, Comin CE. Incidence and prognostic significance of occult tumor cells in lymph nodes from patients with stage IIA colorectal carcinoma. Hum Pathol. 2006;37:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65-71. [PubMed] |
| 10. | Caplin S, Cerottini JP, Bosman FT, Constanda MT, Givel JC. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer. 1998;83:666-672. [PubMed] |
| 11. | Wasif N, Faries MB, Saha S, Turner RR, Wiese D, McCarter MD, Shen P, Stojadinovic A, Bilchik AJ. Predictors of occult nodal metastasis in colon cancer: results from a prospective multicenter trial. Surgery. 2010;147:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Adell G, Boeryd B, Frånlund B, Sjödahl R, Håkansson L. Occurrence and prognostic importance of micrometastases in regional lymph nodes in Dukes’ B colorectal carcinoma: an immunohistochemical study. Eur J Surg. 1996;162:637-642. [PubMed] |
| 13. | Choi HJ, Choi YY, Hong SH. Incidence and prognostic implications of isolated tumor cells in lymph nodes from patients with Dukes B colorectal carcinoma. Dis Colon Rectum. 2002;45:750-755; discussion 755-756. [PubMed] |
| 14. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4899] [Article Influence: 257.8] [Reference Citation Analysis (0)] |
| 15. | Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Murr C, Bergant A, Widschwendter M, Heim K, Schröcksnadel H, Fuchs D. Neopterin is an independent prognostic variable in females with breast cancer. Clin Chem. 1999;45:1998-2004. [PubMed] |
| 17. | Schoedon G, Troppmair J, Adolf G, Huber C, Niederwieser A. Interferon-gamma enhances biosynthesis of pterins in peripheral blood mononuclear cells by induction of GTP-cyclohydrolase I activity. J Interferon Res. 1986;6:697-703. [PubMed] |
| 18. | Furukawa Y, Nishi K, Kondo T, Tanabe K, Mizuno Y. Significance of CSF total neopterin and biopterin in inflammatory neurological diseases. J Neurol Sci. 1992;111:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Widner B, Weiss G, Fuchs D. Tryptophan degradation to control T-cell responsiveness. Immunol Today. 2000;21:250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer. 2002;86:1691-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Rose DP. Tryptophan metabolism in carcinoma of the breast. Lancet. 1967;1:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 128] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett. 2007;111:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 851] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 24. | Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1642] [Cited by in RCA: 1740] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 25. | Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 468] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 27. | Lindboe CF. Lymph node harvest in colorectal adenocarcinoma specimens: the impact of improved fixation and examination procedures. APMIS. 2011;119:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Hitchcock CL, Sampsel J, Young DC, Martin EW, Arnold MW. Limitations with light microscopy in the detection of colorectal cancer cells. Dis Colon Rectum. 1999;42:1046-1052. [PubMed] |
| 29. | Gastl G, Spizzo G, Obrist P, Dünser M, Mikuz G. Ep-CAM overexpression in breast cancer as a predictor of survival. Lancet. 2000;356:1981-1982. [PubMed] |
| 30. | Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424-2426. [PubMed] |
| 31. | Engin AB, Ergun MA, Yurtcu E, Kan D, Sahin G. Effect of ionizing radiation on the pteridine metabolic pathway and evaluation of its cytotoxicity in exposed hospital staff. Mutat Res. 2005;585:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Wachter H, Fuchs D, Hausen A, Reibnegger G, Weiss G, Werner ER, Werner-Felmayer G. Neopterin, Biochemistry, Methods, Clinical Application. 1992;. |
| 33. | Liu X, Newton RC, Friedman SM, Scherle PA. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:938-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Wachter H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J. 1989;262:861-866. [PubMed] |
| 35. | Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 36. | Van den Eynde B. [A new mechanism of tumor resistance to the immune system, based on tryptophan breakdown by indoleamine 2,3-dioxygenase]. Bull Mem Acad R Med Belg. 2003;158:356-363; discussion 364-365. [PubMed] |
| 37. | Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 880] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 38. | Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 40. | Huh JW, Kim HR, Kim YJ. Lymphovascular or perineural invasion may predict lymph node metastasis in patients with T1 and T2 colorectal cancer. J Gastrointest Surg. 2010;14:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |