Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.3056
Peer-review started: May 14, 2015
First decision: July 10, 2015
Revised: July 27, 2015
Accepted: September 30, 2015
Article in press: September 30, 2015
Published online: March 14, 2016
Processing time: 297 Days and 2.8 Hours
Lymphoepithelioma-like gastric carcinoma is a rare type of gastric cancer characterized by a carcinoma with intense stromal lymphocytic infiltration. Although lymphocytic infiltration is closely associated with Epstein-Barr virus (EBV) infection, concomitant occurrence with differentiated adenocarcinoma is relatively rare. The clinical manifestations of lymphoepithelioma-like gastric carcinoma (including EBV-positive and -negative forms) are similar to those of gastric cancer, and the diagnosis is based on pathologic, histologic, and immunohistochemical findings. This report describes the case of a 55-year-old female patient who presented with a 10-year history of recurrent and worsening abdominal pain and melena that had been occurring for 2 mo. An ulcerative lesion was detected in the stomach by endoscopic examination, which raised suspicion of early gastric cancer. A subsequent preoperative endoscopic biopsy showed adenocarcinoma, but the postoperative pathologic, histologic, and immunohistochemical analyses of the resected specimen revealed a final diagnosis of lymphoepithelioma-like gastric carcinoma.
Core tip: Lymphoepithelioma-like gastric carcinoma is a rare subtype of gastric carcinoma with a better survival rate than other gastric cancers. It is similar to gastric cancer, with no obvious early symptoms. Lymphoepithelioma-like gastric carcinoma is difficult to discern from biopsy specimens because of the stromal lymphocyte infiltrates. As preoperative diagnosis is difficult and it is easily misdiagnosed, cases are usually diagnosed pathologically after tumor surgery. In order to gain a detailed understanding of this rare disease, we reviewed the literature and report here on a recent case of epithelioid gastric cancer in our hospital.
- Citation: Wang ZH, Zhao JJ, Yuan Z. Lymphoepithelioma-like gastric carcinoma: A case report and review of the literature. World J Gastroenterol 2016; 22(10): 3056-3061
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/3056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.3056
Gastric cancer is the second leading cause of cancer mortality in the world[1]. Lymphoepithelioma-like gastric carcinoma (LELGC), first described by Watanabe et al[2] in 1976 as gastric carcinoma with a lymphoid stroma, is an uncommon subtype of gastric carcinoma (GC), which has a better survival rate than other GCs. LELGC comprises approximately 4% of all GCs[3,4], and its simultaneous occurrence with differentiated adenocarcinoma is relatively rare. Lymphoepithelial carcinoma[5], a type of undifferentiated nasopharyngeal carcinoma[6] with lymphoid stroma and non-keratinizing squamous cells, has distinguishing clinical, epidemiologic, and etiologic features. Lymphoepithelioma-like carcinomas (LELCs) are well known to occur in the nasopharynx. In comparison, LELGCs, which histologically resemble lymphoepithelioma[7], are not found in the nasopharynx but in various organs such as the stomach, lungs[8], tonsils, esophagus[9], salivary glands[10], thymus, cervix[11], and skin[12]. LELGCs tend to lack continuous sheets of cancer cells, but rather consist of small clusters and aggregates of cancer cells that are broken up by large numbers of intratumoral lymphocytes[7]. In order to acquire better insight into this rare disease, we review the literature here and report on a recent case of epithelioid gastric cancer in a patient from our hospital.
A 55-year-old woman was admitted to our hospital with a 10-year history of persistent abdominal pain, acid regurgitation, and heartburn. Two months prior to admittance to the hospital she had visited the emergency room with complaint of dizziness, fainting spells, cold sweat, amaurosis fugax, and melena. The patient had a history of multiple leiomyoma resection and use of nonsteroidal anti-inflammatory drugs. On admission, she was pale with normotensive blood pressure (105/68 mmHg), normal cardiac rhythm (85 beats/min) and ventilation rate (16 breaths/min).
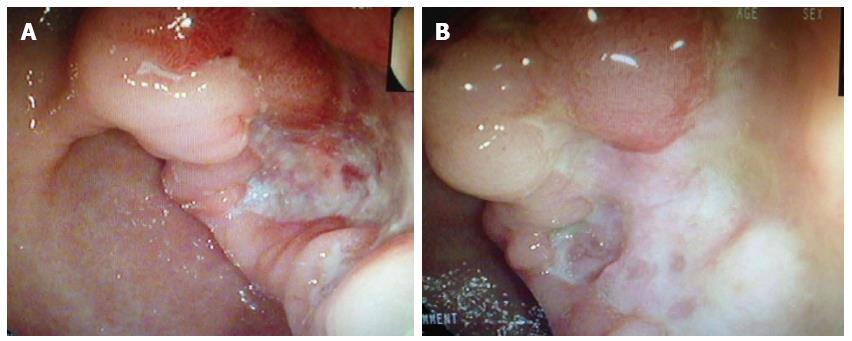
The patient was then transferred to the gastroenterology ward and underwent a physical examination, which was unremarkable except for her pale appearance. Laboratory results revealed decreased hemoglobin (66 g/L) and hematocrit (0.208) levels, but an elevated urea nitrogen level (9.74 mmol/L). Meanwhile, no abnormal findings were detected by the chest radiograph or CT. However, gastroscopic examination revealed that the patient had a typical ulcerative lesion (malignancy could not be excluded) close to the gastric antrum, with an advanced damaged area of 3.0 cm × 3.5 cm (Figure 1A). A subsequent biopsy analysis revealed inflammatory changes. After the standard treatment of blood transfusion, acid suppression and protection of the gastric mucosa, the patient’s symptoms improved and she was discharged from the hospital. As the possibility of GC was not completely eliminated, the patient was asked to continue the drug treatment and return one month later. The follow-up endoscopy showed that the ulcer remained unhealed (Figure 1B), while the biopsy revealed no malignancy. Accordingly, the drug treatment was combined with Helicobacter pylori eradication therapy for another month. Histologic typing at this time revealed an adenocarcinoma.
The patient was transferred to our general surgery unit for surgical treatment. In view of the patient’s general condition, laparoscopic-assisted radical gastrectomy was performed under general anesthesia. Intrasurgical observation revealed that the tumor situated in the antrum had not invaded the serosa. Thus, groups of 1-8 lymph nodes were cleared, and the tumor was treated by distal subtotal gastrectomy. After providing continuous decompression, active antibiotics (cefotaxime sodium), acid-base balance fluid, rehydration, and other supportive treatment, the further postoperative course was uneventful and the patient recovered well.
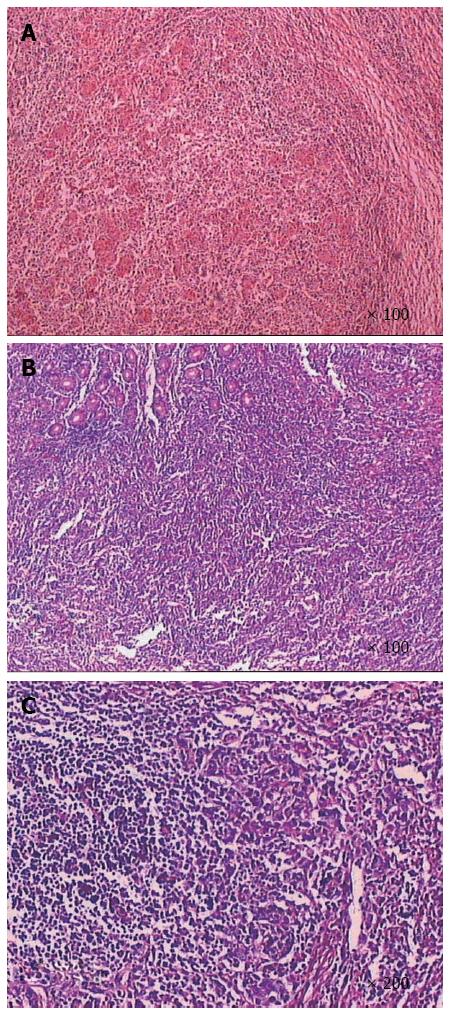
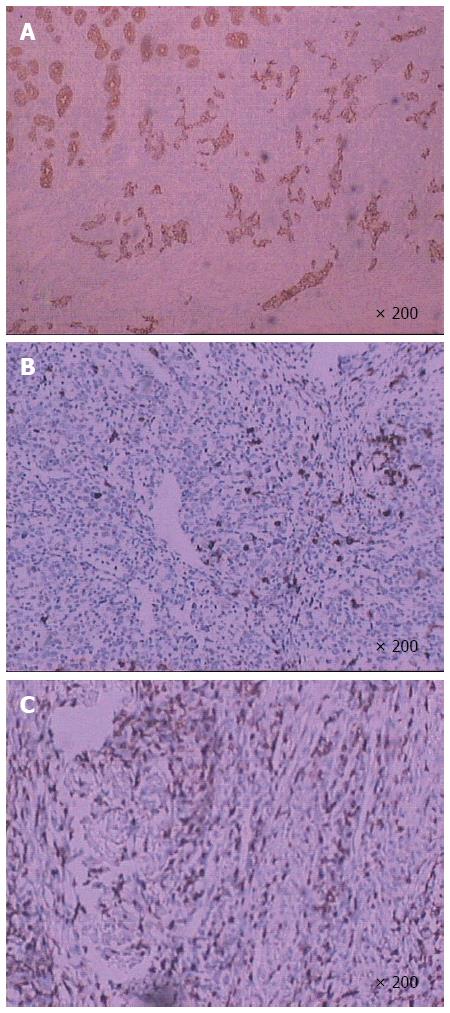
Postoperative pathologic analyses (Figure 2) showed that the tumor was composed of epithelial cell nests surrounded by large numbers of lymphocyte plasma cells, which was consistent with lymphoepithelial carcinoma with deep myometrial invasion (tumor size was about 5.5 cm × 4.0 cm × 1.0 cm). Analysis of the upper and lower margins revealed no cancer tissue. Additionally, no lymph node metastasis was found in the regional lymph nodes. Furthermore, immunohistochemical analysis (Figure 3) indicated that the tumor cells were intensely positive for Ckpan and negative for CD3 and CD20. On the other hand, no expression of chromogranin, synaptophysin, or neuron-specific enolase was observed.
The patient did not receive any adjuvant chemotherapy or radiotherapy postoperatively, and she remains well without further evidence of the disease.
LELGC is a type of GC with distinctive clinicopathologic features, including stromal, intense lymphocytic infiltration. EBV is believed to be a possible factor in the etiology of lymphoepithelioma-like tumors[13]. The worldwide prevalence of EBV infection among GCs has been reported as 8.29% for gastric adenocarcinoma, 7.08% for the intestinal type and 9.82% for the diffuse type[14]. EBV was the first virus found in human neoplastic cells, the Burkitt’s lymphoma cell line, in 1964[15], and subsequent studies have identified the virus in a variety of malignant neoplasms. Two subsets of gastric cancer, namely EBV-positive and microsatellite instability-high cancers, have been linked with a lymphocyte-rich phenotype[16]. Additionally, EBV infection is reported to be associated with a majority of the nasopharyngeal carcinomas and a few of the LELCs[17]. Specifically, more than 90% of LELGCs occurring in areas other than the foregut were reported to be EBV-positive[18]. EBV-associated LELGC occurs more frequently in men[19]. As most cases of gastric cancer, the disease is usually prevalent among the elderly, and although some reports have indicated that tumors occur in younger patients[18,20], a meta-analysis did not confirm these reports[21]. In addition, while it has been shown in LELGC that only EBV-infected progenitor cells exhibit monoclonal proliferation, the exact mechanism whereby EBV contributes to gastric mucosa carcinogenesis, though a hot topic in cancer research, is still uncertain. Our patient refused to do EBV testing because of personal economic reasons.
LELGC is similar to GC in various aspects, such as no obvious early symptoms and occasional abdominal pain as the disease progresses. Additionally, there may be aggravated abdominal pain, loss of appetite, fatigue, weight loss, etc. The occurrence of LELCs with epithelioid granulomas has rarely been reported[22]. Hence, most radiologists are not familiar with these tumors. In the present case, imaging analysis revealed no lymph node or distant organ metastasis. This is because the spread of the tumors through the gastric wall may have been prevented by abundant lymphocytic and granulomatous reactions[22,23]. Because preoperative diagnosis of LELGC is difficult, most cases are diagnosed pathologically after tumor surgery, even if endoscopic biopsy is performed[24]. Indeed, a definitive diagnosis of LELGC could not be established in the present case from the sample obtained from endoscopic biopsy, as this disease is pathologically characterized by prominent lymphocytic infiltration, which often obscures the neoplastic epithelial component. Furthermore, small LELGCs are easily and frequently missed by endoscopic ultrasound fine-needle aspiration. Endoscopic mucosal resection is recommended for diagnosis and potential curative resection of gastric submucosal lesions[25]. In our case, despite thrice performing endoscopic biopsy, we were initially unable to make an accurate diagnosis.
Histopathologic analysis findings from our case were similar to those found in all previously reported cases, including characteristics such as a syncytial growth pattern, round-to-large vesicular nuclei, and prominent nucleoli. The nests of epithelial tumor cells were associated with an intense and accentuated lymphoid infiltrate[26]. Accurate diagnosis was aided by immunohistochemical staining for a panel of epithelial markers and common leukocyte antigens. Generally, immunohistochemical staining shows nests that are positive for cytokeratin, carcinoembryonic antigen, and epithelial membrane antigen, suggesting epithelial origin, and the interstitial lymphoid tissue, or T lymphocyte clones. In the present report, the immunohistochemical profile of the neoplastic cells showed strong positivity for cytokeratin.
In a previous study, the rate of 12-year disease-free survival in patients with LELC was reported to be approximately 95%[27], suggesting that the extensive lymphocytic infiltration contributes to a low risk of tumor spread and a better prognosis. In fact, a lower rate of lymph node involvement has been found in LELC, especially during its early stage within the submucosa[28]. Accordingly, a lymphadenectomy did not seem necessary in our case. Surgical resection of LELGC and adjuvant short-term chemotherapy offered a good prognosis. Homogeneous LELCs have a significantly better prognosis than other types of stomach cancer. Indeed, the 5-year survival rate is significantly higher than that of non-lymphoid epithelioma gastric cancer and there have been reports of a 5-year survival of 59%[28,29], reaching 62% for the pure type[30].
In summary, LELCs of the stomach are rare neoplasms, thought to be associated with EBV infection. Diagnosis is based on pathologic, histologic, and immunohistochemical analyses. LELGC is difficult to identify in biopsy specimens due to the stromal lymphocyte infiltrates. This tumor type generally has a better prognosis than other forms of EBV-associated GCs and conventional GCs[28].
A 55-year-old female patient, whose preoperative endoscopic biopsy showed adenocarcinoma, was finally diagnosed with lymphoepithelioma-like gastric carcinoma (LELGC) according to findings of postoperative pathology and immunohistochemistry of the resected specimen.
LELGC had no obvious early symptoms, and gastroscopy revealed a typical ulcerative lesion.
Lymphoepithelial carcinoma discovered in nasopharynx, which histologically resembles lymphoepithelioma-like carcinoma (LELCs).
A decreased level of hemoglobin (66 g/L) and hematocrit (0.208), and increased urea nitrogen (9.74 mmol/L).
The CT appearance of LELCs with epithelioid granulomas has not been reported previously.
LELCs are pathologically characterized by a pronounced lymphocytic infiltrate, which often obscures the neoplastic epithelial component.
Surgical resection of LELGC and adjuvant short-term chemotherapy has a good prognosis. The patient refused to receive any adjuvant chemotherapy or radiotherapy postoperatively, but did accept and receive oral proton pump inhibitor conservative treatment.
There are a few cases of LELGC reported in the literature.
Immunohistochemical staining is widely used in the diagnosis of abnormal cells, such as those found in cancerous tumors. Specific molecular markers are characteristic of particular cellular events, such as proliferation or cell death (apoptosis).
This case report represents the diagnosis and therapeutic strategy for LELGC, and patients with non-healing ulcers on standard treatment should be evaluated for possible malignant tumors using immunohistochemical analyses for differential diagnosis when necessary.
The authors performed a good literature review. Although this condition is not that rare, it deserves further recognition.
P- Reviewer: Tang SJ S- Editor: Yu J L- Editor: Filipodia E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] |
| 2. | Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38:232-243. [PubMed] |
| 3. | Horiuchi K, Mishima K, Ohsawa M, Aozasa K. Carcinoma of stomach and breast with lymphoid stroma: localisation of Epstein-Barr virus. J Clin Pathol. 1994;47:538-540. [PubMed] |
| 4. | Herath CH, Chetty R. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Arch Pathol Lab Med. 2008;132:706-709. [PubMed] |
| 5. | Aurilio G, Ricci V, De Vita F, Fasano M, Fazio N, Orditura M, Funicelli L, De Luca G, Iasevoli D, Iovino F. A possible connective tissue primary lymphoepithelioma-like carcinoma (LELC). Ecancermedicalscience. 2010;4:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Sashiyama H, Nozawa A, Kimura M, Nomura E, Tamaru JI, Ninomiya E, Koide Y, Iino M, Ozawa K. Case report: A case of lymphoepithelioma-like carcinoma of the oesophagus and review of the literature. J Gastroenterol Hepatol. 1999;14:534-539. [PubMed] |
| 7. | Chetty R. Gastrointestinal cancers accompanied by a dense lymphoid component: an overview with special reference to gastric and colonic medullary and lymphoepithelioma-like carcinomas. J Clin Pathol. 2012;65:1062-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Bildirici K, Ak G, Peker B, Metintaş M, Alataş F, Erginel S, Uçgun I. Primary lymphoepithelioma-like carcinoma of the lung. Tuberk Toraks. 2005;53:69-73. [PubMed] |
| 9. | Chen PC, Pan CC, Hsu WH, Ka HJ, Yang AH. Epstein-Barr virus-associated lymphoepithelioma-like carcinoma of the esophagus. Hum Pathol. 2003;34:407-411. [PubMed] |
| 10. | Sun XN, Xu J, Yang QC, Hu JB, Wang Q. Lymphoepithelioma-like carcinoma of the submandibular salivary gland: a case report. Chin Med J (Engl). 2006;119:1315-1317. [PubMed] |
| 11. | Bais AG, Kooi S, Teune TM, Ewing PC, Ansink AC. Lymphoepithelioma-like carcinoma of the uterine cervix: absence of Epstein-Barr virus, but presence of a multiple human papillomavirus infection. Gynecol Oncol. 2005;97:716-718. [PubMed] |
| 12. | Cavalieri S, Feliciani C, Massi G, Addolorato G, Gasbarrini G, Amerio P, Rotoli M. Lymphoepithelioma-like carcinoma of the skin. Int J Immunopathol Pharmacol. 2007;20:851-854. [PubMed] |
| 13. | Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss LM. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469-474. [PubMed] |
| 14. | Sousa H, Pinto-Correia AL, Medeiros R, Dinis-Ribeiro M. Epstein-Barr virus is associated with gastric carcinoma: the question is what is the significance? World J Gastroenterol. 2008;14:4347-4351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt‘s lymphoma. Lancet. 1964;1:702-703. [PubMed] |
| 16. | Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641-651. [PubMed] |
| 17. | Gulley ML, Amin MB, Nicholls JM, Banks PM, Ayala AG, Srigley JR, Eagan PA, Ro JY. Epstein-Barr virus is detected in undifferentiated nasopharyngeal carcinoma but not in lymphoepithelioma-like carcinoma of the urinary bladder. Hum Pathol. 1995;26:1207-1214. [PubMed] |
| 18. | Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Liang Y, Wang L, Zhu Y, Lin Y, Liu H, Rao H, Xu G, Rong T. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer. 2012;118:4748-4758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Fukayama M, Ushiku T. Epstein-Barr virus-associated gastric carcinoma. Pathol Res Pract. 2011;207:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Liu S, Jin L, Xu X, Lin N, Lei B, Shen H. Pathological and computed tomography findings of lymphoepithelioma-like gastric carcinoma with epithelioid granulomas: A case report. Oncol Lett. 2013;5:549-551. [PubMed] |
| 23. | Tamura T, Hamada T, Sako T, Makihara K, Yamada K, Kashima K, Yokoyama S, Hirata K, Hachiya Y, Fukuyama T. Lymphoepithelioma-Like Carcinoma of the Stomach with Epithelioid Granulomas. Case Rep Gastroenterol. 2010;4:361-368. [PubMed] |
| 24. | Ishihara Y, Ikegami M, Yokoyama T, Matsuda H, Kawamura T, Kawamura T. A case of early lymphocytic infiltrating medullary carcinoma of the stomach with a morphology of a submucosal tumor demanded 3 years for making definite diagnosis. Nippon Rinshogeka Gakkai Zasshi. 2003;64:347-351. |
| 25. | Moon HS, Kang SH, Seong JK, Jeong HY, Song KS. Lymphoepithelioma-like gastric carcinoma resected by endoscopic submucosal dissection (ESD). Endoscopy. 2010;42 Suppl 2:E73-E74. [PubMed] |
| 26. | Tang SJ, Ahmed N, Bhaijee F, Sheehan J, Subramony C, Jackson C, Lewin JR. Endoscopic mucosal resection of an Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Dig Dis Sci. 2012;57:3032-3034. [PubMed] |
| 27. | Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, Kim M, Kim S, Park CK, Kim S. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139:84-92.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Bittar Z, Fend F, Quintanilla-Martinez L. Lymphoepithelioma-like carcinoma of the stomach: a case report and review of the literature. Diagn Pathol. 2013;8:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Singh NG, Mannan AA, Rifaat AA, Kahvic M. Lymphoepithelioma-like carcinoma of the urinary bladder: report of a rare case. Ann Saudi Med. 2009;29:478-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Mori K, Ando T, Nomura T, Sato F, Mimata H. Lymphoepithelioma-like carcinoma of the bladder: a case report and review of the literature. Case Rep Urol. 2013;2013:356576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |