Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3506
Peer-review started: July 30, 2015
First decision: September 9, 2015
Revised: September 28, 2015
Accepted: October 17, 2015
Article in press: October 20, 2015
Published online: March 28, 2016
Processing time: 238 Days and 20.3 Hours
Primary splenic angiosarcoma (PSA) is an unusual and highly malignant vascular tumour with a high rate of metastatic. Moreover, the research on prognosis of the disease is poor. The epidemiology, etiology, clinical diagnosis and treatment of the disease remain challenging, because case reports of the disease are few in number. In accordance with other malignant tumors, PSA is very aggressive, and the majority of patients in which this disease is found are at an advanced stage. Almost all patients die within 12 mo of diagnosis irrespective of treatment. We report here a woman who had complained of upper bellyache and anorexia for 10 d. Magnetic resonance imaging showed enlargement of the spleen with multiple heterogeneous masses in the lower pole of the spleen. A hand-assisted laparoscopic splenectomy was performed which allowed histopathologic diagnosis. The patient was diagnosed with PSA and liver metastasis, and succumbed to the disease 35 d after surgery. The literature was finished combined with the clinical features, diagnosis and management of PSA.
Core tip: Primary splenic angiosarcoma (PSA) is an unusual tumor originating from the blood vessel. To date, very few cases of PSA have been reported. We report a woman who had PSA after splenectomy, and liver metastasis was also detected. The patient died 35 d after surgery. We review the literature and conclude that early diagnosis followed by splenectomy is beneficial for better survival of the patients.
- Citation: Yang KF, Li Y, Wang DL, Yang JW, Wu SY, Xiao WD. Primary splenic angiosarcoma with liver metastasis: A case report and literature review. World J Gastroenterol 2016; 22(12): 3506-3510
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3506.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3506
Primary splenic angiosarcoma (PSA) is an unusual and highly malignant non-hematolymphoid tumor of the spleen. It was described for the first time by Theodor Langhans in 1879[1], and the incidence of SPA has been reported between 0.15 and 0.26 per million people[2,3]. Although it occurs primarily in older patients, individuals of any age can be affected[2,4-8], from 14 mo to 89 years. It has a slight gender difference, such that the ratio of rates in males and females is 4:3[3]. We report a 46-year-old female PSA patient with hepatic metastasis. In addition, we summarize the features and outcomes of PSA according to the clinical characteristics.
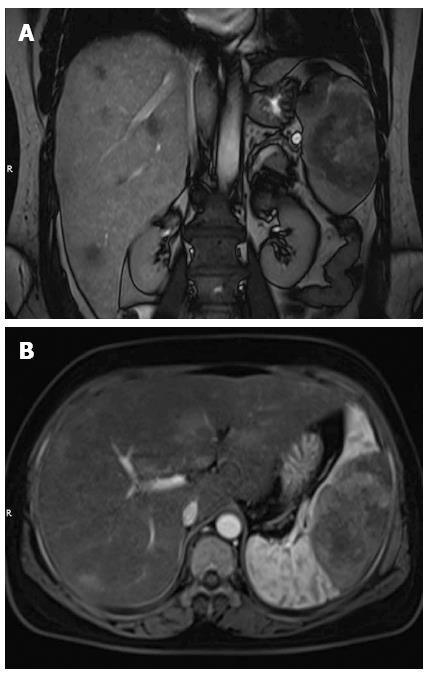
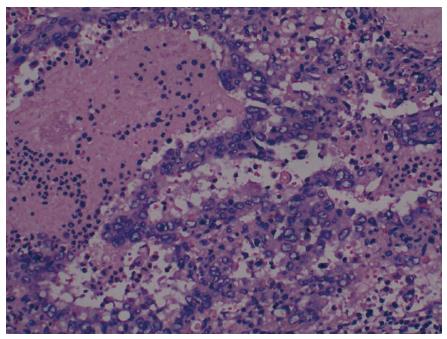
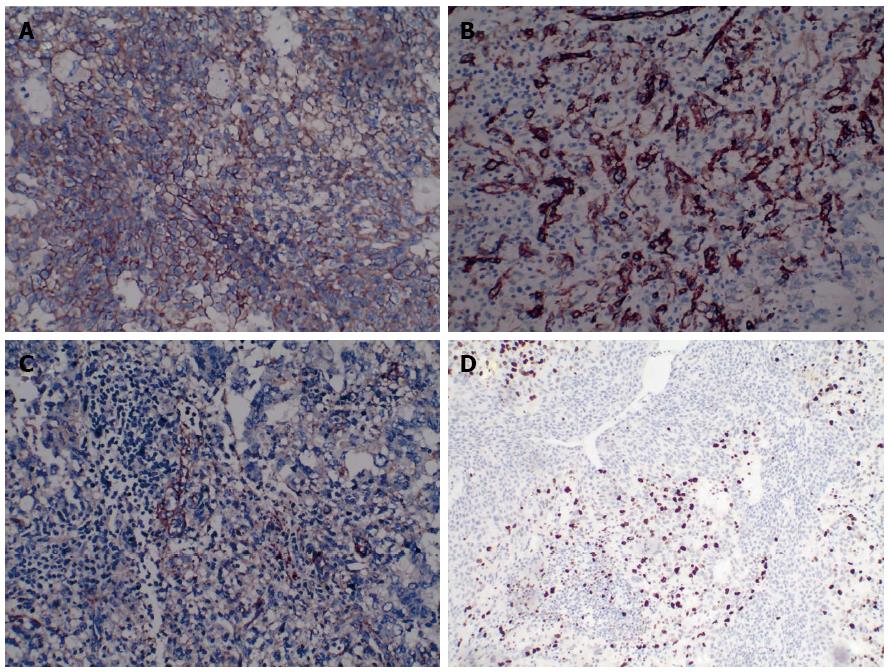
A woman was referred to our hospital from a local clinic on April 16, 2015, complaining of intermittent upper abdominal pain and anorexia for 10 d. Following the occurrence of these symptoms, the patient reported no weight loss. We did not know the medical history of the patience except for laparoscopic resection of an ovarian cyst, which was performed in Fuzhou People’s Hospital (Jiangxi, China) 10 years previously. The patient’s family number was very important, since the patient’s mother and father had all died of cancer. No abnormalities were observed during physical examination. Laboratory examinations on admission revealed: white blood cell (WBC) count of 5.41 × 109/L (normal range 4-10 × 109/L), hemoglobin 75 g/L (normal range 110-150 g/L), platelet count 151 × 109/L (normal range 100-300 × 109/L), alanine aminotransferase 49 U/L (normal range 5-35 U/L), aspartate transaminase 48 U/L (normal range 5-40 U/L), γ-glutamyl transpeptidase 87 U/L (normal range ≤ 32 U/L), and albumin 29.6 g/L (normal range 34-48 g/L). Serum tumor markers, including α-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and carbohydrate-125 (CA-125), were all within normal ranges. A coagulation function test was performed and no positive results were observed. Magnetic resonance imaging (MRI) of the abdomen has shown a well circumscribed heterogeneous mass of 7.8 cm × 5.7 cm and multiple nodules in the liver parenchyma (Figure 1). So the diagnosis of the primary splenic malignancy with hepatic metastasis was suggested based on imaging findings. A hand-assisted laparoscopic splenectomy was then performed not only for the purpose of curing the disease, but also for histopathologic diagnosis. In this process, the patient had received 2 units of fresh frozen plasma and frozen plasma, 3 units of blood cells and 6 units of cryoprecipitate. Intraoperatively, we observed a scleroid tumor mass 6 cm × 5 cm in size in the lower pole of the spleen. A cross-section of the specimen demonstrated a well-circumscribed grayish yellow lesion. In accordance with the imaging findings, laparotomy revealed micrometastases in the liver. Thus, a histopathologic biopsy was carried out. The pathologic diagnosis of the excised spleen was originating from the spleen (Figure 2) and liver metastasis, which were confirmed by pathologic examination of the biopsied specimen. Immunohistochemical analysis showed that the tumor cells were positive for CD31+++ (Figure 3A), CD34+++ (Figure 3B), FVIII (Figure 3C), Ki-67 + 30% (Figure 3D), and were negative for CD68, cytokeratin, epithelial membrane antigen, desmin, actin, smooth muscle actin, and lysozyme. The patient leave the hospital after 15 d with no complications, but succumbed to the disease 35 d after surgery.
Although the spleen play an important role in our life and it is also an important immune organ, it rarely reported as the beginning of tumors. Primary malignant tumors of the spleen are extremely rare and include lymphoma, reticulum cell sarcoma, fibrosarcoma, and angiosarcoma which is also recognized as malignant hemagioendothelioma. It is reported that PSA originates from immature endothelial-type cells, the immunohistochemical techniques has confirmed it[9]. PSA is a very aggressive neoplasm, it could last 4.4-14 mo[2,3]. In the majority of patients with PSA, distant metastasis is present at the time of laparotomy, with the most common sites has occurred in lungs, bones, lymph nodes, adrenal glands and others[2,3,5]. The findings in our case were consistent with these results, as liver metastasis was identified.
The pathogenesis of this highly aggressive malignancy remains unclear. Possible causative factors include ionizing radiation, arsenic, vinyl chloride and chemotherapy for lymphoma[10,11]. However, some reports indicate that angiosarcoma of the spleen develops from previously existing benign tumors, such as hemangioma or hemangioendothelioma[4,6]. None of these factors were involved in our case. Delacruz et al[12] reported a 69-year-old woman who presented with splenic angiosarcoma during the follow-up for a history of liposarcoma of the right buttock. Three years prior to the patient’s admission, she had received neoadjuvant chemotherapy for the liposarcoma. In addition, a remarkable family medical history was noted for a sister with angiosarcoma of the spleen and her mother with breast cancer. Our patient’s family history was significant for the death of her mother due to gastric cancer and the death of her father due to lung cancer. With the information provided, we made an assumption that family history may play a significant role in the pathogenesis of PSA.
The clinical manifestations of PSA vary significantly. Upper abdominal pain is the most common manifestation[3]. Other symptoms include weakness or fatigue, shortness of breath, fever, chest pain, weight loss and anorexia[3,13]. A minority of patients are asymptomatic and PSA is discovered incidentally. In severe cases, patients may develop spontaneous splenic rupture which is one of the most severe complications of this disease with a reported incidence of up to 25%[3]. However, it has been reported that splenic rupture was not combined with the clinical outcome[3]. As we all know, a total of 17 cases of splenic rupture secondary to angiosarcoma had been reported until February 2015. All of these cases had undergone splenectomy, and postoperative survival ranged from 1 d to 7 mo. When do the physical examination, the disease could also presented with the splenomegaly identified, and quadrant abdominal mass[14].
Laboratory findings described combined with the disease include anemia, thrombocytopenia, leukocytosis, and an elevated erythrocyte sedimentation rate[2,3]. None of these abnormalities was present in our patient, with the exception of anemia. Tumor markers (AFP, CEA, CA-125 and CA19-9) are always within normal ranges or only mildly elevated.
While there is still a lack of standardization, the majority of the diagnosis of angiosarcoma is suggested at the imaging of the patients[14]. The most common ultrasonographic findings are represented by splenomegaly, ill-defined solid and others[15,16]. Computed tomography could show the enlarged spleen with solitary or multiple nodular masses of heterogeneous low attenuation. Some of these masses has shown the peripheral enhancement, and the margins of the lesions could not see clearly[13,16,17]. On MRI, areas of increased and decreased signal intensity may be seen on images obtained with both T1- and T2-weighted pulse sequences, and contrast-enhanced MRI reveals heterogeneous enhancement within the tumor[18,19]. Low-signal-intensity areas on MRI probably represent siderotic nodules[20]. In our case, only MRI was performed. Nonspecific clinical presentation and laboratory test results emphasize the essential role of imaging in the diagnosis of PSA.
The therapeutic strategies for PSA are limited as the disease is extremely rare and has highly aggressive characteristics. Splenectomy is the predominant treatment method for PSA without metastasis. Chemotherapy and radiotherapy have also been reported[21,22]. However, the role of these adjuvant therapies still remain to be defined. In addition to patients with distant metastasis, splenectomy is suggested for pediatric angiosarcoma of the spleen. Due to the immune system of infants is not developed very well, so that a complete splenectomy may have negative effects on system. A complete splenectomy may increases postoperative risks, including the possibility of overwhelming post-splenectomy infection (OPSI). OPSI occurs at an estimated incidence of 0.23%-0.42% per year, with a mortality of 38%-69%[23]. Splenic rupture may occur in PSA, thus early diagnosis of PSA followed by splenectomy before rupture may yield a more favorable survival rate[2,24]. Montemayor et al[25] found that patients with splenic angiosarcoma had a longer survival time if splenectomy was performed prior to rupture rather than after rupture (14.4 mo vs 4.4 mo).
Similar to other aggressive neoplasms, PSA is a malignancy with a poor prognosis. It was reported by Neuhauser et al[3] that PSA has a survival of 5 mo, irrespective of the type of treatment administered. We report a case of a 46-year-old woman with PSA and liver metastasis, who died 35 d after surgery. This case report together with the literature review of previous cases provide an alert for clinicians that PSA should be considered when the clinical presentation includes symptoms such as upper abdominal pain, hematology abnormalities (anemia, leukocytosis, thrombocytopenia, and/or elevated erythrocyte sedimentation rate). In addition, imaging examination is essential for early diagnosis of PSA. Considering that early diagnosis followed by splenectomy shows an advantage in terms of survival rate, early diagnosis should be a hot topic in the treatment of this disease.
A 46-year-old woman with a history of laparoscopic resection of an ovarian cyst presented with upper abdominal pain and anorexia for 10 d.
Intermittent upper abdominal pain and anorexia may be nonspecific symptoms. No abnormalities were found during physical examination.
Differential diagnosis included hemangioma, littoral cell angioma, lymphangioma and hemangiopericytoma.
White blood cell count 5.41 × 109/L, hemoglobin 75 g/L, platelet count 151 × 109/L, alanine aminotransferase 49 U/L, aspartate transaminase 48 U/L, γ-glutamyl transpeptidase 87 U/L, and albumin 29.6 g/L. Serum tumor markers were within normal ranges.
Magnetic resonance imaging of the abdomen revealed an enlarged spleen with a well circumscribed heterogeneous mass measuring 7.8 cm × 5.7 cm and multiple nodules in the liver parenchyma.
Histopathologic diagnosis was angiosarcoma originating from the spleen, and liver metastasis. Splenic tumor cells stained positive for CD31, CD34+++, FVIII, Vimentin+++, and Ki-67 + 30%.
A hand-assisted laparoscopic splenectomy was performed not only for the purpose of curing the disease, but also for histopathologic diagnosis.
There are few reports of liver metastasis from splenic angiosarcoma in the literature. However, the liver is the most frequently reported organ in terms of distant metastasis with an incidence of 70%. The etiology of Primary splenic angiosarcoma (PSA) has not been clearly elucidated. Median survival has been reported to be 12 mo.
PSA is a rare splenic tumor of significant malignancy, with a reported incidence between 0.14 and 0.25 per million persons.
Etiology, clinical diagnosis and treatment of the disease remain challenging. Early diagnosis followed by splenectomy result in a favorable survival rate.
The reviewer agrees with the authors that early diagnosis followed by splenectomy, it is beneficial for better survival of the patients because only histopathological examination is essential for diagnosis. Although primary splenic angiosarcoma is quite widely featured, there are two reasons why the article should be published: (1) the manuscript is very well prepared; and (2) it is necessary to remind us about this type of neoplasm.
P- Reviewer: Kamocki Z S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Langhans T. Pulsating cavernous neoplasm of the spleen with metastatic nodules to the liver. Vichows Arch Pathol Anat. 1879;75:273-291. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Falk S, Krishnan J, Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. 1993;17:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, Abbondanzo SL. Splenic angiosarcoma: a clinicopathologic and immunophenotypic study of 28 cases. Mod Pathol. 2000;13:978-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Sordillo EM, Sordillo PP, Hajdu SI. Primary hemangiosarcoma of the spleen: report of four cases. Med Pediatr Oncol. 1981;9:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Chen KT, Bolles JC, Gilbert EF. Angiosarcoma of the spleen: a report of two cases and review of the literature. Arch Pathol Lab Med. 1979;103:122-124. [PubMed] |
| 6. | Alt B, Hafez GR, Trigg M, Shahidi NT, Gilbert EF. Angiosarcoma of the liver and spleen in an infant. Pediatr Pathol. 1985;4:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Myoui A, Aozasa K. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol. 1996;61:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 345] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Takato H, Iwamoto H, Ikezu M, Kato N, Ikarashi T, Kaneko H. Splenic hemangiosarcoma with sinus endothelial differentiation. Acta Pathol Jpn. 1993;43:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Sordillo EM, Sordillo PP, Hajdu SI. Splenic angiosarcoma. Am J Surg Pathol. 1995;19:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Zwi LJ, Evans DJ, Wechsler AL, Catovsky D. Splenic angiosarcoma following chemotherapy for follicular lymphoma. Hum Pathol. 1986;17:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Delacruz V, Jorda M, Gomez-Fernandez C, Benedetto P, Ganjei P. Fine-needle aspiration diagnosis of angiosarcoma of the spleen: a case report and review of the literature. Arch Pathol Lab Med. 2005;129:1054-1056. [PubMed] |
| 13. | Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology. 2005;235:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | De Vriese L, De Coster M, Noyez D. Angiosarcoma of the spleen. Case report and review of literature. Acta Chir Belg. 1989;89:46-48. [PubMed] |
| 15. | Nahman B, Cunningham JJ. Sonography of splenic angiosarcoma. J Clin Ultrasound. 1985;13:354-356. [PubMed] [DOI] [Full Text] |
| 16. | Vrachliotis TG, Bennett WF, Vaswani KK, Niemann TH, Bova JG. Primary angiosarcoma of the spleen--CT, MR, and sonographic characteristics: report of two cases. Abdom Imaging. 2000;25:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Reddy SC, Reddy SC. Hemangiosarcoma of the spleen: helical computed tomography features. South Med J. 2000;93:825-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Imaoka I, Sugimura K, Furukawa M, Kuroda S, Yasui K. CT and MR findings of splenic angiosarcoma. Radiat Med. 1999;17:67-70. [PubMed] |
| 19. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Kaneko K, Onitsuka H, Murakami J, Honda H, Kimura M, Shiraishi N, Masuda K. MRI of primary spleen angiosarcoma with iron accumulation. J Comput Assist Tomogr. 1992;16:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Stutz FH, Tormey DC, Blom J. Hemangiosarcoma and pathologic rupture of the spleen. Cancer. 1973;31:1213-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Serrano OK, Knapp E, Huang K, Baran G, Statter M, McClain D, Gill J. Pediatric primary splenic angiosarcoma: an aggressive multidisciplinary approach to the oncologic management of a rare malignancy. World J Surg Oncol. 2014;12:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Autry JR, Weitzner S. Hemangiosarcoma of spleen with spontaneous rupture. Cancer. 1975;35:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |