Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3441
Peer-review started: September 16, 2015
First decision: November 5, 2015
Revised: December 3, 2015
Accepted: December 19, 2015
Article in press: December 21, 2015
Published online: March 28, 2016
Processing time: 194 Days and 13.7 Hours
AIM: To investigate the relationship between osteopontin plasma concentrations and the severity of portal hypertension and to assess osteopontin prognostic value.
METHODS: A cohort of 154 patients with confirmed liver cirrhosis (112 ethylic, 108 men, age 34-72 years) were enrolled in the study. Hepatic venous pressure gradient (HVPG) measurement and laboratory and ultrasound examinations were carried out for all patients. HVPG was measured using a standard catheterization method with the balloon wedge technique. Osteopontin was measured using the enzyme-linked immunosorbent assay (ELISA) method in plasma. Patients were followed up with a specific focus on mortality. The control group consisted of 137 healthy age- and sex- matched individuals.
RESULTS: The mean value of HVPG was 16.18 ± 5.6 mmHg. Compared to controls, the plasma levels of osteopontin in cirrhotic patients were significantly higher (P < 0.001). The plasma levels of osteopontin were positively related to HVPG (P = 0.0022, r = 0.25) and differed among the individual Child-Pugh groups of patients. The cut-off value of 80 ng/mL osteopontin distinguished patients with significant portal hypertension (HVPG above 10 mmHg) at 75% sensitivity and 63% specificity. The mean follow-up of patients was 3.7 ± 2.6 years. The probability of cumulative survival was 39% for patients with HVPG > 10 mmHg and 65% for those with HVPG ≤ 10 mmHg (P = 0.0086, odds ratio (OR), 2.92, 95% confidence interval (CI): 1.09-7.76). Osteopontin showed a similar prognostic value to HVPG. Patients with osteopontin values above 80 ng/mL had significantly lower cumulative survival compared to those with osteopontin ≤ 80 ng/mL (37% vs 56%, P = 0.00035; OR = 2.23, 95%CI: 1.06-4.68).
CONCLUSION: Osteopontin is a non-invasive parameter of portal hypertension that distinguishes patients with clinically significant portal hypertension. It is a strong prognostic factor for survival.
Core tip: Data presented in our study are based on a 7-year follow-up interval with systematic hemodynamic evaluations of more than 150 cirrhotic patients. We report for the first time a close relationship between osteopontin (OPN) and portal hypertension. Our findings suggest that OPN in plasma could be a marker of clinically significant portal hypertension. Importantly, we found that OPN is a strong prognostic indicator in patients with liver cirrhosis; and, similar to hepatic venous pressure gradient (HVPG) value, it significantly determined survival probability. Moreover, the combination of HVPG and OPN increased the validity of prognosis.
- Citation: Bruha R, Jachymova M, Petrtyl J, Dvorak K, Lenicek M, Urbanek P, Svestka T, Vitek L. Osteopontin: A non-invasive parameter of portal hypertension and prognostic marker of cirrhosis. World J Gastroenterol 2016; 22(12): 3441-3450
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3441.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3441
Osteopontin (OPN), first described in 1979[1], is a multifunctional protein that is physiologically expressed in the kidney and bone[2]. Under pathological conditions, OPN expression has been found in various organs and has been attributed to many pathological conditions, including inflammation, angiogenesis, fibrosis, and carcinogenesis[3]. Hepatic expression of OPN was first described in rats after carbon tetrachloride intoxication[4]. OPN was shown to contribute to the migration of macrophages into the necrotic areas in liver tissue[5] and to serve as a key cytokine within the extracellular matrix; thus, contributing to fibrogenesis[6,7]. OPN is involved in the evolution and progression of various cancers, including hepatocellular carcinoma (HCC)[8] and cholangiocarcinoma[9]. In fact, plasma OPN levels have been found to be significantly elevated in patients with liver cirrhosis and HCC compared to those without HCC[10].
Recently, plasma OPN levels were shown to predict liver fibrosis in various chronic liver diseases, such as non-alcoholic steatohepatitis[11], alcoholic liver disease[12], and chronic viral hepatitis B[13] and C[14]. As OPN levels correlate significantly with the fibrosis stage in alcohol-induced liver disease[12], it follows that OPN levels could be related to the degree of portal hypertension and, hence, serve as a surrogate non-invasive marker of portal hypertension. This relationship has not been studied until now. Portal hypertension, which is pathogenically related to liver injury and fibrosis, leads to major complications of cirrhosis. In the clinical setting, portal hypertension is evaluated by invasive measurement of the hepatic venous pressure gradient (HVPG)[15]. Recently, non-invasive biomarkers of cirrhosis[16] have been suggested as substitutes for invasive measurement of portal pressure in some indications.
The aim of our study was to evaluate the relationship between OPN plasma concentration and the degree of portal hypertension, as measured by HVPG, and to assess the impact of OPN values on prognosis in patients with liver cirrhosis.
A group of 154 patients with confirmed liver cirrhosis and portal hypertension were included in the study. They were recruited from a list of consecutive patients referred to the 4th Department of Internal Medicine of the General University Hospital in Prague between 2007 and 2014 for hemodynamic evaluation of portal hypertension. A total of 286 patients were examined, out of which 154 were included in the study. The main criteria for exclusion from the study were: active uncontrolled alcohol abuse, known HCC, concomitant antiviral treatment, the use of any drug affecting splanchnic hemodynamics or portal pressure within 2 wk before HVPG measurement, portal vein thrombosis, lack of initial clinical data in the database, lack of appropriate blood sample volume in storage, and refusal of patients to store blood samples for future evaluation or to collect clinical data. Blood samples were collected during hepatic vein catheterization and immediately separated and stored at -70 °C until OPN evaluation.
Liver cirrhosis was diagnosed on the basis of biochemical tests, clinical and ultrasound findings, and/or liver biopsies; the presence of portal hypertension was diagnosed by hepatic vein catheterization.
Hepatic vein catheterization and HVPG measurement were performed in all patients. Eighty-three patients included in the study were referred for HVPG measurement in cases of primary or secondary prophylaxis of bleeding from esophageal varices, according to the Baveno criteria[17]. Indications for HVPG measurement in the remaining 71 patients were staging of liver cirrhosis and portal hypertension. The clinical and laboratory data of all patients were collected at the time of hepatic vein catheterization. The follow-up data were collected from the hospital charts at the time of OPN evaluation. The patients were carefully followed up with a special focus on mortality. The data of six transplanted patients were censored at the time of transplantation.
The control group, used for the purposes of comparing OPN levels, consisted of 137 healthy individuals. The control population was recruited from the staff of the university hospital in Prague. These individuals consisted of healthy subjects without a history of liver disease, coronary artery disease, or other chronic diseases and were age- and gender-matched to the patient population.
The study was carried out in full accordance with the Helsinki Declaration of 1975, as revised in 1983, and was approved by the Institutional Ethics Committee. Informed consent for future evaluation of blood samples for scientific purposes was obtained from all subjects at the time of hepatic vein catheterization.
Measurement of the HVPG was performed using the classic wedge technique[18]. Shortly after overnight fasting, patients were transferred to the catheterization room. Under local anesthesia, a 7F catheter introducer was placed in the right jugular vein using the Seldinger technique. Under fluoroscopic control, a 7F balloon-tipped catheter (B. Braun Melsungen AG, Melsungen, Germany) was advanced into the right hepatic vein in order to measure both free hepatic venous pressure and wedged hepatic venous pressure. All measurements were performed in triplicate using a continuous recording unit. HVPG was calculated as the difference between the wedged hepatic venous pressure and the free hepatic venous pressure. Clinically significant portal hypertension was defined according to the Baveno criteria[17] with HVPG > 10 mmHg.
Biochemical and hematology examinations were performed on automatic analyzers (Modular Analyzer; Roche Diagnostics GmbH, Mannheim, Germany) using standard laboratory assays. The severity of liver disease was evaluated by Child-Pugh scoring and the model end-stage liver disease (MELD) score and then evaluated separately according to platelet count, presence of esophageal varices, ascites, and hepatic encephalopathy.
OPN levels in plasma were measured using an enzyme-linked immunosorbent assay (ELISA) kit (DOST00, R&D Systems, Minneapolis, MI, United States), according to the manufacturer’s instructions. The control group consisted of 137 healthy age- and sex-matched individuals. In each ELISA kit, both controls and patients were included to minimize the effect of inter-assay error due to group comparison.
Other non-invasive markers of portal hypertension were compared to OPN for the detection of clinically significant portal hypertension, including platelet count, platelet count/spleen diameter ratio[19], and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio[20].
The results are presented as mean values with standard deviation. Either a two-sample t-test or the Mann-Whitney rank test for non-Gaussian distributed variables was used to estimate intergroup differences. The correlations between different parameters were evaluated by calculation of Pearson or Spearman correlation coefficients and linear regression analyses. Logistic regression was used to assess the predictive value for mortality of risk factors. In order to prevent model over-adjustment, we merged both predictors (HVPG and OPN), as they seemed to act independently and exhibit similar ORs. All tests were two-sided, with P < 0.05 considered as statistically significant. Receiver operating characteristic curve analysis was used to assess the utility of OPN and other parameters when distinguishing between patients with and without clinically significant portal hypertension. Survival probability was determined using the Kaplan-Meier method. The statistical analyses were performed using BMDP Statistical Software (Release 8.1) and Statistica 12 CZ.
One hundred and fifty four patients were included in the study - 108 males and 46 females. Both gender groups exhibited no differences with regard to age, biochemical parameters, and/or the etiology and stage of cirrhosis (data not shown). Patients were divided into two groups depending on the absence or presence of ascites (compensated, n = 91; decompensated, n = 63). The clinical and laboratory parameters of the whole group as well as patients with and without ascites are given in Table 1. There were no statistical differences between patients and controls in respect to age and gender (data not shown). There were no differences in OPN values between patients with alcoholic or other etiology: 110 (73-159) ng/mL vs 107 (75-146) ng/mL, P = 0.95 (values as median and interquartile (IQ) range).
| Parameter | All patients (n = 154) | Compensated patients (without ascites) (n = 91) | Decompensated patients (with ascites) (n = 63) | P value1 |
| Age (yr) | 54.7 ± 11.1 | 54.5 ± 11.2 | 55.4 ± 11.3 | 0.276 |
| Gender (M/F) (%) | 74/26 | 73/27 | 77/23 | 0.340 |
| Etiology of cirrhosis (alcohol/viral/other incl. NASH) Number of patients | 112/22/20 | 63/15/13 | 47/9/7 | 0.490 |
| Child-Pugh A/B/C (%) | 41/34/25 | 69/27/4 | 14/39/47 | < 0.001 |
| MELD score | 12.5 ± 4.9 | 10.9 ± 4.5 | 14.3 ± 4.8 | < 0.001 |
| Bleeding from varices (%) | 30 | 28 | 33 | 0.288 |
| Bilirubin (µmol/L) | 31 (18-55) | 25 (15-44) | 40 (23-62) | 0.011 |
| Albumin (g/L) | 33.6 ± 7.4 | 39.9 ± 6.7 | 30.1 ± 6.6 | < 0.001 |
| Creatinine (µmol/L) | 83 ± 29 | 81.6 ± 27.2 | 97.8 ± 22.3 | < 0.001 |
| ALT (μkat/L) | 0.65 (0.5-1.4) | 0.71 (0.6-1.7) | 0.58 (0.4-0.8) | 0.282 |
| AST (μkat/L) | 0.96 (0.7-1.6) | 1.11 (0.8-1.6) | 0.75 (0.6-1) | 0.193 |
| Platelets (× 109/L) | 107 (74-163) | 98 (68-142) | 130 (82-203) | 0.015 |
| Arterial mean blood pressure (mmHg) | 91 ± 11.5 | 92 ± 13 | 90 ± 10 | 0.483 |
| Ascites (%) | 41 | - | - | - |
| Encephalopathy (%) | 12 | 6 | 19 | < 0.001 |
| HVPG (mmHg) | 16.0 ± 5.4 | 14.2 ± 5.1 | 26.9 ± 6.1 | < 0.001 |
| Varices (none/small/large) (%) | 18/35/47 | 25/30/45 | 10/40/50 | < 0.001 |
| Spleen length (mm) | 143 ± 22 | 142 ± 19 | 146 ± 26 | 0.534 |
| Diameter of the portal vein (mm) | 13.3 ± 2.1 | 13.2 ± 1.8 | 13.5 ± 2.4 | 0.729 |
| Portal flow velocity (cm/s) | 16 ± 5.8 | 18.8 ± 6 | 13.5 ± 4 | < 0.001 |
| Diameter of the lienal vein (mm) | 10.1 ± 3 | 10.25 ± 2.7 | 9.8 ± 3.5 | 0.348 |
| Follow-up (yr) | 3.7 ± 2.6 | 4.13 ± 2.4 | 2.88 ± 2.6 | 0.004 |
| Osteopontin (ng/mL) | 107 (73.7-154) | 85.7 (65.7-129) | 138 (106-194) | < 0.001 |
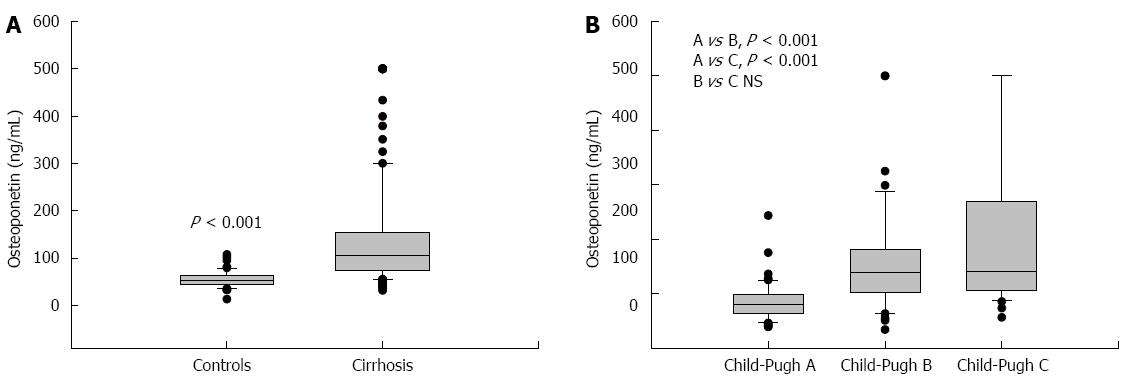
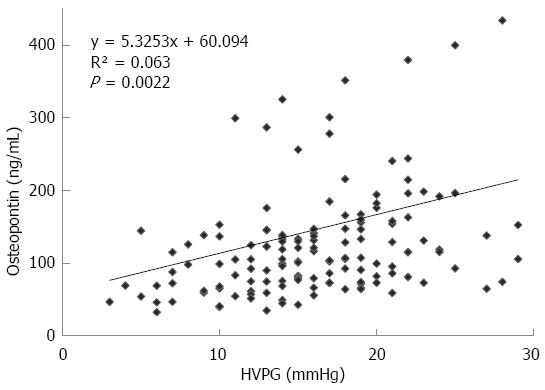
The mean value of HVPG in patients with cirrhosis was 16.18 ± 5.6 mmHg. The plasma values of OPN in cirrhotic patients were significantly higher than values in controls: 107 (74-154) ng/mL vs 55 (42-67) ng/mL, P < 0.001, values as median and IQ range; Figure 1A). Plasma levels of OPN were closely and positively related to HVPG values (P = 0.002, r = 0.25, Figure 2). Plasma levels of OPN above 80 ng/mL distinguished patients with HVPG > 10 mmHg with 75% sensitivity and 63% specificity (AUC 0.763, confidence interval (CI) 49.8-83.7). The positive predictive value (PPV) and negative predictive value (NPV) for the OPN cut-off of 80 of ng/mL concentration in discriminating patients with significant portal hypertension was 92% (95%CI: 85%-96%) and 31% (95%CI: 18%-47%), respectively. A cut-off value of 90 ng/mL distinguished patients with HVPG > 12 mmHg (a marker of increased risk of variceal bleeding) to 71% sensitivity and 62% specificity (area under the curve (AUC), 0.725, 95%CI: 57.3-85.1). When calculated for patients without ascites only, plasma levels of OPN above 80 ng/mL distinguished patients with clinically significant portal hypertension with similar results (sensitivity 65%, specificity 64%, AUC, 0.69; 95%CI: 42-89). The sensitivity and specificity of other non-invasive parameters for the discrimination of patients with clinically significant portal hypertension were as follows: platelet count/spleen size ratio - 32%, 50% (AUC, 0.392, 95%CI: 0.17-0.51), respectively; platelet count - 45%, 40% (AUC, 0.392, 95%CI: 0.3-0.62), respectively; AST/ALT ratio - 74%, 50% (AUC, 0.696, 95%CI: 0.53-0.89), respectively.
No relation of OPN or HVPG to ultrasound portal hemodynamic parameters or laboratory parameters (portal vein diameter, spleen size, platelet count, and serum concentration of albumin) was found, with the exception of portal vein flow velocity. Portal vein flow velocity correlated negatively with HVPG (P = 0.008, r = -0.356) and OPN levels (P = 0.002, r = -0.412). There was no relationship between plasma values of OPN and age, neither in patients (P = 0.9) nor in controls (P = 0.6). Under multivariate analysis performed with HVPG above/below 10 mmHg, OPN values still differed significantly in comparison with other commonly examined laboratory parameters of portal hypertension (Table 2).
| Parameter | P value |
| Osteopontin | 0.04 |
| Platelet count | 0.05 |
| AST/ALT ratio | 0.49 |
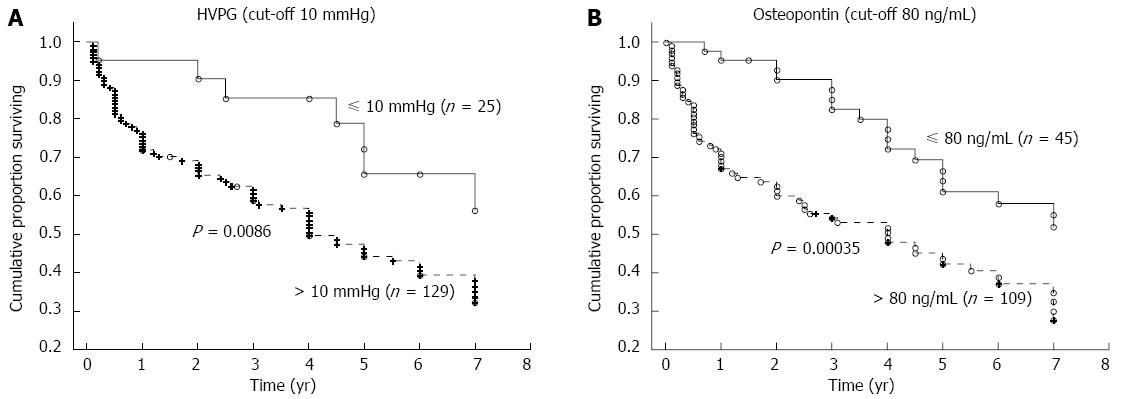
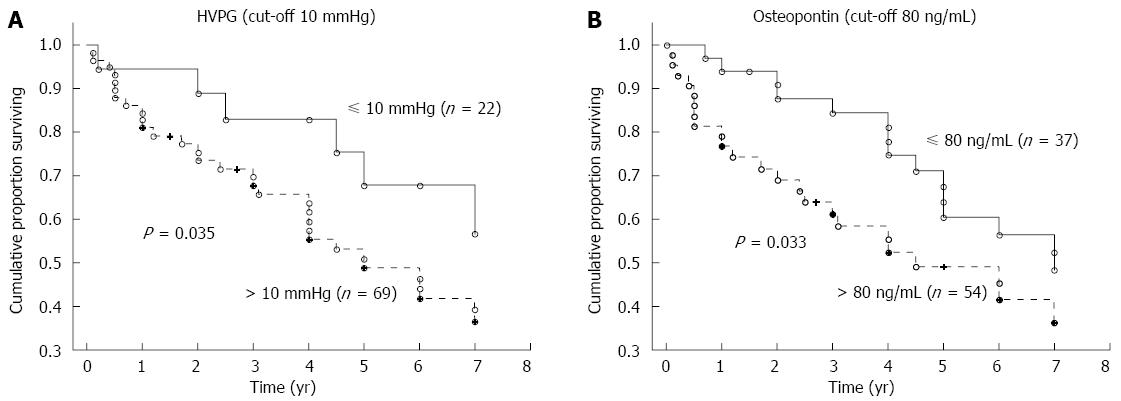
The mean time of follow-up was 3.7 ± 2.6 years (a range of 1 mo to 7 years). During the follow-up, 62 patients died, 77 patients were alive at the time of evaluation (six of whom were transplanted), and 15 patients were lost for follow-up. The HVPG cut-off value of 10 mmHg (i.e., the threshold for clinically significant portal hypertension) divided patients into two groups with significantly different probabilities of cumulative survival (39% for those with HVPG > 10 mmHg compared to 65% for those with HVPG ≤ 10 mmHg; P = 0.0086, OR = 2.92, 95%CI: 1.09-7.76; Figure 3A). When survival probability was calculated as a function of OPN, the plasma cut-off value of OPN 80 ng/mL distinguished two different groups of patients with significantly different probabilities of cumulative survival (37% for those with OPN above 80 ng/mL compared to 56% for those with OPN below 80 ng/mL, P = 0.00035, OR = 2.23, 95%CI: 1.06-4.68; Figure 3B). This difference was significant, independent of HVPG value. Mortality in patients with at least one risk factor (HVPG > 10 mmHg or plasma OPN > 80 ng/L) was more than twice as high compared to patients without any risk factors (OR = 2.34); in those with both risk factors, mortality was more than five times as high (OR = 5.10) compared to patients without any risk factors (Table 3). When considering patients with compensated cirrhosis only (i.e., without ascites), both the plasma cut-off value of OPN 80 ng/mL and the HVPG value of 10 mmHg divided patients into two groups with significantly different probabilities of cumulative survival (Figure 4A and B).
Plasma values of OPN differed among the individual Child-Pugh groups of patients. The plasma values of OPN were 84.8 ± 34.9 ng/mL in Child-Pugh A, 158.8 ± 98.2 ng/mL in Child-Pugh B, and 205.2 ± 142.8 ng/mL in Child Pugh C. Significant differences were found between Child-Pugh A vs B and A vs C groups (Figure 1B). The same significance was found among Child-Pugh groups according to HVPG values (Table 4).
| Parameter | Child-Pugh A | Child-Pugh B | Child-Pugh C | P value |
| Age (yr) | 55.6 ± 10.7 | 52.9 ± 11.2 | 56.3 ± 10.7 | NS |
| Osteopontin (ng/mL) | 84.8 ± 34.9 | 158.8 ± 98.2 | 205.2 ± 142.8 | A vs B, P < 0.001, A vs C, P < 0.001, B vs C, NS |
| HVPG (mmHg) | 14.1 ± 5.1 | 17.1 ± 4.8 | 19.5 ± 4.5 | A vs B, P < 0.001, A vs C, P < 0.001, B vs C, NS |
| Survival (yr) | 4.6 ± 2.3 | 3.3 ± 2.5 | 2.5 ± 2.6 | A vs C, P < 0.001, A vs B, NS, B vs C, NS |
Plasma OPN concentrations correlated significantly with platelet count (r = 0.231; P = 0.009) and presence of ascites (P < 0.001) but not with the size of varices or history of variceal bleeding (Table 5). HVPG correlated with all of above-mentioned clinical parameters, including size of varices and history of variceal bleeding (Table 5). Information about the presence or absence of HCC during the follow-up period was available in the case of 81 patients, of whom HCC developed in six patients (7.4%). Neither plasma OPN levels nor HVPG values correlated with the occurrence of HCC.
| Parameter | HVPG | Osteopontin | ||
| r | P value | r | P value | |
| Survival | 0.220 | 0.009 | 0.230 | 0.006 |
| Platelet counts | 0.247 | 0.005 | 0.231 | 0.009 |
| Size of varices | 0.352 | < 0.001 | 0.009 | 0.927 |
| History of bleeding from varices | < 0.001 | 0.506 | ||
| Presence of ascites | < 0.001 | < 0.001 | ||
The main finding of our study is that plasma concentration of OPN is in close relation to portal hypertension. Although there is much evidence available regarding the role of OPN in hepatic fibrogenesis, the relationship of OPN to portal hypertension (evaluated by HVPG measurement) has not been described previously. HVPG measurement is used in different clinical situations in routine praxis; distinguishing between patients with clinically significant portal hypertension (i.e., HVPG > 10 mmHg) and those at risk of bleeding (HVPG > 12 mmHg)[17] is of most importance. Our data suggest that, in both these groups of patients, a single laboratory parameter, i.e., plasma OPN levels, was sufficient to estimate values to quite a satisfactory sensitivity and specificity. From a clinical point of view, it is important to note that this differentiation was also observed in the group of compensated patients without ascites (which is usually the basic clinical parameter for showing the presence of clinically significant portal hypertension). The performance of OPN values in the discrimination of patients with clinically significant portal hypertension was better than those of common markers, such as platelet count/spleen size ratio, platelet count, and AST/ALT ratio.
In an experimental model of liver fibrosis, OPN was shown to serve as a key cytokine within the extracellular matrix protein network, contributing to scarring and liver fibrosis[6]. OPN also delays liver fibrosis resolution due to sustained fibrillar collagen-I deposition in mice after thioacetamide-induced fibrosis[7]. Recently, plasma OPN levels have been found to predict liver fibrosis in various chronic liver diseases, such as NASH[11], alcoholic liver disease[12], and chronic viral hepatitis B[13] and C[14]. Pereira et al[21] have also demonstrated that OPN secretion could be stimulated by Schistosoma mansoni and that serum OPN levels correlated with splenic vein pressure and liver fibrosis stage in patients with schistosomiasis.
Portal hypertension is pathogenically related to liver injury and fibrosis, which leads to major complication of cirrhosis, and has been evaluated to date by invasive measurement of portal pressure (HVPG)[15]. The HVPG is a prognostic factor for long-term survival in the case of cirrhosis[22] and can even reflect progression of the disease in the pre-cirrhotic stage. In fact, there is an association between the severity of hepatic inflammation and fibrosis and the HVPG even before cirrhosis develops[23]. Longitudinal studies are needed to assess whether OPN, as a key mediator of the alcohol-induced effects on hepatic stellate cell functions and liver fibrogenesis[24], could give similar information.
Another important finding from our study relates to the prognostic value of OPN.
Data are abound in the literature on the ability of HVPG to predict overall liver-related outcomes, in particular liver cirrhosis decompensation[25] and variceal hemorrhage[26]. In a study by Ripoll et al[25], patients with an HVPG < 10 mmHg had a 90% probability of not developing clinical decompensation after a 4-year median follow-up; however, the survival data were not clearly shown. A reduction in the HVPG to less than 12 mmHg or a reduction of more than 20% from the baseline value was associated with a decreased risk of variceal hemorrhage and improved survival[27,28]. In one study, the HVPG was shown to have better efficacy for predicting 1- and 2-year mortality in cirrhotic patients than that from results obtained using the MELD score[29]. Nevertheless, the clear relationship between single HVPG measurement and overall survival of patients with cirrhosis is supported by very few studies. We observed in our patients that a HVPG cut-off value of 10 mmHg, obtained during a single measurement, stratified cirrhotic patients into two groups with different prognoses regarding survival probability. Surprisingly, we found OPN to be a strong predictor of survival in patients with cirrhosis, with the same validity as the HVPG. A cut-off value of 80 ng/mL revealed two groups of cirrhotic patients with different probabilities of survival, even in the group of compensated patients, which suggests that OPN determination might benefit from being implemented in routine clinical settings. As described previously, common clinical parameters, such as platelet count or ultrasonographic parameters of portal hypertension, correlate with the degree of portal hypertension, but none of these simple parameters could be used for staging portal hypertension or patient risk stratification. The most frequently used (and proved) prognostic parameters in cirrhosis are based on the evaluation of liver function (Child-Pugh classification, MELD score). The only prognostic parameter related to portal hypertension that is independent of other factors was shown to be the HVPG. Our study suggests that plasma OPN levels could stratify patients into two groups with different prognoses, similar to the HVPG. The survival of patients with plasma OPN concentrations below the cut-off value of 80 ng/mL was significantly longer compared to those patients with higher OPN levels (56% vs 37%).
The mean follow-up interval of our patients was 3.7 years, an interval sufficiently long to consider our data statistically significant and reliable for identification of survival differences.
Most patients in our study had cirrhosis of ethylic etiology (none of them presented with uncontrolled abuse of alcohol). The number of patients with other etiologies did not enable us to find a prognostic role, but based on literature data[26] we would not expect a significant difference.
As predicted, HVPG values correlated with different clinical parameters of portal hypertension, such as platelet count, size of esophageal varices, history of variceal bleeding, and presence of ascites. Surprisingly, plasma OPN levels correlated only with platelet count and presence of ascites but not with “variceal-related” parameters (size of varices or history of variceal bleeding).
However, it remains to be answered whether OPN plasma concentration reflects the actual value of HVPG and whether it in turn changes continuously with HVPG changes. If indeed it does, this would enable us, for example, to assess the effect of pharmacology treatment on portal hypertension or to evaluate other continuous changes in portal hypertension, otherwise made possible only by invasive HVPG measurement until now[30]. Further studies are needed to address these questions.
Another important issue is the relation of plasma OPN concentration and HCC. Using proteomic profiling of plasma from patients with cirrhosis and HCC, Shang et al[10] identified OPN to be significantly upregulated in HCC cases compared to cirrhosis controls. Subsequently, plasma concentrations of OPN measured in cirrhotic patients, with and without HCC, revealed significantly higher concentrations in individuals with HCC compared with those without tumors. Recently, Nabih et al[31] suggested OPN as a tumor marker, which could be used as a screening test for the diagnosis of HCC in patients with liver cirrhosis caused by the hepatitis C virus. No relationship of plasma OPN or HVPG levels to occurrence of HCC was found in our cohort of patients. This could be partly due to the limited number of patients with available clinical data with regard to HCC (81 of 154 patients) and partly due to the low incidence of HCC in our patients, which is in concordance with the generally low incidence of HCC in the Czech Republic[9].
Some limitations of our study to consider are the lack of a validation cohort and the strong regional focus on enrolled patients in this study. The main influence within our examined region mainly pertains to the high percentage of alcoholic cirrhosis in our patients. Another limitation is the lack of liver stiffness measurement in our patients, which has been shown to perform well in the detection of clinically significant portal hypertension, especially in combination with the platelet count/spleen diameter ratio[32].
In conclusion, we report a close relationship between plasma concentrations of OPN and portal hypertension in cirrhotic patients, a fact not known until now. OPN could be used to detect significant portal hypertension even in compensated patients without ascites. Our results also indicate that OPN is an independent prognostic parameter of overall survival in cirrhotic patients and that it could be incorporated into prognostic models in patients with liver cirrhosis. The role of OPN in the evaluation of responses to portal hypertension treatments should be explored in future studies.
Portal hypertension leads to major complications of cirrhosis. Until now, invasive measurement of the hepatic venous pressure gradient (HVPG) has been the only method used for the exact evaluation of portal hypertension. Recently, osteopontin (OPN) has emerged as a new marker through its possible relation to fibrosis and cirrhosis.
Although the relationship between OPN and liver fibrosis has been described previously, the relationship to portal hypertension has never been studied.
The close relation of OPN plasmatic levels to portal hypertension has never been described. OPN is a strong prognostic indicator in patients with liver cirrhosis and, similar to HVPG values, significantly determines survival probability even in compensated patients. Moreover, the combination of HVPG and OPN increases the validity of prognosis.
OPN could be used as a marker of clinically significant portal hypertension and a prognostic parameter in patients with cirrhosis.
Clinically significant portal hypertension was defined as HVPG > 10 mmHg.
The authors provide interesting information on the value of OPN measurement as a non-invasive biomarker of portal hypertension. As noted by the authors, this association had not previously been described. The patient size is reasonably large, and the authors have made a good attempt to exclude indications associated with elevated circulation OPN, such as alcohol abuse and hepatocellular carcinoma, in their patient populations.
P- Reviewer: Lalor P, Morales-Ruiz M S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885-893. [PubMed] |
| 2. | Oldberg A, Franzén A, Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci USA. 1986;83:8819-8823. [PubMed] |
| 3. | Nagoshi S. Osteopontin: Versatile modulator of liver diseases. Hepatol Res. 2014;44:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Kawashima R, Mochida S, Matsui A, YouLuTuZ Y, Ishikawa K, Toshima K, Yamanobe F, Inao M, Ikeda H, Ohno A. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem Biophys Res Commun. 1999;256:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008;103:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, Ge X, Fiel MI, Nieto N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology. 2012;55:594-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Leung TM, Wang X, Kitamura N, Fiel MI, Nieto N. Osteopontin delays resolution of liver fibrosis. Lab Invest. 2013;93:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52:19-24. [PubMed] |
| 9. | Terashi T, Aishima S, Taguchi K, Asayama Y, Sugimachi K, Matsuura S, Shimada M, Maehara S, Maehara Y, Tsuneyoshi M. Decreased expression of osteopontin is related to tumor aggressiveness and clinical outcome of intrahepatic cholangiocarcinoma. Liver Int. 2004;24:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, Philips G, Chan IS, Karaca GF. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Patouraux S, Bonnafous S, Voican CS, Anty R, Saint-Paul MC, Rosenthal-Allieri MA, Agostini H, Njike M, Barri-Ova N, Naveau S. The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PLoS One. 2012;7:e35612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Xie H, Wang J, Han Y, Liu Z. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int J Clin Pract. 2008;62:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Huang W, Zhu G, Huang M, Lou G, Liu Y, Wang S. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. Clin Chim Acta. 2010;411:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407. [PubMed] |
| 16. | Buck M, Garcia-Tsao G, Groszmann RJ, Stalling C, Grace ND, Burroughs AK, Patch D, Matloff DS, Clopton P, Chojkier M. Novel inflammatory biomarkers of portal pressure in compensated cirrhosis patients. Hepatology. 2014;59:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1030] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 18. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [PubMed] |
| 20. | Shimada M, Hashimoto E, Kaneda H, Noguchi S, Hayashi N. Nonalcoholic steatohepatitis: risk factors for liver fibrosis. Hepatol Res. 2002;24:429-438. [PubMed] |
| 21. | Pereira TA, Syn WK, Machado MV, Vidigal PV, Resende V, Voieta I, Xie G, Otoni A, Souza MM, Santos ET. Schistosome-induced cholangiocyte proliferation and osteopontin secretion correlate with fibrosis and portal hypertension in human and murine schistosomiasis mansoni. Clin Sci (Lond). 2015;129:875-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. Portal hypertension and gastrointestinal bleeding. Semin Liver Dis. 2008;28:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Burroughs AK, Groszmann R, Bosch J, Grace N, Garcia-Tsao G, Patch D, Garcia-Pagan JC, Dagher L. Assessment of therapeutic benefit of antiviral therapy in chronic hepatitis C: is hepatic venous pressure gradient a better end point? Gut. 2002;50:425-427. [PubMed] |
| 24. | Seth D, Duly A, Kuo PC, McCaughan GW, Haber PS. Osteopontin is an important mediator of alcoholic liver disease via hepatic stellate cell activation. World J Gastroenterol. 2014;20:13088-13104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 810] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 26. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2133] [Article Influence: 112.3] [Reference Citation Analysis (3)] |
| 27. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 29. | Suk KT, Kim CH, Park SH, Sung HT, Choi JY, Han KH, Hong SH, Kim DY, Yoon JH, Kim YS. Comparison of hepatic venous pressure gradient and two models of end-stage liver disease for predicting the survival in patients with decompensated liver cirrhosis. J Clin Gastroenterol. 2012;46:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Nabih MI, Aref WM, Fathy MM. Significance of plasma osteopontin in diagnosis of hepatitis C virus-related hepatocellular carcinoma. Arab J Gastroenterol. 2014;15:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Cho EJ, Kim MY, Lee JH, Lee IY, Lim YL, Choi DH, Kim YJ, Yoon JH, Baik SK. Diagnostic and Prognostic Values of Noninvasive Predictors of Portal Hypertension in Patients with Alcoholic Cirrhosis. PLoS One. 2015;10:e0133935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |