Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3451
Peer-review started: May 25, 2015
First decision: June 25, 2015
Revised: August 9, 2015
Accepted: October 23, 2015
Article in press: October 26, 2015
Published online: March 28, 2016
Processing time: 303 Days and 18.5 Hours
AIM: To study differences in the visceral sensitivity of the colonic mucosa between patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and those with ulcerative colitis (UC) in remission and to relate these differences with changes in the 5-hydroxytryptophan (5-HT) signaling pathway.
METHODS: Gastrointestinal symptoms were used to determine the clinical symptom scores and rectal visceral sensitivity of patients with IBS-D and patients with UC in remission. Blood levels of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were measured using an HPLC-electrochemical detection system. The levels of 5-HT 3 receptor (3R), 4R, and 7R mRNAs in colonic biopsy samples were detected using reverse transcription-polymerase chain reaction. The protein expression of TPH1 was analyzed by Western blot and immunohistochemistry.
RESULTS: Abdominal pain or discomfort, stool frequency, and the scores of these symptoms in combination with gastrointestinal symptoms were higher in the IBS-D and UC groups than in the control groups. However, no significant differences were observed between the IBS-D and UC remission groups. With respect to rectal visceral sensitivity, the UC remission and IBS-D groups showed a decrease in the initial perception threshold, defecating threshold and pain threshold. However, these groups exhibited significantly increased anorectal relaxation pressure. Tests examining the main indicators of the 5-HT signaling pathway showed that the plasma 5-HT levels, 5-HIAA concentrations, TPH1 expression in the colonic mucosa, and 5-HT3R and 5-HT5R expression were increased in both the IBS-D and the UC remission groups; no increases were observed with respect to 5-HT7R expression.
CONCLUSION: The IBS-D and UC groups showed similar clinical symptom scores, visceral sensitivity, and levels of serotonin signaling pathway indicators in the plasma and colonic mucosa. However, the pain threshold and 5-HT7R expression in the colonic mucosa were significantly different between these groups. The results reveal that (1) IBS-D and UC are related to visceral sensitivity pathogenesis and the clinical manifestations of these conditions and (2) the observed differences in visceral hypersensitivity are possibly due to differences in levels of the 5-HT7 receptor, a component of the 5-HT signaling pathway.
Core tip: Irritable bowel syndrome (IBS) is among the most common functional gastrointestinal disorders, but its pathogenesis is not understood. Ulcerative colitis (UC) is a chronic non-specific inflammatory gastrointestinal disease. Visceral hypersensitivity is the most well-known cause of abdominal pain related to diarrhea-predominant IBS (IBS-D) and UC. The 5-hydroxytryptophan (5-HT) signaling pathway is important for both sensory signal transduction in gastrointestinal motility and the development of visceral hypersensitivity. This study examined visceral sensitivity differences in the colonic mucosa between IBS-D and UC remission groups with respect to the 5-HT signaling pathway. We offer a new theoretical basis for Chinese medical treatment for the two types of common intestinal diseases related to 5-HT signaling pathways. These data will also provide new insights into future methods for the application of traditional Chinese medicine.
- Citation: Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Comparison of 5-hydroxytryptophan signaling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J Gastroenterol 2016; 22(12): 3451-3459
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3451.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3451
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders, but its pathogenesis is poorly understood. Ulcerative colitis (UC) is a chronic non-specific inflammatory gastrointestinal disease. The pathogenesis of UC may be related to genetic factors, intestinal flora, immune disorders, dietary allergies, and anxiety.
Abdominal pain is an important symptom in UC patients, and visceral hypersensitivity is the most widely known cause of abdominal pain. The 5-hydroxytryptophan (5-HT) signaling pathway is important for both sensory signal transduction in gastrointestinal motility and the development of visceral hypersensitivity[1-4].
In this study, we determined the blood levels of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) using a high performance liquid chromatography (HPLC)-electrochemical detection system. We used reverse transcription-polymerase chain reaction (RT-PCR) to assay the transcriptional levels of 5-HT 3 receptor (3R), 4R, and 7R in biopsied colonic tissue samples. The protein expression of tryptophan hydroxylase 1 (TPH1) was determined using Western blot, and serotonin levels were detected via immunohistochemistry.
These assays were performed using samples from normal, diarrhea-predominant IBS (IBS-D), and UC patients. The results obtained will provide a new theoretical basis for the Chinese medical treatment of the two common intestinal diseases that are related to 5-HT signaling. These data will also provide new insights into future applications of traditional Chinese medicine.
The diagnostic criteria for IBD were based on the Consensus Norms for Chinese Diagnosis and Treatment of Inflammatory Bowel Disease, which were issued in 2008 by the Inflammatory Bowel Disease Collaborative Group of the Chinese Medical Association Digest Credits. UC patients were not included in this group[5,6]. UC activity was assessed according to Mayo’s Disease Activity Index (DAI), which considers the frequency of bowel movements, blood in the stool, colonic mucosal inflammation and the physician’s overall evaluation. Cases of UC were judged to be in remission if the evaluation score was ≤ 2.
Inclusion criteria were: (1) meeting the UC or IBS-D criteria; (2) being 19-60 years of age; and (3) signing an informed consent form. Exclusion criteria were: (1) having colon cancer; (2) having other autoimmune diseases, such as systemic lupus erythematosus and multiple sclerosis; and (3) having other tumors or digestive issues.
We selected patients treated at University City Branch of Guangdong Provincial Hospital and Nanfang Hospital from June 2012 to January 2014. The normal control group consisted of healthy individuals. We selected 33 patients with UC in remission, 30 IBS-D patients and 30 healthy participants. There were 18 males and 15 females with UC in remission, and they were aged from 22 to 56 years. The average age was 39 ± 17 years, and the average duration of the condition was 1.8 ± 18.2 years. No significant differences were observed in age, sex, or duration of remission between the analyzed groups (P > 0.05) (Table 1).
| Healthy group | UC remission group | IBS-D group | |
| Cases | 30 | 33 | 30 |
| Age (yr), mean age | 23-62, (42.5 ± 19.5) | 22-56, (39 ± 17) | 18-60, (39 ± 21) |
| Sex | 15 females, 15 males | 15 females, 18 males | 16 males, 14 females |
| Duration (yr) | None | 1.8 ± 18.2 | 1.6 ± 20.4 |
The gastrointestinal symptoms rating scale (GSRS)[2] was used. For symptom rating, the considered symptoms/complaints included the degree of abdominal pain or discomfort, the frequency of stool passage, abnormal stool frequency, abnormal bowel movement frequency (bowel problems, defecation), and frequency of mucus in the stool. The severity was scored as follows: 0, symptom not present; 1, mild; 2, moderate; and 3, severe. Frequency rating was as follows: 0, symptom did not occur; 1, occasionally (symptomatic between 1% and 24% of the time); 2, often (symptomatic between 25% and 50% of the time); and 3, sustained (> 50% of the time).
A Synectics Visceral Stimulator (CTD-Synectics Medical Company, Sweden) was used as a whole digestive tract detector. This device consists of an electronic pressure pump for monitoring gastrointestinal tension as well as a capillary perfusion digestive pressure monitoring system. The catheter used for anorectal manometry had an outer diameter of 0.8 cm, with a 10 cm × 8 cm air sac at the front end. The gas injection channel and the balloon pressure channel at the opening of the balloon were connected with an electronic pressure pump during the procedure. Four perfusion manometry channels (1 cm apart) were located 14 cm from the catheter tip and were connected with a PC Polygraf apparatus during testing. Patients undergoing anorectal manometry kept a normal diet and received a fecal enema a few days prior to testing. The patient was placed in the left lateral supine position, and the catheter was inserted following anal dilation until the four perfusion pressure measurement channels were in the high-pressure zone. The manometry catheter was then fixed. First, the anal sphincter resting pressure was recorded, and the patient was then asked to contract the anus for detection of maximum diastolic blood pressure and to defecate to detect sphincter diastolic blood pressure. We tested patient sensation and compliance using the electronic pressure pump. Gas was injected into a balloon using a 20 mL gas injection gradient at a rate of 38 mL/h. The patients’ initial sensory thresholds, defecation thresholds and pain thresholds were observed. The pressure between the wall of the balloon and the intestine during gas injection was detected, and a compliance curve was made to determine the maximum compliance value.
Three milliliters of venous blood were obtained from all of the subjects for HPLC analysis. Colonoscopies were also carried out for all subjects. The specimen collection was approved by the hospital ethics committee. A polyclonal antibody for the serotonin transporter (SERT) was purchased from Beijing Bioss. The rabbit and mouse anti-human immunohistochemistry kits and the DAB reagent kit were purchased from Beijing Zhongshan Golden Bridge Company. A rabbit-anti-rat TPH1 antibody (Santa Cruz, United States), a mouse-anti-rat β2-actin antibody (Ab-cam, United States), mouse and rabbit secondary antibodies (KPL, United States) and TRIzol Reagent (Invitrogen, United States) were used. SDS-PAGE apparatus (BIO-RAD, United States), a DY2CZ-40B electrophoretic transfer tank (Beijing Liuyi, China) and image analysis system (UVP) were used for protein detection. RNA extraction was performed according to the kit’s instructions, and OD260 was measured using a UV spectrophotometer (UV-1601). RT-PCR primers for the examined genes and the internal control (GAPDH) were synthesized by SANGON. A tissue RNA extraction kit was purchased from Invitrogen. A reverse transcription kit was purchased from PROMEGA, and RNase AWAY was purchased from QIAGEN. All of these procedures were carried out according to the kits’ instructions.
5-HT and 5-HIAA in serum were detected using an HPLC-electrochemical detector system.
The specimens were cut into small pieces of 1 mm3 and washed thoroughly with PBS. After the addition of lysis buffer, the samples were incubated for 2 h at 4 °C. The samples were then centrifuged and denatured. The supernatant was immediately analyzed or stored at -80 °C.
After electrophoresis, the proteins were transferred to a PVDF membrane using wet transfer. The membrane was blocked in 5% skim milk for 2 h at room temperature and then incubated with rabbit anti-TPH1 (1:200) or mouse anti-β2 actin (1:1000) antibody at 4 °C overnight. The membrane was incubated at room temperature with an anti- rabbit or mouse secondary antibody, as appropriate (1:1000 and 1:500) for 2 h. Photographs were taken after ECL coloration. Image analysis was carried out, and the gray ratio of TPH1 to β-actin was measured.
Immunohistochemical detection of serotonin in the colonic mucosa: Specimens were processed using conventional immobilization, embedding and sectioning. Serotonin levels were detected in each group using immunohistochemistry. Briefly, the SERT protein antigen was retrieved via hot fixation with EDTA (130-160 °C, 1-2 min). PBS was employed as a negative control. Under a microscope (magnification × 200), three areas of positively stained cells were randomly selected for each slice to observe and calculate the average gray value. A digital image acquisition system and an HPIAS-1000 high-resolution, color pathology report analysis system were used for this procedure. The gray value was inversely proportional to the expression level, i.e., the higher the gray value, the lower the level of expression.
The obtained tissue blocks (approximately 100 mg) were homogenized, and TRIzol was added (approximately 2 mL TRIzol per 100 mg tissue). Frozen homogenized tissue was transferred to Eppendorf tubes and incubated for 5 min at 15-30 °C. Chloroform was added (0.2 mL chloroform/1 mL TRIzol), after which the samples were shaken for 15 s and incubated for 2-3 min at 15-30 °C. The samples were then centrifuged at 12000 r/min for 15 min at 4 °C. The supernatant was transferred to a new Eppendorf tube, and isopropyl alcohol was added (0.5 mL isopropanol/1 mL TRIzol). The samples were incubated for 10 min at 15-30 °C and centrifuged at 12000 r/min (4 °C) for 10 min. The supernatant was discarded, and 75% ethanol was used to wash the precipitate (at least 1 mL 75% ethanol/1 mL TRIzol). Then, the sample was centrifuged at 500 r/min for 5 min at 4 °C, and the ethanol was removed. The precipitate was dried for 5-10 min in air (the sample was not allowed to dry completely). DEPC-treated water was added to dissolve the RNA, which was stored at -80 °C. The RNA was reverse transcribed to cDNA. The cDNA was used as a template for PCR amplification with two pairs of primers. The first pair recognized the 5-HT3R gene (forward: 5’-CAAGCCACCAAGACTGATGA-3’; reverse: 5’-AACCAGGGTGATGCTGTAGG-3’). The expected amplified fragment length was 290 bp. The second primer pair recognized GAPDH (forward: 5’-GAGTCAACGGA1TITI1GGTCGT-3’; reverse: 5’-CCATCCACAGTCTrCTGGGT-3’), and the expected amplified fragment length was 577 bp. The reaction conditions were as follows: 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 59 °C for 30 s, and 72 °C for 1 min; and final extension for 5 min at 72 °C. The primers for the 5-HT7R gene were 5 -GCTCATCACGCTGCTGACGAT-3 (forward) and 5’-CGCCAGGGACACAATCAGG-3 (reverse), amplifying a 106-bp fragment. The specific steps were carried out according to kit instructions. Eight microliters of the PCR product and 2 μL DNA loading buffer were mixed to perform 2% agarose gel electrophoresis, and a 100-bp molecular weight standard was added as a control marker. The electrophoresis data were analyzed using a gel imaging system. The location of the desired product was determined, and the gray area densities on the gel images were used to represent gene expression. The mRNA levels of GAPDH and the 5-HT3R, 4R, and 7R genes were determined in a semi-quantitative manner.
SPSS 13.0 software was used to analyze the results. The statistical data are expressed as the mean ± SD and were analyzed using single-factor analysis of variance (ANOVA).
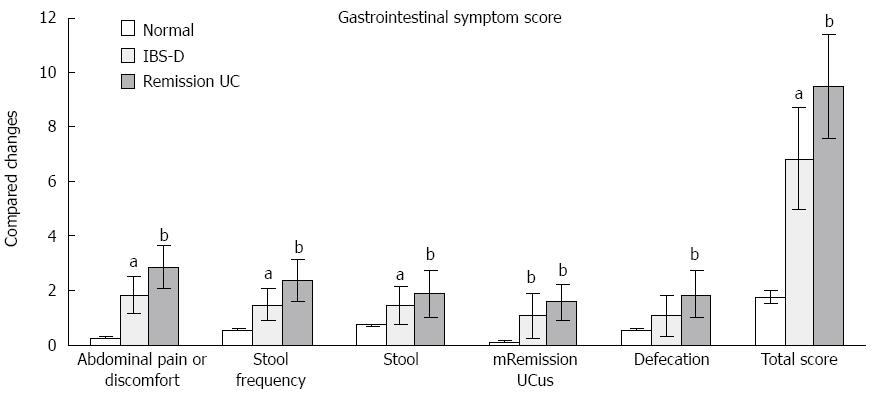
The IBS-D and UC remission groups exhibited increased abdominal pain or discomfort, stool frequency, stool mucus integration and total gastrointestinal symptom scores compared with the normal control group. Various gastrointestinal symptom scores and the total symptom score of UC patients were higher than those for the IBS-D group, but no significant differences were observed between these two groups (Figure 1, Table 2).
| Symptom | Normal | IBS-D | UC in remission |
| Abdominal pain or discomfort | 0.25 ± 0.05 | 1.84 ± 0.67a | 2.84 ± 0.81b |
| Stool frequency | 0.55 ± 0.05 | 1.46 ± 0.58a | 2.34 ± 0.76b |
| Stool | 0.75 ± 0.05 | 1.44 ± 0.67a | 1.89 ± 0.87b |
| Mucus | 0.10 ± 0.03 | 1.04 ± 0.84b | 1.58 ± 0.67b |
| Defecation | 0.55 ± 0.05 | 1.06 ± 0.74 | 1.86 ± 0.86b |
| Total score | 1.75 ± 0.23 | 6.83 ± 1.86a | 9.51 ± 1.91b |
The anorectal pressure measurement results for each group are shown in Table 3. The initial sensory thresholds, defecation threshold and pain thresholds in the UC remission and IBS-D groups were significantly lower compared with those in the healthy control group (P < 0.05). In addition, anorectal relaxation pressure was significantly higher in both the UC remission and IBS-D groups compared to the control (P < 0.05). There were no significant differences in the initial sensory thresholds, defecation threshold or anorectal relaxation pressure between the UC remission and IBS-D groups, although these values were lower in the IBS-D group (P > 0.05). A significant difference was observed, however, for pain thresholds between these two groups (P < 0.05).
| Normal | IBS-D | UC in remission | |
| Initial sensory threshold (mL) | 50.18 ± 4.19 | 23.91 ± 10.15a | 20.10 ± 9.17b |
| Defecation threshold (mL) | 69.15 ± 10.75 | 35.17 ± 12.71a | 21.28 ± 10.32b |
| Pain threshold (mL) | 105.90 ± 20.15 | 70.10 ± 11.25a | 50.33 ± 12.30b |
| Maximum compliance (mL/mmHg) | 4.95 ± 1.81 | 5.02 ± 1.75 | 4.98 ± 1.91 |
| Resting pressure (mmHg) | 62.15 ± 15.21 | 65.54 ± 13.15 | 63.27 ± 10.15 |
| Relaxation pressure (mmHg) | 105.15 ± 60.15 | 140.50 ± 55.50a | 138.69 ± 52.82a |
| Relaxation pressure (mmHg) | 43.18 ± 22.50 | 45.15 ± 20.65 | 46.10 ± 19.97 |
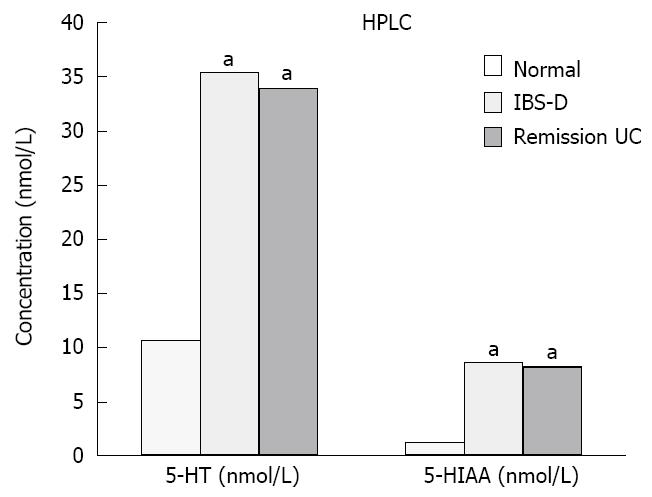
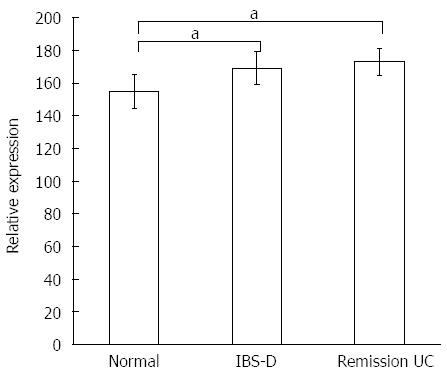
Plasma concentrations of 5-HT and 5-HIAA in patients with IBS-D and UC in remission were significantly increased relative to the controls (P < 0.05). The differences between the IBS-D and UC remission groups were not significant (P > 0.05) (Figure 2, Table 4).
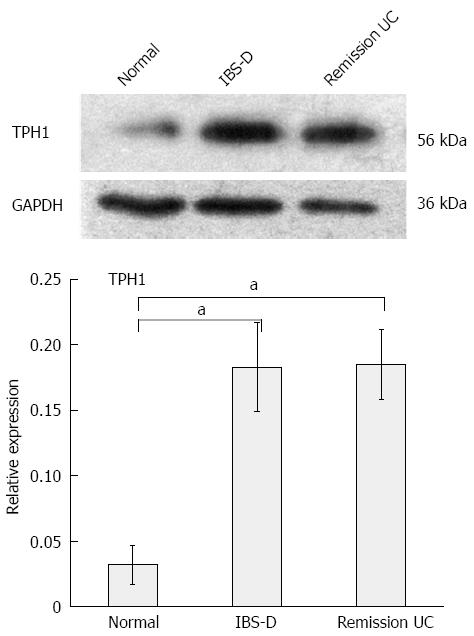
TPH1 expression was significantly increased in patients with IBS-D and UC in remission (P < 0.05) compared with the control group. The difference between the IBS-D group and the UC remission group was not significant (P > 0.05) (Figure 3, Table 5).
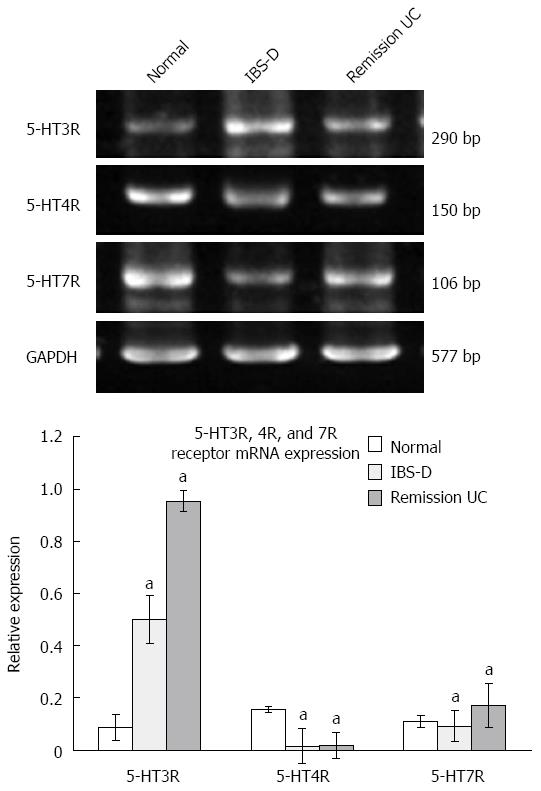
The 5-HT3R expression levels in the colonic mucosa were significantly higher in both the IBS-D and UC remission groups compared with those in the normal control group, although there was no significant difference (P > 0.05) between the UC group and the IBS-D group. 5-HT7R expression in the UC group was elevated compared with the IBS-D and normal control groups, and these differences were significant (P < 0.05). The difference in 5-HT7R expression between the IBS-D group and the normal control group was not significant (P > 0.05). 5-HT4R expression levels in the colonic mucosa of patients with IBS-D and UC in remission were significantly lower than those in the normal control group (P < 0.05), although there was no significant difference between the IBS-D group and UC remission group (P > 0.05) (Figure 4).
The SERT is widely expressed in the membrane and cytoplasm of cells in the colon. In this study, cells with positive expression were stained brown. Compared with the control group, the number of positive cells and staining density were both lower in the IBS-D and the UC groups. The PBS negative control group showed no positive staining. The gray values of each group fell within a normal distribution (P = 0.2). All of the results are shown in Table 6, and the gray values of the IBS-D and UC groups were significantly higher than those in the control (P < 0.05), while no statistical significance was observed between these two groups (P > 0.05) (Figure 5, Table 7).
IBS is one of the most common clinical functional gastrointestinal disorders, but the pathogenesis of IBS is unclear. UC is a chronic non-specific inflammatory gastrointestinal disease, and its pathogenesis may be related to genetic factors, intestinal flora, immune disorders, dietary allergies and anxiety. Abdominal pain is a frequent symptom in UC patients, and visceral hypersensitivity is currently the most widely used explanation for abdominal pain. Research[3] shows that 5-HT plays an important role in the formation of intestinal peristalsis, signal transduction and visceral hypersensitivity[4].
Ohman et al[7] used an enema containing acetic acid in SD rats. After 7 d, a histological examination of myeloperoxidase staining revealed normal results, but the rats showed a high sensitivity to intestine expansion; after constant pressure stimulation, defecation was higher than that in the normal control group. This previous study revealed that both intestinal damage and high levels of intestinal smooth muscle tension were associated with high visceral sensitivity.
Another study found that visceral sensitivity was involved in UC disease activity, stage and involvement scope[8].
5-HT is both an important signaling molecule in the gastrointestinal tract and a neurotransmitter. Approximately 95% of 5-HT is in the intestine, where 90% is stored in enterochromaffin cells (ECs). The 5-HT transporter (SERT) is present in the central nervous system and digestive tract, where it is expressed to a high degree in neurons and intestinal epithelial cells of the intestinal tract. In these cells, SERT can mediate the effects of 5-HT by rapidly uptaking 5-HT. Abnormalities in the 5-HT signal system[9] can cause gastrointestinal motility problems, secretion function abnormalities and high visceral sensitivity. Moreover, abnormalities in this signaling pathway are closely related to abdominal pain, chronic constipation, diarrhea, IBS, functional dyspepsia and other diseases. SERT is the most important protein in mediating the biological activity of 5-HT. Selective serotonin reuptake inhibitors block 5-HT reuptake by acting on the SERT, increasing 5-HT concentrations in the synaptic cleft. We found that such drugs can effectively relieve some of the symptoms, especially abdominal pain, of IBS patients[10-16]. In summary, SERT plays an important role in the generation of visceral hypersensitivity. 5-HT functions through a variety of 5-HT receptors. The multiple types of 5-HT receptors in the intestine have different functions on smooth muscle cells. Among these receptors, both 5-HT3 and 5-HT4 are closely related to the pathogenesis of IBS-D. The 5-HT3 receptor is a ligand-gated anion channel that is expressed in the external sensory neurons of the intestinal tract. This receptor transmits injury signals to the central nervous system, acting as a rapid onset excitatory neurotransmitter on 5-HT neurons, and is closely related to the regulation of visceral sensitivity. The 5-HT4 receptor is a G protein-coupled metabotropic receptor. The opening of voltage-sensitive calcium channels stimulates the release of other neurotransmitters that play an important role in the gastrointestinal tract, such as calcitonin gene-related peptide and substance P. These neurotransmitters thereby affect gastrointestinal motility and visceral sensation[4,5]. This study shows that visceral hypersensitivity usually manifests as hyperalgesia and allodynia, which have been linked to 5-HT1A, 5-HT2A, 5-HT3R, 5-HT4R, and 5-HT7R[16].
Compared with the healthy group, patients in both disease groups experienced abdominal pain or discomfort and passed stools frequently. The scores of these symptoms coupled with the gastrointestinal symptoms were higher in the IBS-D and UC groups than in the control group. However, there was no significant difference between the IBS-D and UC remission groups. In the rectal visceral sensitivity test, the UC remission and IBS-D groups showed decreases in the initial perception threshold, the defecating threshold and the pain threshold; however, these groups exhibited significantly increased anorectal relaxation pressure. With respect to the primary indicators of the 5-HT signaling pathway, the IBS-D and UC remission groups both exhibited increased levels of plasma 5-HT and 5-HIAA, elevated TPH1 expression in the colonic mucosa, and higher expression of 5-HT3R and 5-HT5R. No such increase was observed in the expression of 5-HT7R.
In summary, we have found that the IBS-D and UC groups showed similar changes in clinical symptom scores, visceral sensitivity and indicators of serotonin signaling pathway in the plasma and colonic mucosa. However, both the pain threshold and 5-HT7R expression in the colonic mucosa were significantly higher in UC patients. This study examined colonic visceral sensitivity differences between the IBS-D and UC remission groups with respect to the 5-HT signaling pathway. We offer a new theoretical basis for Chinese medicine for treatment of the two common intestinal diseases that are related to 5-HT signaling. These data will also provide novel insights into potential traditional Chinese medicine applications for the treatment of these conditions.
Irritable bowel syndrome (IBS) is one of the most common clinical functional gastrointestinal disorders. Ulcerative colitis (UC) is a chronic, non-specific inflammatory gastrointestinal disease, the pathogenesis of which is not fully understood. The present study demonstrates that visceral hypersensitivity is the most common cause of abdominal pain in patients with IBS-D and UC. 5-HT signaling pathways play an important role in both the sensory signal transduction of gastrointestinal motility and the development of visceral hypersensitivity.
This study of IBS and UC reveals that these two diseases have the same pathogenic mechanism in terms of visceral sensitivity. Showing that the 5-HT signaling pathway is involved in visceral sensitivity is an extremely important result. This study reveals that both IBS and UP are related to visceral sensitivity, which represents both the pathogenic mechanism and clinical manifestation of these conditions. These results reveal how the 5-HT signaling pathway differs between these syndromes in terms of visceral hypersensitivity. These data will promote the exploration of visceral sensitivity and the evolution of gastrointestinal dynamics in the context of these two conditions.
This multi-level project shows that the 5-HT signaling pathway is involved in the pathogenic mechanism of visceral hypersensitivity in the two disease models. This study aims to make theoretical contributions to the field and to stimulate innovation with respect to understanding and treating the pathogenic mechanisms of the considered diseases.
The results suggest that patients with IBS-D and UC groups exhibit similar symptoms with respect to clinical symptom scores, visceral sensitivity, and indicators of the serotonin signaling pathway in the plasma and colonic mucosa. However, the pain threshold and 5-HT7R expression in the colonic mucosa were significantly higher for both groups relative to the control. The results reveal that both conditions are associated with visceral sensitivity and clinical manifestations, and evidence was provided showing that visceral hypersensitivity may be due to differences in levels of the 5-HT7 receptor, which is a component of the 5-HT signaling pathway.
5-HT is an important signaling molecule in the central nervous system and is involved in a variety of physiological and psychological functions. There is a close relationship between 5-HT signaling and functional gastrointestinal diseases. Therefore, it is necessary to study the role of this signaling pathway in terms of its functional role in the pathogenesis of UC and for guiding clinical treatment.
This is a well-designed descriptive study in which the authors have analyzed the inhibitory effect of the 5-HT signaling pathway (especially 5-HT7R) and its important role in both the sensory signal transduction of gastrointestinal motility and the development of visceral hypersensitivity.
P- Reviewer: Hardy T, Vela S S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Basilisco G. [Pathogenesis of irritable bowel syndrome: current understanding]. Recenti Prog Med. 2007;98:543-547. [PubMed] |
| 2. | Dinan TG, O’Keane V, O’Boyle C, Chua A, Keeling PW. A comparison of the mental status, personality profiles and life events of patients with irritable bowel syndrome and peptic ulcer disease. Acta Psychiatr Scand. 1991;84:26-28. [PubMed] |
| 3. | Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, Stanghellini V, Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G141-G154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 5. | La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791-2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Lucas A, Cobelens PM, Kavelaars A, Heijnen CJ, Holtmann G, Haag S, Gerken G, Langhorst J, Dobos GJ, Schedlowski M. Disturbed in vitro adrenergic modulation of cytokine production in inflammatory bowel diseases in remission. J Neuroimmunol. 2007;182:195-203. [PubMed] |
| 7. | Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39:201-215. [PubMed] |
| 8. | Greenwood-Van Meerveld B, Venkova K, Hicks G, Dennis E, Crowell MD. Activation of peripheral 5-HT receptors attenuates colonic sensitivity to intraluminal distension. Neurogastroenterol Motil. 2006;18:76-86. [PubMed] |
| 9. | Narboux-Nême N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology. 2008;55:994-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Chen YL, Huang XQ, Xu SJ, Liao JB, Wang RJ, Lu XF, Xie YL, Zhou FS, Su ZR, Lai XP. Relieving visceral hyperalgesia effect of Kangtai capsule and its potential mechanisms via modulating the 5-HT and NO level in vivo. Phytomedicine. 2013;20:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994;39:1239-1248. [PubMed] |
| 12. | O’Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut. 2007;56:186-194. [PubMed] |
| 13. | Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067-1076. [PubMed] |
| 14. | El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383-2391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Barbara G. Revival of 5-HT3 antagonism as treatment of IBS-D? Gut. 2014;63:1530-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |