Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3363
Peer-review started: August 10, 2015
First decision: November 13, 2015
Revised: November 19, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: March 28, 2016
Processing time: 229 Days and 7.2 Hours
AIM: To investigate the role of tolvaptan in regulating aquaporin (AQP)-2 expression and fecal water content in cirrhotic rats with ascites.
METHODS: Cirrhosis with ascites was induced in rats by repetitive dorsal injection of CCl4 for 14 wk. In total, 84 cirrhotic rats with ascites divided into three groups (vehicle, 3 mg/kg and 5 mg/kg tolvaptan), and then further divided into five subgroups (days 1, 2, 3, 4, and 5). Blood samples were obtained to measure vasopressin and sodium concentrations. Rats were killed and colonic mucosa was scraped for analysis of protein expression and AQP-2 transcriptional level. The whole layer was fixed for hematoxylin&eosin (HE) staining and feces were collected for determination of fecal water content.
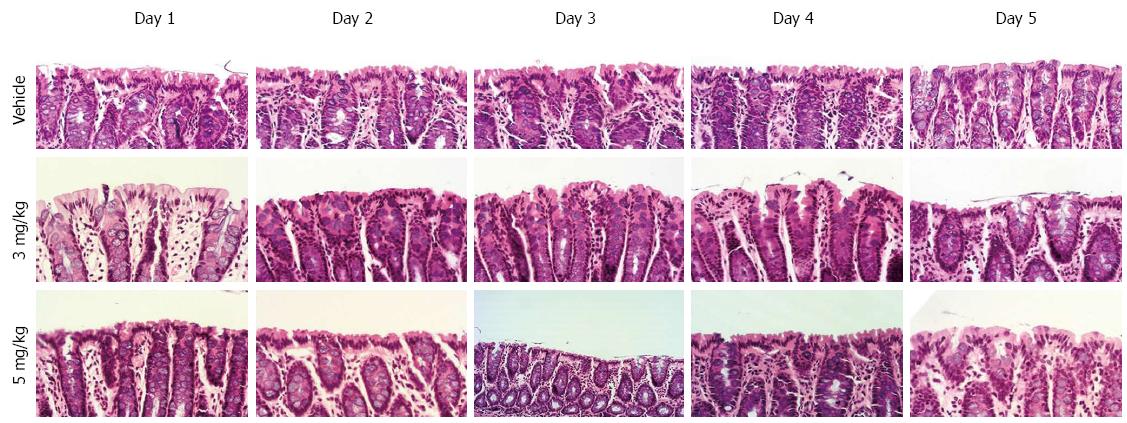
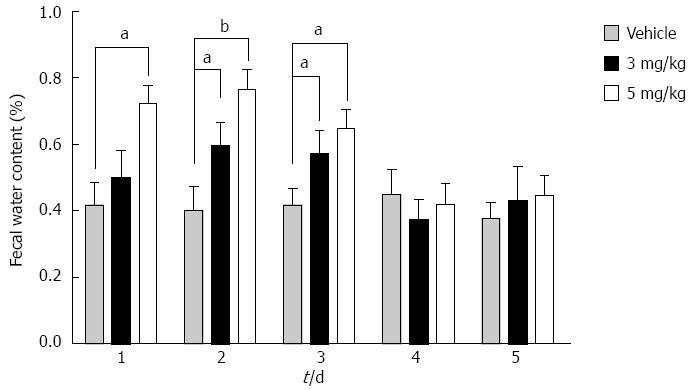
CONCLUSION: Compared with vehicle, vasopressin decreased significantly in the tolvaptan groups from day 2 to a similar level in each treatment group. AQP-2 showed significant upregulation in cirrhotic rats with ascites compared with an untreated control group (100% ± 22.9% vs 22.2% ± 10.23%, P < 0.01). After administration of tolvaptan, AQP-2 expression began to decrease significantly from day 2 in each treatment group, but no significant difference was finally found between the treatment groups. Fecal water content in the distal colon was increased by 5 mg/kg tolvaptan on day 1 (66.8% ± 9.3% vs 41.4% ± 6.3%, in the vehicle group, P < 0.05). Fecal water content returned to baseline at day 4 at the latest in both treatment groups, and did not correspond to the change in AQP-2 expression. HE staining of the colonic mucosa showed no mucosal damage related to tolvaptan.
CONCLUSION: Upregulation of AQP-2 in the distal colon is found in cirrhotic rats with ascites. Tolvaptan inhibits its expression and may decrease water reabsorption and induce diarrhea.
Core tip: Aquaporin (AQP)-2 is mainly expressed in the kidneys, although it has been detected in the distal colon. We confirmed its expression in the distal colon and its upregulation in cirrhotic rats with ascites. Tolvaptan is a highly potent and selective AQP-2 antagonist and is used to treat cirrhotic ascites and hyponatremia. It blocked AQP-2 expression in the distal colon after oral administration, and temporarily increased fecal water content. Fecal water content returned to baseline quickly. The results suggest that tolvaptan inhibited AQP-2 expression in the distal colon and may induce diarrhea to clear water.
- Citation: Chen C, Chen RP, Lin HH, Zhang WY, Huang XL, Huang ZM. Tolvaptan regulates aquaporin-2 and fecal water in cirrhotic rats with ascites. World J Gastroenterol 2016; 22(12): 3363-3371
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3363.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3363
Ascites is a serious complication often seen in patients with advanced liver cirrhosis with portal hypertension. The onset and development of cirrhotic ascites are multifactorial. Liver function deterioration, portal hypertension, and systemic vasodilation lead to activation of the renin-angiotensin-aldosterone system, sympathetic nervous system, and vasopressin[1]. Ascites and water retention are associated with increased mortality rates[2], and redistribution of liquid is a major component of the physiological and pathological changes.
The colon is a vital organ for regulating water and salt balance. A total of 6-8 L of digestive fluids is secreted into the intestinal tract every day, and mainly comes from plasma and tissue fluid, but little is excreted. Water and salt homeostasis are mainly regulated by the colonic epithelium. The aquaporin (AQP) family plays an important role in modulating absorption and secretion of water and electrolytes. The major AQPs in the colon include AQP-1, 2, 3, 4, and 8[3-5]. AQP-2 is mainly expressed in the kidneys, and was first discovered by Preston et al[6,7] in 1992. Since then, it has been found in human and rat colons. In the kidneys, activation of the vasopressin V2 receptor increases water reabsorption, both by short- and long-term regulation[1]. Whether AQP-2 regulates the water balance in the intestinal tract remains obscure.
Tolvaptan is a novel vaptan and is characterized as a highly effective and selective non-peptide V2 receptor antagonist. It binds to the V2 receptor and induces aquaresis in humans and rats after oral administration[8]. Tolvaptan increases free water clearance and shows its unique curative effect in kidney and cardiovascular diseases[9-11]. Tolvaptan has been shown to alleviate the syndrome of inappropriate antidiuretic hormone or ascites[12-14].
In our clinical cases, some patients with hyponatremia appeared to have diarrhea during aquaretic therapy. We suspected that they might respond to tolvaptan administration, and we designed the present animal study to investigate the correlation between tolvaptan and fecal water content.
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University in 2013 (No. wydw2013-0071). Tolvaptan was a gift from Otsuka Pharmaceutical Company, Japan.
The study was performed in 84 conscious adult male Sprague-Dawley rats with cirrhosis and ascites induced by dorsal subcutaneous injection of CCl4 (1 mg/kg) dissolved in paraffin (1:1; v/v) twice weekly (Monday and Friday) for 14 wk. Rats weighing 200-250g were fed ad libitum with standard chow and filtrated water containing phenobarbital (0.3g/L) as drinking fluid. After 1 wk phenobarbital induction, CCl4 treatment began. One hundred and thirty rats were submitted to the model protocol, but 46 of these could not be included in the study for the following reasons: 20 died during the protocol and 26 failed to develop ascites. After developing ascites, all rats were observed for one more week to judge the stability of the ascites. All the animals with ascites were kept in metabolic cages for 3 d before tolvaptan (dissolved in 1% hydroxypropyl methylcellulose) treatment.
In total, 84 cirrhotic rats with ascites were included in this protocol and randomly assigned to three groups (vehicle, 3 mg/kg and 5 mg/kg tolvaptan). Each group was further divided into five subgroups (1, 2, 3, 4, and 5 d). Tolvaptan was applied by oral gavage. Body weight was measured daily at 09:00 am prior to treatment.
At the end of the experiment, rats were anesthetized intraperitoneally with 10% chloral hydrate; blood samples were obtained by postcaval puncture, and the descending colon was removed rapidly, feces were collected, and the colon was washed with phosphate-buffered saline at 4 °C three times. Animals were then killed by cervical dislocation. Colonic mucosa was isolated from underlying serosa by scraping with a glass slide and placing it into liquid nitrogen and then storingat -80 °C for western blotting and real-time polymerase chain reaction (PCR); the whole layer was fixed in 4% paraformaldehyde for 24 h and prepared for hematoxylin&eosin (HE) staining. Blood was collected in centrifuge tubes containing EDTA (1.5 mg/mL) and proteinase inhibitor (Roche, Switzerland), and then kept on ice until centrifugation at 4 °C to obtain plasma. The plasma was stored at -20 °C for subsequent assay of plasma vasopressin and ion concentrations.
Hormone levels were determined by avidin-biotin ELISA using commercially available kits, vasopressin ELISA kit (Westang Bio-Tech, Shanghai, China) for plasma vasopressin concentration. Na+ concentration was determined using a potentiometric method (AU5800; Beckman Coulter, CA, United States).
Colonic mucosal epithelium was obtained by scraping with a glass slide, and homogenized in lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) with a glass homogenizer. The successive process followed the instructions of the Mem-PER Eukaryotic Membrane Protein Extraction Kit (Thermo Scientific). The supernatant was collected and its protein concentration was analyzed by BCA kit (Tiangen Biotech, Beijing, China), and then diluted with loading buffer (FUDE Biological Technology, Hangzhou, China) to 4 μg/μL. Each mixture was boiled at 100 °C for 5 min and chilled on ice. The mixture was stored at -20 °C and equal volumes were loaded in SDS-PAGE, together with molecular markers. The protein was transferred from the gel to a PVDF membrane, and the membrane was blocked for 1h at room temperature using 5% bovine serum albumin. Membranes were incubated with AQP-2 antibody (Cell Signaling Technology, Danvers, MA, United States) overnight at 4 °C. After washing the membrane with Tris-buffered saline containing 0.1% Triton X-100, horseradish peroxidase was added for color development. Images were obtained by darkroom development techniques for chemiluminescence by ECL Plus (Advansta, Menlo Park, CA, United States).
Total RNA was isolated from the colonic mucosal epithelium samples using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). Reverse transcription was carried out using a Fist-Strand cDNA Synthesis kit (Thermo Scientific) in a total volume of 20 μL. The resultant cDNA was amplified using a SYBR Green qPCR kit (Applied Biosystems, Carlsbad, CA, United States), and quantified using an ABI PRISM 7500 sequence detection system (Applied Biosystems). For PCR, the following sense and antisense primers were designed from rat AQP-2 cDNA sequence: sense, 5’-TGGGTTGCCATGTCTCCTTC-3’; and antisense, 5’-GCGTTGTTGTGGAGAGCATT-3’. The PCR system consisted of 1 μL cDNA, 0.5 μL each of specific primers (10 μmol/L), 5 μL 2 × SYBR Green Mix and 3 μL water in a total volume of 10 μL. The PCR cycling conditions for AQP-2 and GAPDH were as follows: an initial denaturation and activation at 95 °C for 10 min, followed by 40 amplification cycles of 95 °C for 15 s and 60 °C for 60 s. mRNA expression of the target genes was standardized by reference gene GAPDH. The relative expression of each gene was calculated by the comparative Ct method. The 2-ΔΔCt method was adopted to quantify the mRNA expression level between each group, and ΔΔCt was defined by the following equation: ΔΔCt = [(Ct of AQP-2 - Ct of GAPDH)tolvaptan - (Ct of AQP-2 - Ct of GAPDH)vehicle].
Colonic mucosal epithelium samples from cirrhotic rats with ascites were fixed overnight in 4% paraformaldehyde at 4 °C and routinely processed for paraffin embedding. Histological sections (5 μm) were then prepared and stained with HE. Images (× 400 magnification) of the distal colon were acquired with a biological imaging microscope (Olympus, Tokyo, Japan).
Fecal samples were collected at the time of sacrifice, and dried in anoven at 60 °C for 24 h. The fecal water content was calculated based on the following equation: [wet weights (g)-dry weights (g)]/wet weights (g) × 100%.
The results were expressed as the mean ± SEM, and the analyses were performed using SPSS version 17.0 software. The Student t test was used for two-group comparisons. Differences were considered statistically significant at P < 0.05. All the statistical analyses were performed by a biomedical statistician.
In the pathological examination, all liver specimens from cirrhotic rats with ascites showed cirrhosis, but we failed to distinguish any significant differences between rats receiving tolvaptan or vehicle. HE staining of the colon tissue sections showed no mucosal damage to the colon attributable to the administration of tolvaptan (Figure 1).
Table 1 shows the effect of different doses of tolvaptan at different times on cirrhotic rats, and we chose the first 5 d to observe plasma vasopressin, sodium concentration, and body weight. No significant differences were observed in body weight and no change was identified for sodium and vasopressin concentrations based on different doses of tolvaptan and vehicle.
| 1 d | 2 d | 3 d | 4 d | 5 d | ||
| Body weight (g) | Vehicle | 486.7 ± 12.57 (5) | 492.1 ± 10.12 (5) | 472.8 ± 18.21 (5) | 475.2 ± 9.21 (5) | 482.8 ± 13.24 (5) |
| Tolvaptan (3 mg/kg) | 502.3 ± 11.91 (6) | 487.3 ± 12.43 (6) | 465.6 ± 16.59 (6) | 465.6 ± 13.24 (6) | 476.3 ± 16.00 (5) | |
| Tolvaptan (5 mg/kg) | 493.4 ± 16.34 (6) | 485.2 ± 20.21 (6) | 490.1 ± 13.12 (6) | 472.1 ± 12.12 (6) | 490.1 ± 11.12 (6) | |
| Vasopressin(pg/L) | Vehicle | 13.25 ± 1.24 (5) | 14.15 ± 1.41 (5) | 15.44 ± 1.38 (5) | 14.99 ± 0.94 (5) | 13.05 ± 0.85 (5) |
| Tolvaptan (3 mg/kg) | 15.29 ± 0.93 (6) | 13.37 ± 1.22 (6) | 16.87 ± 0.73 (6) | 15.16 ± 1.17 (6) | 14.33 ± 0.99 (5) | |
| Tolvaptan (5 mg/kg) | 14.85 ± 1.39 (6) | 14.23 ± 0.81 (6) | 15.12 ± 1.21 (6) | 13.21 ± 1.21 (6) | 15.23 ± 0.81 (6) | |
| Serum sodium (mol/L) | Vehicle | 141.2 ± 1.31 (5) | 141.2 ± 1.11 (5) | 139.2 ± 0.75 (5) | 140.1 ± 0.92 (5) | 140.2 ± 1.31 (5) |
| Tolvaptan (3 mg/kg) | 139.2 ± 1.27 (6) | 141.3 ± 1.20 (6) | 138.2 ± 1.85 (6) | 140.1 ± 1.23 (6) | 138.2 ± 1.87 (5) | |
| Tolvaptan (5 mg/kg) | 141.2 ± 1.21 (6) | 140.9 ± 1.12 (6) | 142.2 ± 1.22 (6) | 138.1 ± 2.21 (6) | 139.1 ± 1.55 (6) |
After administration of both doses of tolvaptan, there was a significant decrease in body weight on day 1, as well on successive days (Table 2). After drug treatment for 4-5 d, ascites was eliminated (confirmed by abdominal laparotomy when sacrificed). Vasopressin decreased from day 2, and the successive days.
| 1 d | 2 d | 3 d | 4 d | 5 d | ||
| Body weight (g) | Vehicle | 492.6 ± 11.22 (5) | 498.5 ± 12.21 (5) | 490.3 ± 16.21 (5) | 500.1 ± 16.21 (5) | 493.1 ± 11.12 (5) |
| Tolvaptan (3 mg/kg) | 482.2 ± 20.21 (5) | 466.2 ± 10.19 (6)a | 450.1 ± 18.21 (5)a | 443.1 ± 20.12 (6)a | 440.1 ± 20.12 (5)a | |
| Tolvaptan (5 mg/kg) | 460.2 ± 12.31 (5)a | 455.1 ± 16.12 (5)a | 440.2 ± 23.12 (6)a | 437.3 ± 22.21 (5)a | 446.1 ± 21.21 (4)a | |
| Vasopressin (pg/L) | Vehicle | 12.97 ± 0.92 (5) | 13.81 ± 1.30 (5) | 15.91 ± 0.89 (5) | 16.97 ± 1.38 (5) | 14.02 ± 0.98 (5) |
| Tolvaptan (3 mg/kg) | 12.25 ± 0.82 (5) | 11.41 ± 0.62 (6) | 10.21 ± 0.91 (5)a | 9.77 ± 1.21 (6)a | 7.12 ± 1.32 (5)b | |
| Tolvaptan (5 mg/kg) | 12.01 ± 1.21 (5) | 10.12 ± 0.92 (5)a | 8.12 ± 1.22 (6)a | 7.01 ± 1.63 (5)b | 7.33 ± 1.13 (4)a | |
| Serum sodium (mol/L) | Vehicle | 140.2 ± 1.25 (5) | 140.2 ± 1.51 (5) | 141.2 ± 0.65 (5) | 141.1 ± 1.61 (5) | 142.2 ± 0.81 (5) |
| Tolvaptan (3 mg/kg) | 143.2 ± 2.32 (5) | 144.2 ± 1.17 (6)a | 146.1 ± 1.13 (5)a | 147.2 ± 2.11 (6)b | 146.1 ± 2.47 (5)a | |
| Tolvaptan (5 mg/kg) | 144.1 ± 1.22 (5)a | 147.1 ± 2.51 (5)a | 147.9 ± 2.21 (6)a | 146.2 ± 1.71 (5)a | 147.2 ± 1.12 (4)b |
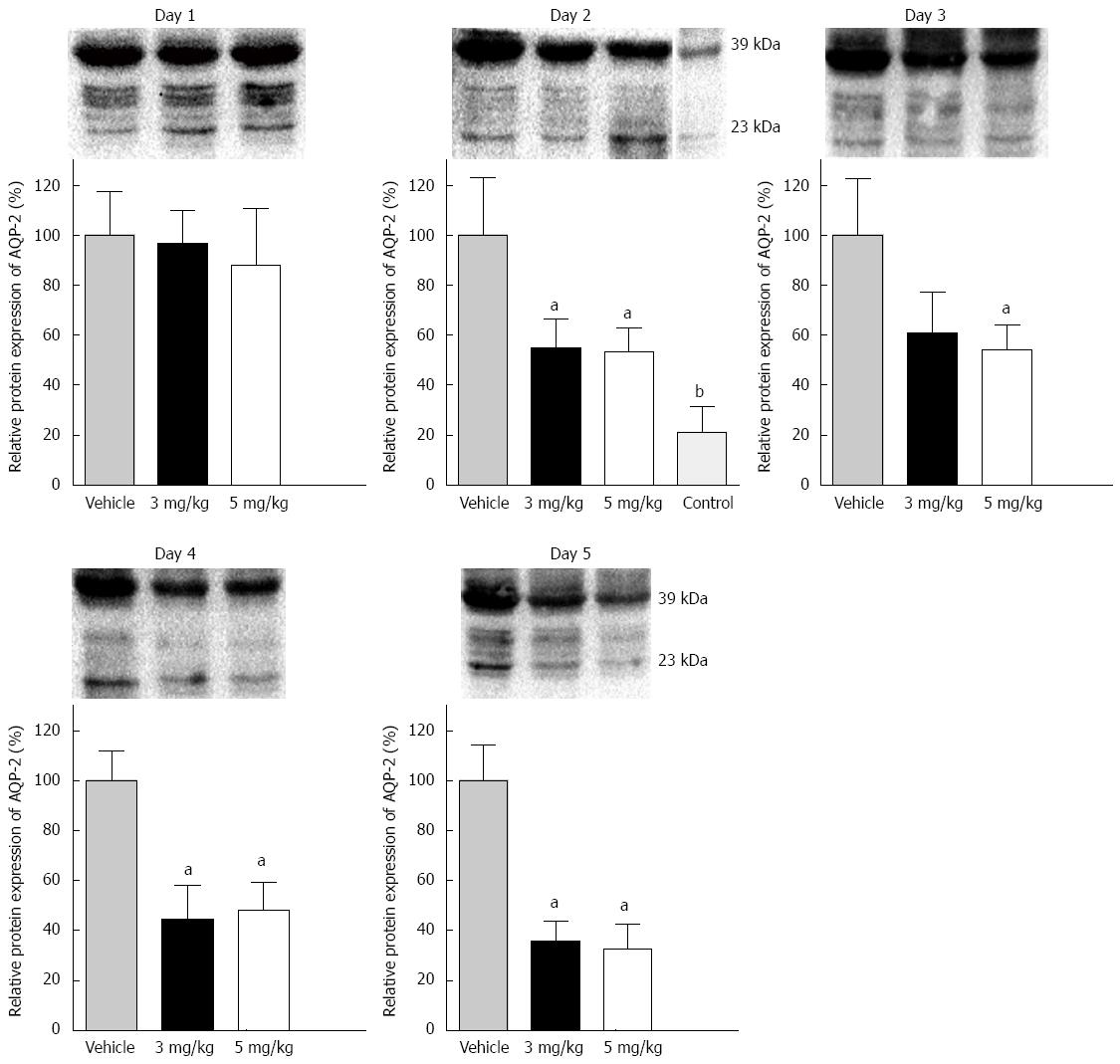
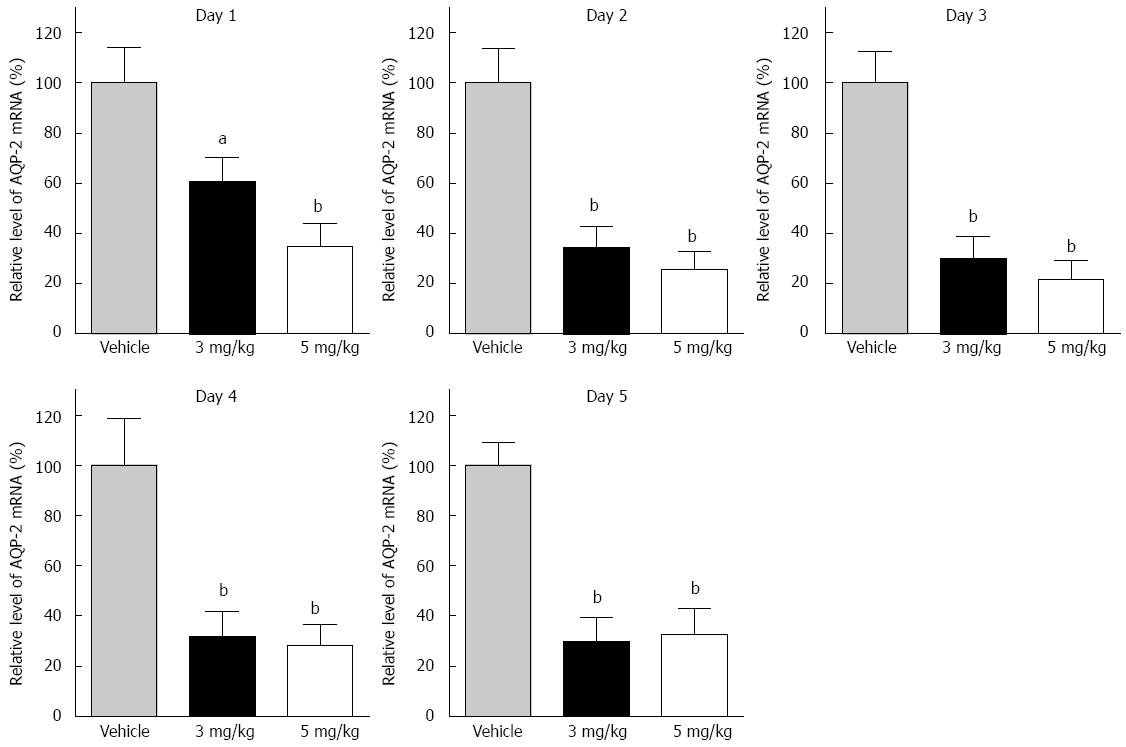
AQP-2 expression in the distal colon was detected in each group. Two bands of AQP-2 protein were detected by western blotting. One band appeared at 23 kDa and the other at 39 kDa, representing the nonphosphorylated and phosphorylated forms, respectively. The total expression of the two bands was analyzed as the protein level in the distal colon (Figure 2). The protein expression level of AQP-2 after 1 d tolvaptan administration showed no significant difference compared to that with vehicle. Compared with vehicle, significant AQP-2 downregulation was found on day 2 (3 mg/kg: 100% ± 22.9% vs 54.7% ± 11.7%, P < 0.05; 5 mg/kg: 100% ± 22.9% vs 53.0% ± 9.4%, P < 0.01), and on successive days with both doses of tolvaptan. However, no difference was found in AQP-2 expression between the 3 mg/kg and 5 mg/kg groups. Transcriptional AQP-2 in the distal colon was also measured (Figure 3). Compared with vehicle, there was a significant reduction on day 1 with tolvaptan (3 mg/kg: 100 ± 16.3% vs 68.5 ± 10.0%, P < 0.05; 5 mg/kg: 100 ± 16.3% vs 34.1 ± 15.1%, P < 0.01), as well as on successive days. Expression of AQP-2 mRNA before and after administration of tolvaptan showed a consistent trend to reflect protein expression.
Fecal water content after tolvaptan administration increased on days 2 and 3 with both doses of tolvaptan, but only the higher dose caused an increase on day 1 (Figure 4). After 3 d of tolvaptan administration, the fecal water content returned to baseline.
The effect of tolvaptan on AQP-2 in the distal colon has been reported in rats in order to explore the roles of vasopressin and aldosterone in physiological adaption[15,16]. In our study, different doses of tolvaptan were administered for different times to investigate the colonic expression of AQP-2 in cirrhotic rats with ascites, and to explore the role of tolvaptan in regulation of fecal water content. The findings presented here indicated that AQP-2 expression in the distal colon increased in cirrhotic ratswith ascites, and it was downregulated by oral administration of tolvaptan. Furthermore, the fecal water content increased temporarily during tolvaptan therapy.
Every AQP protein consists of six transmembrane domains, and the AQP family selectively transports water, glycerin, and other compounds through the membrane. It is generally accepted in the kidneys that transmembrane free water is regulated by water channel, namely AQP-2. Both water deprivation[17,18] and deamino-arg8-vasopressin (dAVP)[19,20] infusion increases AQP-2 expression in the kidney. AQP-2 was first observed in the distal colon in dehydrated rats by Gallardo et al[3] in 2001. They suggested that the colonic expression of AQP-2 in apical membranes of rats helps regulate transepithelial water transport from the intestinal side to the vascular side. This mechanism was strongly involved in water absorption and fecal dehydration.
In our study, colonic expression of AQP-2 was confirmed and we found that it was upregulated in cirrhotic rats with ascites. Ascites is a state of water retention, which is a combined effect of endocrine and hemodynamic systems. We attributed the increase in AQP-2 expression to the development of ascites. A high hemodynamic state accelerates hypothalamus secretion of excessive vasopressin[21], and deterioration of liver function reduces the vasopressin and aldosterone metabolism. Both of these contribute to the high levels of vasopressin and aldosterone in the bloodstream. Excessive vasopressin stimulates V2 receptors and activates adenylyl cyclase, which increases the concentration of cAMP in the cytoplasm, thus facilitating phosphorylation of AQP-2. The accumulation of AQP-2 is induced by vesicle trafficking from storage vesicles to apical surface membranes within several tens of minutes (short-term regulation)[22-25], and in the long-term, upregulation of AQP-2 in response to vasopressin plays a major role[26,27].
Tolvaptan is a highly effective and selective non-peptide V2 receptor antagonist. It was reported that oral tolvaptan (1 and 3 mg/kg) promoted a marked diuretic effect in dimethylnitrosamine-induced cirrhotic rats with ascites[28]. Cristia et al[16] reported that 3 mg/kg tolvaptan significantly inhibited AQP-2 expression in the distal colon. In our preliminary experiment, we selected different doses of tolvaptan and found that 1 mg/kg showed aquaresis but had no significant effect on colonic AQP-2 expression. So, we tried higher doses of 3 mg/kg and 5 mg/kg. Ascites was eliminated at the end of the protocol in most of the rats receiving tolvaptan therapy. Serum sodium increased and no hypernatremia was present. Some rats died of dehydration. We confirmed elevation of vasopressin in cirrhotic rats[21] and different doses of tolvaptan decreased it to a similar level. It seems that in the cirrhotic ascites model, elevation of vasopressin is mainly induced by a non-osmotic mechanism, because hyponatremia should inhibit vasopressin excretion in healthy individuals. After the circulation improved, vasopressin levels decreased.
As in the kidneys, the effect of vasopressinon AQP-2 regulation in the colon is a response to V2 receptor activation. Our results confirmed that the reduction of colonic expression of AQP-2 was a direct response to oral tolvaptan administration. In the present study, both doses of tolvaptan affected AQP-2 regulation but different doses eventually resulted in similar expression levels in the distal colon.
It is reported that the colon could be a target for action by vasopressin, as it has been shown that vasopressin stimulates Na+ and water absorption[15]. Water is removed from luminal feces by the surface mucosa and the crypts[29]. Cristià et al[16] suggested that the stimulation of myofibroblast growth and the increase in AQP-2 expression are consistent with the antidiuretic role of tolvaptan in the distal colon. The studies above discussed the colonic microscopic function and electrochemical changes of tolvaptan, but the biological function has not been explored.
We showed that the fecal water content increased and we attributed it to tolvaptan administration. It is known that mucosal damage in the colon causes diarrhea[30,31]. We were not sure whether a higher dose of tolvaptan would cause mucosal damage. So, we performed HE staining of the colon tissue sections, and no mucosal damage was found that was attributable to administration of tolvaptan. However, after a few days of therapy, the fecal water content returned to normal (4-5 d). This phenomenon did not correspond to the alteration of AQP-2 expression in the distal colon. As tolvaptan was the only variable in this experiment, we suggest there would be compensatory mechanisms of water balance.
Vasopressin-dependent water flow occurs via AQP-2 from the intestinal tract, and outflow to the intercellular space via AQP-3 and/or AQP-4. However, it appears that neither AQP-3 nor AQP-4 is regulated by vasopressin in the distal colon[32,33]. It has been suggested that AQP-4 has little or no effect on colonic fluid secretion or fecal dehydration. However, AQP-3 is the most dominantly expressed AQP in the colon and plays an important role in the absorption of water[34,35], and its abundance appears to be regulated on a long-term basis in a manner similar to the long-term regulation of AQP-2. When exposed to an environment of possible water overload[36], AQP-3 could serve as a water channel to reabsorb water from the enteric cavity. When AQP-3 was specifically blocked by HgCl2 or CuSO4, diarrhea was induced[37]. We speculate that AQP-3 upregulation may be one of the mechanisms to balance water absorption in the distal colon after tolvaptan administration.
Our experiment explored fecal water content in response to oral tolvaptan administration in the therapy of cirrhotic ascites. Tolvaptan reduces water reabsorption and may promote clearance of redundant water. This mechanism could induce diarrhea in some conditions. However, the precise mechanism needs to be further explored. Besides long-term regulation, short-term regulation should also be investigated in the distal colon and its biological function should be confirmed.
Aquaporin (AQP)-2 is one of the AQP family and marked free-water clearance is induced when its effects are blocked. Tolvaptan is a potent and selective antagonist, and, can be used to treat cirrhotic ascites and hyponatremia. AQP-2 is found in the distal colon but its biological function has not been established. This study was designed to determine the colonic expression of AQP-2 and to investigate the correlation between tolvaptan administration and fecal water content.
AQP-2 is a member of the AQP family, and helps to absorb water in the kidneys. It is also found in the colon and is involved in water and electrolyte transportation. Tolvaptan is a specific antagonist of AQP-2 and has widespread clinical application.
This is the first study to explore colonic AQP-2 expression in cirrhotic rats with ascites, and tolvaptan inhibited its expression and increased fecal water content.
This study suggests that tolvaptan may be useful in upregulating fecal water content and indicates a way to clear water.
It is an interesting study with appropriate methodology and the results are clear and of great importance.
P- Reviewer: Lee SH, Tekin F, Wang JS S- Editor: Qi Y L- Editor: Cant MR E- Editor: Zhang DN
| 1. | Ikeda M, Matsuzaki T. Regulation of aquaporins by vasopressin in the kidney. Vitam Horm. 2015;98:307-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 611] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Gallardo P, Cid LP, Vio CP, Sepúlveda FV. Aquaporin-2, a regulated water channel, is expressed in apical membranes of rat distal colon epithelium. Am J Physiol Gastrointest Liver Physiol. 2001;281:G856-G863. [PubMed] |
| 4. | Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. Aquaporins in the digestive system. Med Electron Microsc. 2004;37:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Koyama Y, Yamamoto T, Tani T, Nihei K, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K. Expression and localization of aquaporins in rat gastrointestinal tract. Am J Physiol. 1999;276:C621-C627. [PubMed] |
| 6. | Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385-387. [PubMed] |
| 7. | Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88:11110-11114. [PubMed] |
| 8. | Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860-867. [PubMed] |
| 9. | Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ, van der Jagt EJ, Kappert P, Ouyang J, Engels GE, van Oeveren W. Short-term Effects of Tolvaptan in Individuals With Autosomal Dominant Polycystic Kidney Disease at Various Levels of Kidney Function. Am J Kidney Dis. 2015;65:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Suzuki S, Yoshihisa A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Abe Y, Saito T, Ohwada T, Suzuki H. Vasopressin V2 receptor antagonist tolvaptan is effective in heart failure patients with reduced left ventricular systolic function and low blood pressure. Int Heart J. 2015;56:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kinugawa K, Inomata T, Sato N, Yasuda M, Shimakawa T, Bando K, Mizuguchi K. Effectiveness and adverse events of tolvaptan in octogenarians with heart failure. Interim analyses of Samsca Post-Marketing Surveillance In Heart faiLurE (SMILE study). Int Heart J. 2015;56:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Schäffler A, Lindner U. [Syndrome of inadequate ADH secretion: pitfalls in diagnosis and therapy]. Dtsch Med Wochenschr. 2015;140:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Mitsuhashi N, Nemoto S, Satoh Y, Aoki Y, Teranaka R, Sasaki K, Shimazaki R, Ueda A, Nishino H, Akiyama T. [Effect of tolvaptan on ascites due to malignancy]. Gan To Kagaku Ryoho. 2015;42:201-205. [PubMed] |
| 14. | Bordi P, Tiseo M, Buti S, Regolisti G, Ardizzoni A. Efficacy and safety of long-term tolvaptan treatment in a patient with SCLC and SIADH. Tumori. 2015;101:e51-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Cristià E, Amat C, Naftalin RJ, Moretó M. Role of vasopressin in rat distal colon function. J Physiol. 2007;578:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Moretó M, Cristià E, Pérez-Bosque A, Afzal-Ahmed I, Amat C, Naftalin RJ. Aldosterone reduces crypt colon permeability during low-sodium adaptation. J Membr Biol. 2005;206:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 749] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Kishore BK, Krane CM, Miller RL, Shi H, Zhang P, Hemmert A, Sun R, Nelson RD. P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol. 2005;288:F1164-F1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kishore BK, Mandon B, Oza NB, DiGiovanni SR, Coleman RA, Ostrowski NL, Wade JB, Knepper MA. Rat renal arcade segment expresses vasopressin-regulated water channel and vasopressin V2 receptor. J Clin Invest. 1996;97:2763-2771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996;271:F414-F422. [PubMed] |
| 21. | Kim JK, Summer SN, Howard RL, Schrier RW. Vasopressin gene expression in rats with experimental cirrhosis. Hepatology. 1993;17:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA. 1995;92:7212-7216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Tajika Y, Matsuzaki T, Suzuki T, Aoki T, Hagiwara H, Tanaka S, Kominami E, Takata K. Immunohistochemical characterization of the intracellular pool of water channel aquaporin-2 in the rat kidney. Anat Sci Int. 2002;77:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol. 2008;130:197-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Yamamoto T, Sasaki S, Fushimi K, Kawasaki K, Yaoita E, Oota K, Hirata Y, Marumo F, Kihara I. Localization and expression of a collecting duct water channel, aquaporin, in hydrated and dehydrated rats. Exp Nephrol. 1995;3:193-201. [PubMed] |
| 26. | Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Radin MJ, Yu MJ, Stoedkilde L, Miller RL, Hoffert JD, Frokiaer J, Pisitkun T, Knepper MA. Aquaporin-2 regulation in health and disease. Vet Clin Pathol. 2012;41:455-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Miyazaki T, Fujiki H, Yamamura Y. Tolvaptan, an orally active non-peptide arginine vasopressin V2 receptor antagonist, reduces ascites in rats with chronic liver injury. Hepatol Res. 2013;43:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Bleakman D, Naftalin RJ. Hypertonic fluid absorption from rabbit descending colon in vitro. Am J Physiol. 1990;258:G377-G390. [PubMed] |
| 30. | Cuzzocrea S, Ianaro A, Wayman NS, Mazzon E, Pisano B, Dugo L, Serraino I, Di Paola R, Chatterjee PK, Di Rosa M. The cyclopentenone prostaglandin 15-deoxy-delta(12,14)- PGJ2 attenuates the development of colon injury caused by dinitrobenzene sulphonic acid in the rat. Br J Pharmacol. 2003;138:678-688. [PubMed] |
| 31. | Xue H, Sawyer MB, Field CJ, Dieleman LA, Baracos VE. Nutritional modulation of antitumor efficacy and diarrhea toxicity related to irinotecan chemotherapy in rats bearing the ward colon tumor. Clin Cancer Res. 2007;13:7146-7154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269:F663-F672. [PubMed] |
| 33. | Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol. 1995;269:F775-F785. [PubMed] |
| 34. | Itoh A, Tsujikawa T, Fujiyama Y, Bamba T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol. 2003;18:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Tsujikawa T, Itoh A, Fukunaga T, Satoh J, Yasuoka T, Fujiyama Y. Alteration of aquaporin mRNA expression after small bowel resection in the rat residual ileum and colon. J Gastroenterol Hepatol. 2003;18:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J Histochem Cytochem. 1999;47:1275-1286. [PubMed] |
| 37. | Ikarashi N, Kon R, Iizasa T, Suzuki N, Hiruma R, Suenaga K, Toda T, Ishii M, Hoshino M, Ochiai W. Inhibition of aquaporin-3 water channel in the colon induces diarrhea. Biol Pharm Bull. 2012;35:957-962. [PubMed] |