Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.2993
Peer-review started: September 1, 2015
First decision: October 15, 2015
Revised: November 15, 2015
Accepted: December 12, 2015
Article in press: December 14, 2015
Published online: March 14, 2016
Processing time: 185 Days and 22.1 Hours
AIM: To investigate the long-term survival and prognostic factors in hepatocellular carcinoma (HCC) patients undergoing radiofrequency ablation (RFA) as a first-line treatment.
METHODS: From 2000 to 2013, 316 consecutive patients with 404 HCC (1.0-5.0 cm; mean: 3.2 ± 1.1 cm) underwent ultrasonography-guided percutaneous RFA as a first-line treatment. There were 250 males and 66 females with an average age of 60.1 ± 10.8 years (24-87 years). Patients were followed for 1 year to > 10 years after RFA (234, 181, 136, and 71 for 3, 5, 7, and 10 years, respectively). Overall local response rates and long-term survival rates were assessed. Survival results were generated using Kaplan-Meier estimates, and multivariate analysis was performed using the Cox regression model.
RESULTS: In total, 548 RFA sessions were performed and major complications occurred in 10 sessions (1.8%). Local tumor progression and/or new tumor development were observed in 43.3% (132/305) of the patients during the follow-up period. Overall 5- and 10-year survival rates were 49.7% and 28.4%, respectively. Based on multivariate analysis, three factors were identified as independent prognostic factors for overall survival: Child-Pugh classification (HR = 4.054, P < 0.001), portal vein hypertension (HR = 2.743, P = 0.002), and tumor number (HR = 2.693, P = 0.003). The local progression-free 5- and 10-year survival rates were 42.7% and 19.5%. In addition to the Child-Pugh classification and the number of tumors, the number of RFA sessions (HR = 1.550, P = 0.002) was associated with local progression-free survival.
CONCLUSION: RFA can achieve acceptable outcomes for HCC patients as a first-line treatment, especially for patients with Child-Pugh class A, patients with a single tumor and patients without portal vein hypertension.
Core tip: Numerous large series have shown that percutaneous radiofrequency ablation (RFA) is safe and effective, with minimal morbidity and mortality in hepatocellular carcinoma (HCC) treatment. However, few studies had follow-up time that was adequate to rival that of surgery and percutaneous ethanol injection. In our long-term follow-up study on a large group of HCC patients, we further confirmed that RFA could achieve a 10-year survival in HCC patients as a first-line treatment, especially for patients with liver function of Child-Pugh class A, a single tumor, and without portal vein hypertension.
- Citation: Yang W, Yan K, Goldberg SN, Ahmed M, Lee JC, Wu W, Zhang ZY, Wang S, Chen MH. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol 2016; 22(10): 2993-3005
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/2993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.2993
Advances in approaches to the diagnosis and management of hepatocellular carcinoma (HCC) are improving patient survival. Although surgical resection is the standard treatment modality for HCC, its use is usually limited because the majority of patients, even those with small tumors, have associated severe liver dysfunction[1,2]. Therefore, many nonsurgical image-guided ablation methods have been developed, and radiofrequency ablation (RFA) is currently the most widely used percutaneous ablation therapy. Some centers now use RFA as a first-line treatment option, even in patients feasible for surgery.
Recently, more and more evidence has shown the longer-term efficacy of RFA in HCC patients[3-7]. However, among the various clinical studies of RFA, only a few have focused on percutaneous RFA for HCC as a first-line treatment option[8], and reports on the 10-year survival of post-RFA HCC patients are rare, with very few patients reported for this endpoint[9,10]. The present study aimed to investigate long-term survival and prognostic factors in HCC patients undergoing percutaneous RFA as a first-line treatment based on our 14 years of clinical experience.
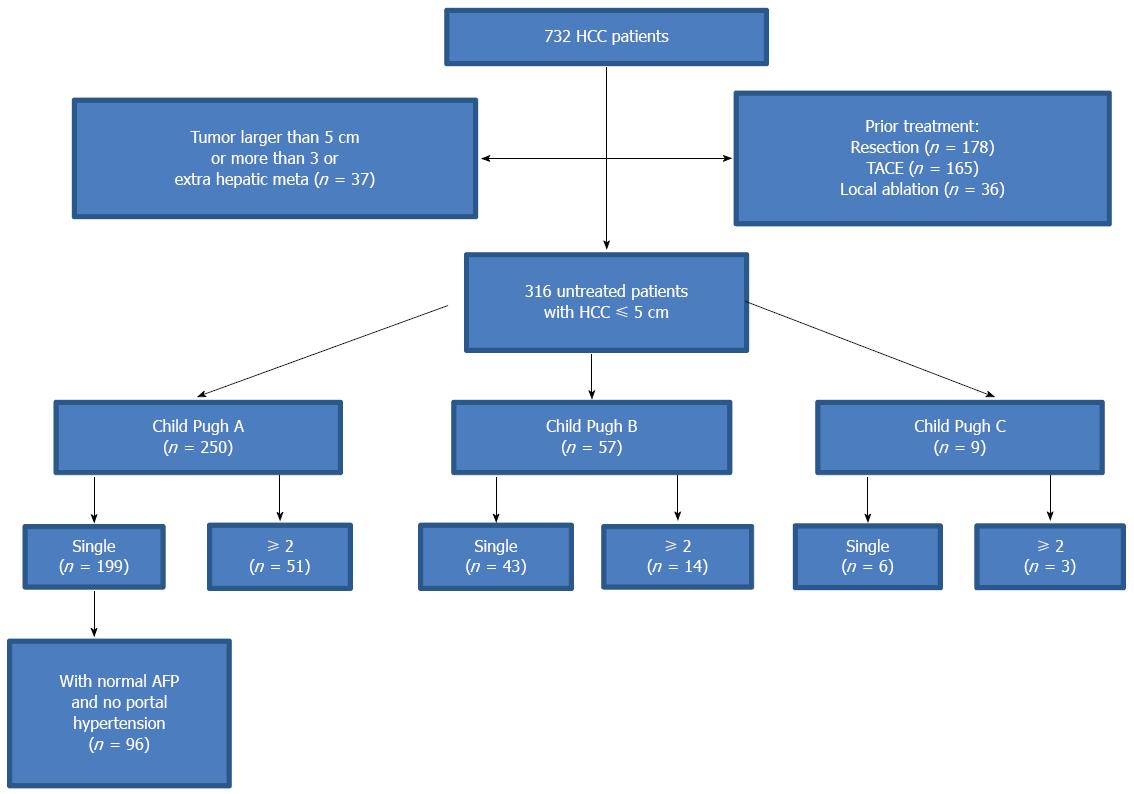
This was a cohort study conducted as a retrospective analysis of a prospective database in a single center. In our group, there were 732 consecutive HCC patients who underwent ultrasound-guided percutaneous RFA in our center from January 2000 to January 2013. The inclusion criteria for this study were as follows: (1) a tumor size ≤ 5 cm and tumor number ≤ 3; (2) an absence of significant direct tumor invasion of adjacent organs or tumor thrombi in the main or lobar portal system; (3) a tumor not invading a main bile duct or being obviously exophytic; (4) a liver function of Child-Pugh A and B or Child-C (whose liver function was improved after liver protection therapy and tumor size <3 cm, no obvious ascites next to the puncture site of liver); (5) a tumor accessible via a percutaneous approach; (6) international standard ratio < 1.6 and platelet count > 50000/μL; (7) No extrahepatic metastasis before RFA; and (8) a follow-up of at least one year after the first RFA treatment.
Among our study subjects, 416 patients did not meet the inclusion criteria and were excluded from the study. The remaining 316 patients, who met the inclusion criteria and received percutaneous RFA as a first-line treatment were enrolled in this study (Figure 1). These patients underwent percutaneous RFA due to severe liver cirrhosis, advanced age, chronic respiratory/cardiac diseases or preferred minimally-invasive therapy. There were 250 men and 66 women. Patients age ranged from 24 to 87 years (mean, 60.1 ± 10.8 years). Two hundred and fifty patients had liver function of Child-Pugh class A, whereas 57 patients had class B, and 9 class C. A total of 278 (88.0%) patients were found with liver cirrhosis, which was caused by hepatitis B in 231 patients, hepatitis C in 37, alcohol abuse in 4 and other causes in 6. The remaining 38 patients had fatty liver (n =24) and normal liver (n = 14) (Table 1).
| Characteristics | Value |
| Age (yr) | |
| Mean (range) | 60.1 ± 10.8 (24-87) |
| Patients aged ≥ 60 yr | 140 (44.3) |
| Sex | |
| Males | 250 (79.1) |
| Females | 66 (20.9) |
| Etiology of cirrhosis | |
| HBV | 231 (86.6) |
| HCV | 37 (10.2) |
| Alcohol | 4 (1.9) |
| Others | 6 (1.3) |
| Pre-RFA serum AFP | |
| < 20 ng/mL | 177 (56.0) |
| ≥ 20 ng/mL | 139 (44.0) |
| Child-Pugh class | |
| A | 250 (77.0) |
| B | 57 (20.1) |
| C | 9 (2.8) |
| Serum ALT/AST | |
| Normal | 229 (72.5) |
| Elevated | 87 (27.5) |
| Tumor no | |
| Single | 248 (78.4) |
| ≥ 2 | 68 (21.6) |
| Tumor size (cm) | |
| Mean (range) | 3.2 ± 1.1(1.0-5.0) |
| Tumor > 3 cm | 169 (53.4) |
The diagnosis of HCC was histologically proven (n = 204) or established according to noninvasive criteria (n = 112)[11]. Liver biopsies were performed when the noninvasive criteria were not satisfied.
All patients underwent a baseline evaluation, which included an enhanced CT or MRI scan of the abdomen and pelvis within one month before the treatment. Serum laboratory tests consisting of a complete blood count, coagulation profile, liver and kidney functional tests (such as ALT, AST) and serum tumor markers (such as AFP) were performed in the two weeks before the treatment. Contrast-enhanced ultrasound (CEUS) was regularly performed to confirm tumor coverage and tumor number prior to RFA. SonoVue (Bracco SpA, Italy) was used as the contrast agent in this study.
All RFA procedures were performed by two of four radiologists (YW, WW, CMH, and YK), all of them had more than 5 years of experience in ultrasound-guided interventional procedures. The tumor size, shape and border were obtained mainly using ultrasound scans, and enhanced CT/MRI was used as a reference. If liver cirrhosis was present, a full evaluation of the portal vein system was performed. We evaluated the related ultrasound parameters for the diagnosis of portal hypertension in the liver cirrhosis patients[12]. “Risky procedure location” was defined as a tumor located adjacent to (≤ 0.5 cm) the diaphragm, bowel, gallbladder, main bile duct or large vessels (i.e., potentially critical structures).
Currently, in clinical practice, most RFA devices are able to create an ablation sphere having a maximum diameter of up to 5 cm in the liver. When treating spherical tumors larger than 3 cm in diameter with 5-cm ablation spheres, we generally used multiple overlapping ablations based on a mathematical protocol[13]. Ablative margins covered 0.5-1.0 cm beyond the original tumor with the exception of tumors adjacent to major structures such as the diaphragm, gastrointestinal tract, or gallbladder. For these latter cases, the ablative margin was limited; thus, an individualized protocol was developed[14-16].
During RFA, intravenous moderate sedation provided by an anesthesiologist was induced using 2.5-5.0 mg of midazolam (Roche; Basel, Switzerland) and 50-100 μg fentanyl (Fentaini; Renfu, Yichang, China). The patient was conscious when the RFA electrode was placed, and vital signs and oxygen saturation were continuously monitored during the procedure. After RFA, the patient was observed for 2-4 h and discharged if no evidence of active bleeding was found.
Selection of the RFA device was based upon the tumor size, morphology and location. In the present study, three types of RFA systems were used: multi-tined (RITA Medical System, United States), multi-polar (Celon Lab Power, Germany) and internally-cooled (Tyco Healthcare, United States) electrodes. Real-time Aloka ultrasound systems (Alokaα-10, Tokyo, Japan) and GE systems (E9, GE, United States) were used for scanning with 3.5-5.0 MHz convex probes with needle guide devices for all ablation procedures. Track ablation was performed when withdrawing the RFA electrode in all patients.
To evaluate the technical success of the RFA therapy, contrast-enhanced CT/MRI was performed 1 mo after treatment. Primary technical success was defined as the absence of contrast enhancement in the target tumor[17]. Residual tumor was retreated if the patient was physically strong enough to tolerate another RFA session. Secondary technical success was defined as the absence of contrast enhancement after re-ablation. Complete ablation that was achieved after a maximum of three iterative procedures within 3 mo after initial RFA was regarded as a treatment success. Therefore, persistence of active tumor foci that were untreatable by RFA after a maximum of three iterative procedures was regarded as treatment failure.
Subsequently, patients were followed with repeat CT/MRI every 2-3 mo during the first year and then every 4-6 mo. Contrast enhancement that was detected in the ablation zone on follow-up CT/MRI scans was considered to represent local tumor progression[18]. New nodules in other sites of the liver were considered new tumor occurrence. When local tumor progression or new tumors were identified, additional RFA treatment was considered according to the same criteria used at the time of the initial RFA. When these criteria were not met, liver transplantation, resection, transarterial chemoembolization (TACE), or other treatments were considered if possible.
Fifteen potential prognostic factors were considered in this study, and their classification scores are listed in Table 2. Transplanted patients were censored from this study at the date of transplantation. The probability of local progression-free survival was defined as the interval between the treatment and the date of local tumor progression or death. Overall survival duration was counted in months from the date of RFA to death. Survival curves were plotted using a Kaplan-Meier model and compared using a log-rank test. A univariate Cox proportional hazards model was fitted to each variable. All significant variables were subject to multivariate analysis to assess their value as independent prognostic factors. SPSS 21.0 software (SPSS, Chicago, IL, United States) was used to perform the analysis, and P < 0.05 was considered to be statistically significant.
| Variables | Definition | Class scores1 |
| X1 | Sex | Male (1), Female (2) |
| X2 | Age (yr) | 18-60 (1), ≥ 60 (2) |
| X3 | Pathological grade | Well-Moderate differentiated (1), Poor-differentiated (2) |
| X4 | Etiology of cirrhosis | HBV (1), HCV (2), Alcohol (3), Others (4) |
| X5 | Portal vein hypertension | No (1), Yes (2) |
| X6 | Liver function enzyme | Normal (1), Elevated (2) |
| (serum AST/ALT) | ||
| X7 | Child-Pugh classification | A (1), B (2), C (3) |
| X8 | Serum AFP (ng/mL) | Normal < 20 (1), Abnormal ≥ 20 (2) |
| X9 | CEUS pre-RFA | No (1), Yes (2) |
| X10 | Number of tumors | Single (1), ≥ 2 (2) |
| X11 | Tumor size (cm) | ≤ 3.0 (1), 3.0-5.0 (2) |
| X12 | Risky location | No (1), Yes (2) |
| X13 | Type of RFA electrodes | Umbrella (1), single (2) |
| X14 | Primary technical success | Yes (0), No (1) |
| X15 | Number of RFA sessions | 1 (0), ≥ 2 (1) |
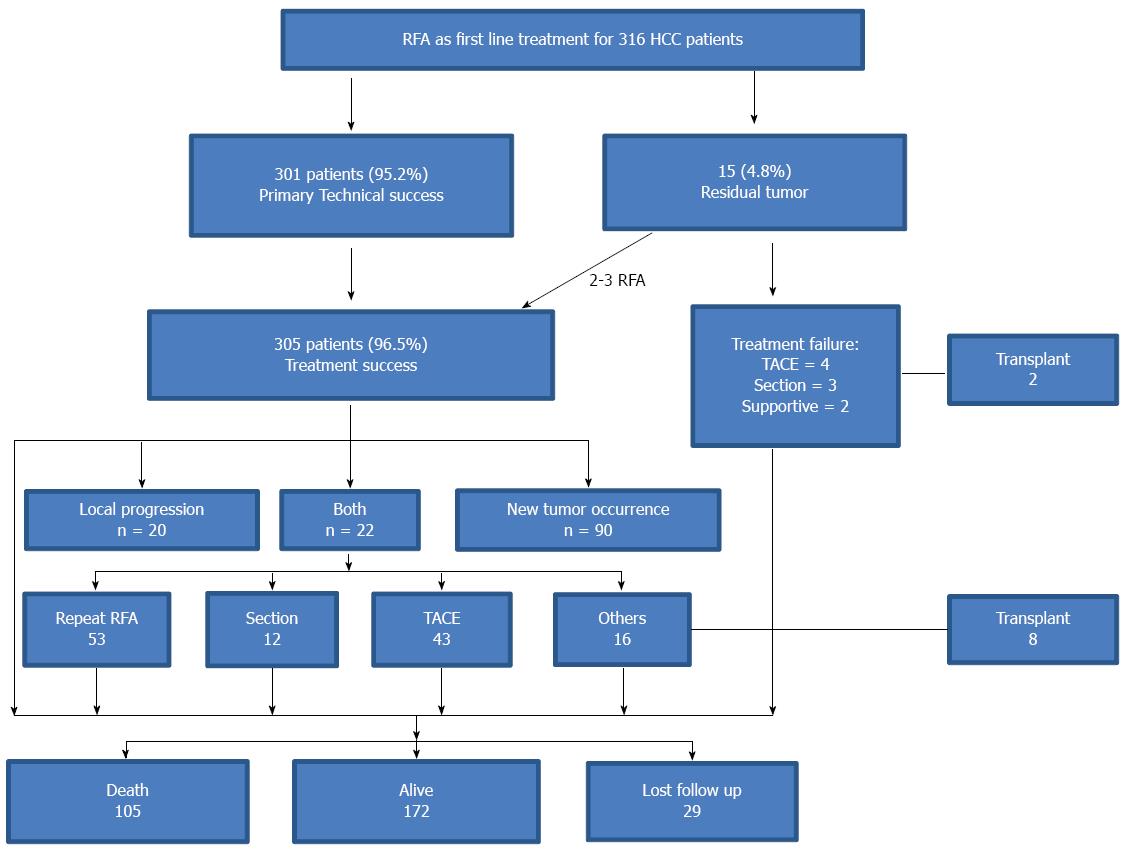
For the treatment of initial tumors, primary technical success was achieved in 301 patients (95.2%). Residual tumor was observed in 15 nodules in 15 patients, including 3 nodules close to the main branch of the portal vein, 2 close to the gallbladder, 2 close to the diaphragm, 2 close to the bowel, 4 larger than 4 cm, and 2 with poorly defined margins during ultrasound imaging. Among the 15 patients, four patients (26.7%) had secondary technical success after 2-3 RFA sessions. The other 11 patients did not receive repeated RFA or still had residual tumor after re-ablation due to difficulty with respect to the ablation location, poor liver function, or development of multiple new tumors (Figure 2).
Local tumor progression and/or new tumor development was observed in 43.3% (132/305) of the patients during the follow-up period. Twenty-two patients had local tumor progression and new tumors, whereas 20 patients had local progression only, and 90 patients had new tumors only. A total of 53 patients underwent repeated RFA sessions (2-5 sessions); 43 received TACE, 12 received resection, 8 liver transplantion, and the other 16 patients received other treatments such as PEI, radiotherapy or supportive treatment (Figure 2).
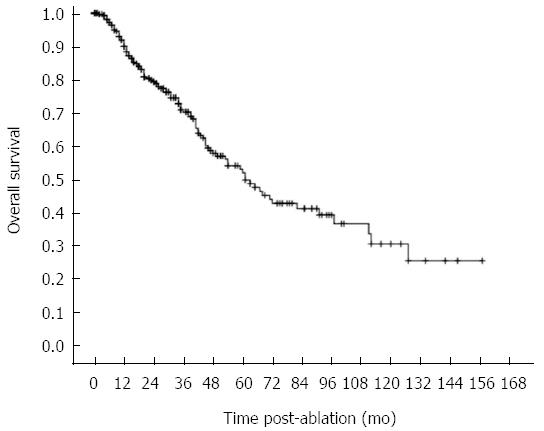
Follow-up ranged from 12 to 158 mo (median: 72.5 mo; mean: 71.8 mo), (Table 3). In all, 105 patients died, 10 received transplants, and 172 were alive without transplants. The remaining 29 patients (9.2%) were lost to follow-up (mean 20.4 mo), largely due to logistic and geographic challenges in making follow-up visits (Figure 2). Overall, 100 deaths were related to HCC progression or cirrhosis complications, and 5 were unrelated to liver disease. The overall estimated 1, 3, 5, 7 and 10 year survival rates were 90.0%, 70.8%, 49.7%, 41.1%, and 28.4%, respectively. Median overall survival was 61.0 mo (Figure 3). When comparing the different periods of enrollment, the overall survival in the second enrollment from 2007 to 2013 was significantly better than survival in the first enrollment from 2000 to 2007 (median 65 mo vs 44 mo, P < 0.001).
| Follow-up (yr) | No. of patients | Cumulative frequency1 |
| 1 | 82 | 316 (100) |
| 3 | 53 | 234 (74.1) |
| 5 | 45 | 181 (57.3) |
| 7 | 65 | 136 (43.0) |
| 10 | 71 | 71 (22.5) |
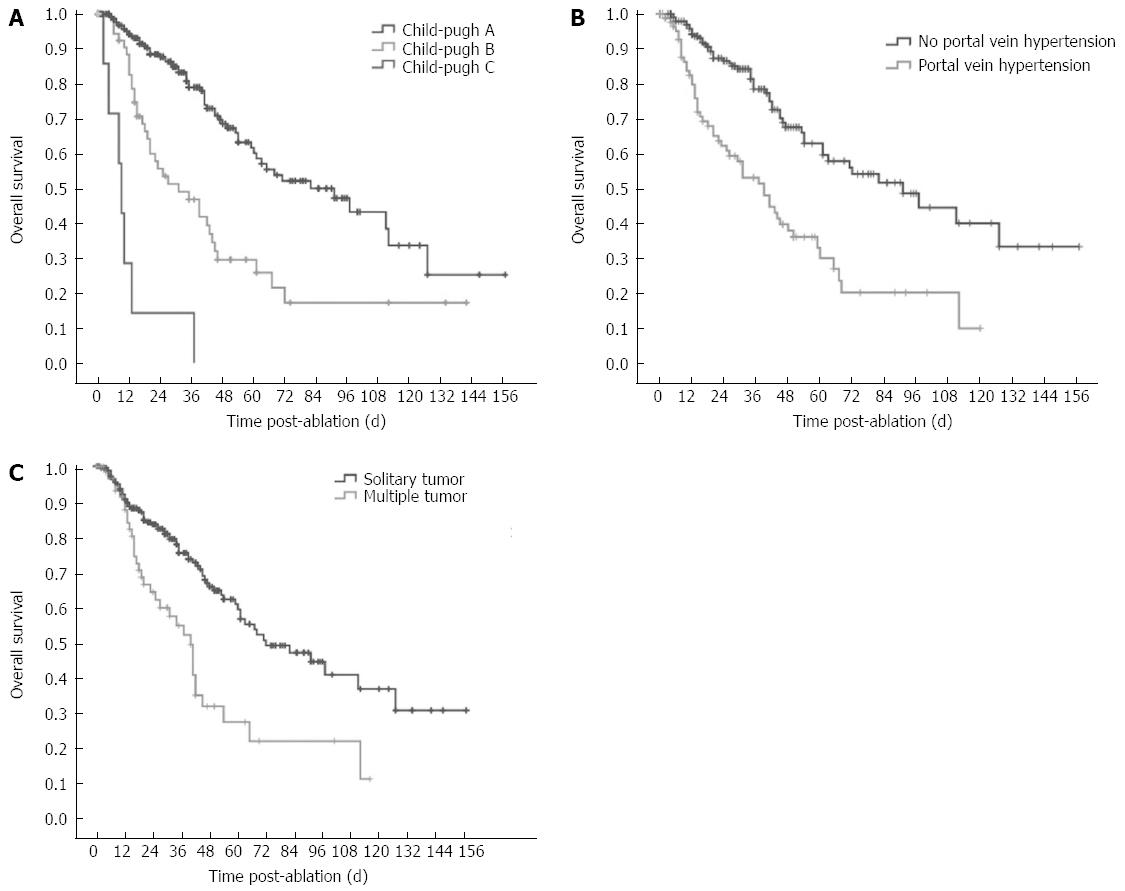
The factors associated with overall survival are reported in Table 4 and Table 5. Univariate analysis identified 8 factors that were related to the post-RFA overall survival rate. Patient-related factors included Child-Pugh classification (HR = 3.210, P < 0.001) and portal vein hypertension (HR = 2.686, P < 0.001). The tumor-related factors included the number of tumors (HR = 2.293, P < 0.001), Serum AFP (HR = 1.501, P = 0.039) and pathological grade of tumor (HR = 2.509, P < 0.001). Procedure-related factors included CEUS pre-RFA (HR = 0.438, P < 0.001), choice of RFA electrodes (HR = 0.191, P < 0.001), and primary technical success (HR = 2.592, P = 0.009). The factors that showed statistical significance from the univariate analysis were included in the multivariate analysis. Among them, Child-Pugh classification, portal vein hypertension, and number of tumors were independent prognostic factors for overall survival (Table 5 and Figure 4).
| Variable | No. of patients | Survival rate (%) | P value | ||||
| 1-yr | 3-yr | 5-yr | 7-yr | 10-yr | |||
| Sex | 0.570 | ||||||
| Male | 250 | 89.8 | 70.4 | 53.2 | 44.8 | 33.0 | |
| Female | 66 | 90.7 | 72.1 | 37.8 | 23.6 | - | |
| Age (yr) | 0.163 | ||||||
| < 60 | 176 | 90.3 | 67.6 | 48.0 | 31.7 | 26.4 | |
| ≥ 60 | 140 | 89.6 | 75.8 | 58.0 | 55.4 | 25.2 | |
| Pathology grade | < 0.001 | ||||||
| Well-Moderate differentiated | 126 | 94.4 | 82.5 | 64.2 | 53.0 | 42.5 | |
| Poor-differentiated | 78 | 83.6 | 51.9 | 33.1 | 26.5 | 16.1 | |
| Etiology of cirrhosis | 0.474 | ||||||
| HBV | 231 | 91.2 | 72.8 | 55.1 | 36.1 | 21.7 | |
| HCV | 37 | 95.8 | 76.7 | 57.5 | 57.5 | - | |
| Portal vein hypertension | < 0.001 | ||||||
| No | 211 | 94.2 | 78.5 | 63.0 | 51.8 | 36.2 | |
| Yes | 103 | 79.8 | 53.2 | 30.2 | 20.4 | 10.2 | |
| Liver function enzyme | 0.489 | ||||||
| Normal | 229 | 89.0 | 73.5 | 54.4 | 42.1 | 30.0 | |
| Elevated | 87 | 92.2 | 64.1 | 45.6 | 40.5 | - | |
| Child-Pugh classification | < 0.001 | ||||||
| A | 250 | 94.1 | 78.9 | 60.1 | 50.5 | 33.6 | |
| B | 57 | 82.5 | 46.8 | 25.9 | 17.2 | 17.2 | |
| C | 9 | 28.6 | 14.3 | 0 | 0 | 0 | |
| Serum AFP (ng/mL) | 0.037 | ||||||
| Normal | 177 | 88.4 | 75.1 | 59.2 | 53.3 | 35.4 | |
| Abnormal | 139 | 91.8 | 66 | 42.1 | 25.7 | 22.1 | |
| CEUS pre-RFA | < 0.001 | ||||||
| No | 83 | 80.1 | 56.2 | 37.7 | 26.1 | 18.0 | |
| Yes | 233 | 93.8 | 77.1 | 59.7 | 54.4 | - | |
| Number of tumors | < 0.001 | ||||||
| Single | 248 | 90.6 | 75.3 | 59.3 | 46.8 | 34.5 | |
| ≥ 2 | 68 | 87.6 | 54.6 | 27.0 | 21.6 | 10.8 | |
| Tumor size (cm) | 0.178 | ||||||
| ≤ 3.0 | 147 | 94.0 | 71.6 | 59.2 | 49.0 | 18.4 | |
| 3.0-5.0 | 169 | 86.8 | 69.8 | 47.3 | 36.4 | 25.2 | |
| Risky location | 0.668 | ||||||
| No | 152 | 91.4 | 71.5 | 48.6 | 41.6 | 32.7 | |
| Yes | 164 | 88.6 | 70.0 | 53.1 | 40.6 | 27.7 | |
| Type of RFA electrodes | <0.001 | ||||||
| Umbrella | 213 | 86.8 | 64.3 | 45.7 | 36.7 | 23.6 | |
| Single | 103 | 97.5 | 94.1 | 62.7 | 62.7 | - | |
| Primary technical success | 0.006 | ||||||
| Yes | 301 | 89.9 | 69.1 | 51.4 | 39.6 | 30.6 | |
| No | 15 | 54.5 | 36.4 | 18.2 | 0 | 0 | |
| Number of RFA sessions | 0.793 | ||||||
| 1 | 256 | 90.2 | 70.1 | 53.4 | 40.3 | 31.0 | |
| ≥ 2 | 60 | 89.3 | 73.4 | 49.0 | 42.4 | 30.3 | |
| Characteristic | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex | 1.149 | 0.709-1.862 | 0.572 | |||
| Age | 0.754 | 0.506-1.125 | 0.166 | |||
| Pathology grade | 2.509 | 1.593-3.952 | < 0.001 | |||
| Etiology of cirrhosis | 0.862 | 0.720-1.031 | 0.105 | |||
| Portal vein hypertension | 2.686 | 1.826-3.951 | < 0.001 | 2.743 | 1.462-5.149 | 0.002 |
| Liver function enzyme (AST/ALT) | 1.162 | 0.758-1.780 | 0.491 | |||
| Child-Pugh classification | 3.210 | 2.297-4.488 | < 0.001 | 4.054 | 2.346-7.005 | < 0.001 |
| Serum AFP | 1.501 | 1.021-2.206 | 0.039 | |||
| CEUS pre-RFA | 0.438 | 0.295-0.651 | < 0.001 | |||
| Number of tumor | 2.293 | 1.518-3.463 | < 0.001 | 2.693 | 1.399-5.185 | 0.003 |
| Tumor size | 1.318 | 0.879-1.978 | 0.181 | |||
| Risky location | 1.090 | 0.735-1.616 | 0.670 | |||
| Type of RFA electrodes | 0.191 | 0.077-0.473 | < 0.001 | |||
| Primary technical success | 2.592 | 1.269-5.296 | 0.009 | |||
| Number of RFA sessions | 0.955 | 0.703-1.298 | 0.769 | |||
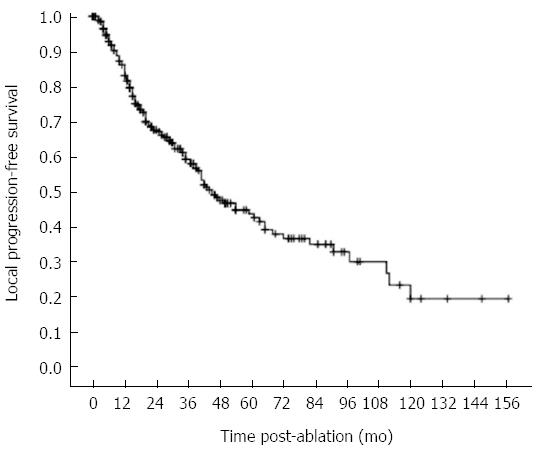
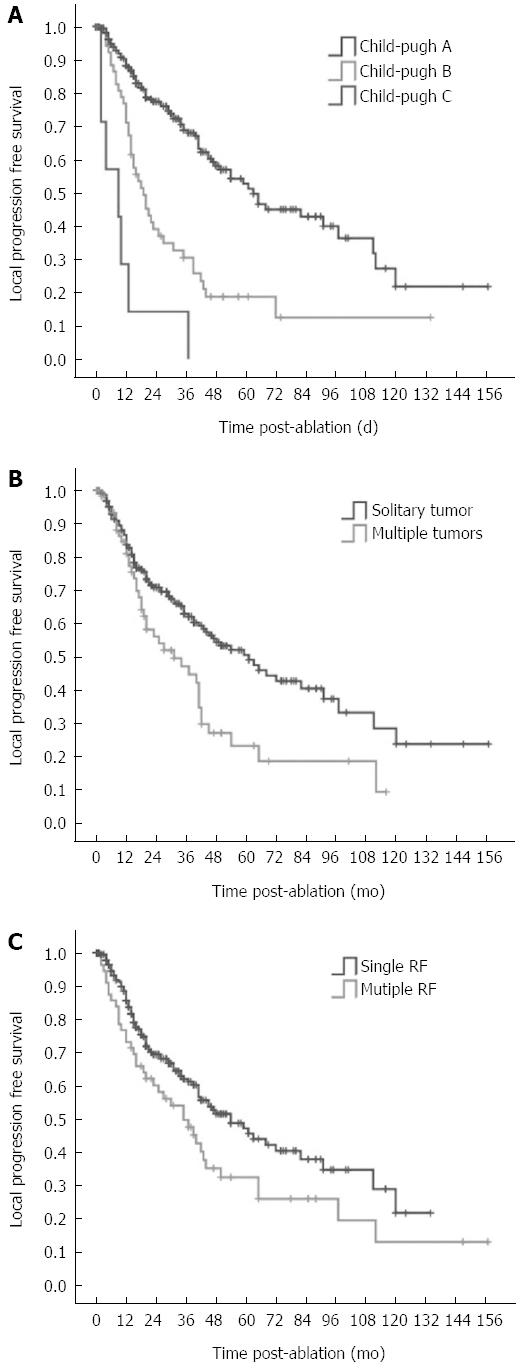
The 1, 3, 5, 7 and 10 year local progression-free survival rates were 83.2%, 59.3%, 42.7%, 35.1%, and 19.5%, respectively (Figure 5). Median local progression-free survival was 45.0 mo. The factors associated with the local progression-free survival are shown in Table 6. Univariate analysis showed 11 factors that were related to the local progression-free survival rate. Of them, 7 factors were the same as above including pathological grade, portal vein hypertension, Child-Pugh classification, number of tumors, serum AFP, CEUS pre-RFA, type of RFA electrodes. The 4 additional factors were the etiology of cirrhosis (HR = 0.851, P = 0.048), number of RFA sessions (HR = 1.574, P = 0.001), tumor size (HR = 1.587, P = 0.013), and risky location (HR = 1.442, P = 0.042). Multivariate analysis showed that Child-Pugh liver function classification (HR = 2.416, P < 0.001), number of tumors (HR = 1.588, P = 0.036) and number of RFA sessions (HR = 1.550, P = 0.002) were strongly related to the local progression-free survival (Table 6, Figure 6).
| Characteristic | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex | 1.073 | 0.691-1.667 | 0.753 | |||
| Age | 0.796 | 0.559-1.133 | 0.205 | |||
| Pathological grade | 2.258 | 1.506-3.385 | < 0.001 | |||
| Etiology of cirrhosis | 0.851 | 0.725-0.999 | 0.048 | |||
| Portal vein hypertension | 1.826 | 1.288-2.589 | 0.001 | |||
| Liver function enzyme | 1.082 | 0.740-1.583 | 0.684 | |||
| (AST/ALT) | ||||||
| Child-Pugh classification | 2.930 | 2.169-3.958 | < 0.001 | 2.416 | 1.713-3.409 | < 0.001 |
| Serum AFP | 1.425 | 1.010-2.011 | 0.044 | |||
| CEUS pre-RFA | 0.421 | 0.297-0.598 | < 0.001 | |||
| Number of tumors | 1.765 | 1.212-2.570 | 0.003 | 1.588 | 1.031-2.447 | 0.036 |
| Tumor size | 1.587 | 1.104-2.283 | 0.013 | |||
| Risky location | 1.442 | 1.013-2.054 | 0.042 | |||
| Type of RFA electrodes | 0.449 | 0.264-0.764 | 0.003 | |||
| Primary technical success | 1.772 | 0.877-3.583 | 0.111 | |||
| Number of RFA sessions | 1.574 | 1.201-2.061 | 0.001 | 1.550 | 1.183-2.033 | 0.002 |
Ten major complications (1.8%) occurred after ablation in this group as summarized in Table 7. One death (0.2%) related to RFA occurred. This patient had a tumor located close to the colon and had a bowel perforation the day after RFA. After surgical repair, he ultimately died of septic shock within one month. Minor complications included mild skin burns (n = 1), moderate pleural effusion (n = 4), slight bowel adhesion without obstruction (n = 8), mild dilation of the biliary duct (n = 4), and inflammation or thickening of the gallbladder wall (n = 3).
| Type of procedure-related complications1 | No. of events | Managements |
| Major complications | 10 (1.8) | |
| Bowel perforation | 1 | Surgically repaired |
| Seeding metastasis | 3 | Surgical resection |
| Pneumothorax or hemothorax | 3 | Drainage |
| Intraperitoneal hemorrhage | 1 | Ablation in site |
| Liver abscess | 1 | PTCD2 |
| Biliary stenosis | 1 | PTCD |
| No. of deaths | 1 (0.2) | (Bowel perforation) Surgically repaired |
Numerous large series have shown that RFA is safe and had minimal morbidity and mortality in the treatment of HCC. Although several promising and optimistic short- and mid-term follow-up studies using RFA for HCC have been reported[5,8,19], it should be noted that very few studies had a follow-up time that was adequate to rival that of surgery and percutaneous ethanol injection. The results from recent retrospective studies of long-term survival with RFA treatment have been promising[9,10,15,20,21]. However, only a few studies have covered 10-year outcomes with a sufficient number (> 20% patients had over 10 years of follow-up) of patients to draw firm conclusions.
In this single-center study, we assessed 10-year survival and prognostic factors in 316 consecutive HCC patients who received RFA as a first-line treatment for up to three ≤ 5 cm diameter HCC tumors (404 tumors; mean diameter: 3.2 ± 1.1 cm). Follow-up ranged from 12 to 158 mo (median: 72.5 mo) with 5-, 7-, 10-year overall survival rates of 49.7%, 41.1% and 28.4%, and local progression-free survival rates of 42.7%, 35.1% and 19.5%, respectively. In the present study in China, HCC patients have higher rates of chronic HBV infection and serve liver cirrhosis, larger tumor sizes, and greater baseline AFP levels than in other survival studies. Taking into account this discrepancy in demographics, our long-term survival outcome after percutaneous RFA for HCC, which was 49.7% at 5 years and 28.4% at 10 years, appears to be comparable with the survival outcomes of surgical resection[22,23].
Evaluation of the prognostic factors is also important when selecting RFA patients, planning RFA protocols and improving treatment efficacy.
As previously reported[10,24,25], we observed that liver function and portal hypertension strongly influenced overall and local progression-free survival rates. In our cohort, there was a high percentage of patients with HBV-related cirrhosis. These patients had significantly better local progression-free survival compared with patients with HCV cirrhosis. In an Italian study involving 187 patients with Child-Pugh class A or B cirrhosis and early-stage HCC, overall survival rates at 1, 3, 5 years were 97%, 71% and 48%, respectively[5]. The most significant prognostic factor identified in that study was Child-Pugh class. Similarly, we also identified host factors as prognostic factors. It is often that the final cause of their death is not HCC itself, but liver failure or portal hypertension related severe bleeding. Therefore, during RFA, normal liver tissue should be reserved as much as possible and anti-virus therapy is necessary.
Although RFA has been shown to be a safe local therapy for HCC with less liver function damage, data with respect to its application in patients with poor liver function are limited[26,27]. The survival of HCC patients with decompensated liver cirrhosis and without target therapy is poor (approximately 2-5 mo)[28,29]. Our results showed that the mean survival time after RFA in HCC patients with Child-Pugh class C cirrhosis was 12 mo, which suggests that RFA provided an opportunity to treat these HCC patients, and prolonged their survival.
Mulier et al[30], demonstrated that the possibility of achieving technical success substantially decreases when the tumor size exceeds 3 cm. In previous series, technical success rates for larger HCCs (3-5 cm) after RFA appeared unfavorable, and ranged from 61.3% to 82.5%[31,32]. In the present cohort, an important observation was that despite the fact that tumor size was a prognostic factor for local progression-free survival by univariate analysis, tumor size did not significantly impact overall survival. Recently, higher-powered RFA generators and modifications to the electrodes have enabled an increase in ablation size. These advances have opened the door to more patients who were previously considered untreatable with RFA[33,34]. In the past 14 years, our team has made great effort to improve the outcome of patients with liver tumors larger than 3 cm. In our study, the overall 5-, 10- years survival rates for HCC patients with a tumor size of 3-5 cm were 47.3% and 25.2%, respectively, which appeared fairly good. Additionally, the fairly good outcome for tumors larger than 3 cm was likely because we repeated RFA when the tumor progressed at a limited stage. It should be noted that tumor size influenced the local progression. Therefore, combination therapy was recommended by some studies to improve completed ablation of larger tumors.
Like tumor size, the number and distribution of the tumors represented the biological behavior of these tumors. When more than one tumor was observed in the liver, intrahepatic metastasis or multicentric HCC was likely to occur. Regarding to tumor location, tumors in risky locations reduced local progression-free survival. Tumor location near important structures easily resulted in incomplete ablation or complications, which influenced survival.
Not surprisingly, a high serum AFP level was predictive of HCC recurrence and poor prognosis. In the present cohort, the impact of AFP levels on the prognosis after RFA therapy demonstrated that patients with high serum AFP levels had a significantly poorer overall survival and a reduced local progression-free survival. Yamamoto et al[35] suggested that serum AFP levels may reflect tumor biology, thereby determining the prognosis of HCC patients after therapy. This implies that monitoring serial serum AFP levels before and after therapy is mandatory for HCC patients undergoing RFA. Our result also showed that well to moderately differentiated HCC had a better prognosis than poorly differentiated HCC, most likely because poorly differentiated HCC tends to grow more aggressively, resulting in a poorer prognosis.
In addition to tumor factors and patient factors, procedural factors were prognostically analyzed in this study. In a univariate analysis of the prognostic factors for overall survival, CEUS application was determined to be helpful in improving outcome after RFA. Solbiati et al[36] first reported their experience in using CEUS in RFA treatment and pointed out CEUS represented a significant improvement in the detection of tumors, in the selection of patients and in all of the steps of tumor ablative treatment. In our previous study[37], the complete necrosis rate after RFA treatment in CEUS group was greater than in control group (92.2% vs 83.0%, P = 0.036), which may be due to the ability of CEUS to have a higher sensitivity for identifying small tumors, tumor invasive range and feeding vessel before treatment.
With the univariate analysis, our study also found that the type of RFA electrodes had an effect on the local progression-free survival. Single electrodes (both internally cooled and multipolar) had some advantages over an umbrella electrode, such as easy operation, more ideal visualization of the needle tip, and a thinner needle diameter, particularly for the treatment of tumors in challenging locations. However, the choice of optimal equipment should be determined based on the tumor condition and the experience of the operator.
There were several limitations to our study. The source of study population in this study may result in selection bias, as we are a specialization unit, more patients with unfavorable tumor conditions for RFA may have been referred to us than to other centers. Therefore, caution is required when extrapolating our findings to the general population of HCC patients. Additionally, this study was a 10-year retrospective investigation, and the data obtained in the early years and in later years were analyzed together. Therefore, it is acknowledged that the better survival results observed in the later years may be partly due to our experience and because RFA equipment had improved. Additionally, anti-virus therapy has been a focus as a method to prolong survival in HCC patients in recent years and should be further analyzed in the next study.
In conclusion, this long-term follow-up study on a large group of HCC patients confirmed that RFA can achieve favorable outcomes in HCC patients as a first-line treatment, especially for patients with Child-Pugh class A, a single tumor, and without portal vein hypertension. Understanding the prognostic factors, including tumor characteristics, liver function Child-Pugh classification, and application of CEUS prior to RFA, are critical for patient care.
We are grateful to Yang Lei, PhD, Beijing Office for Cancer Prevention and Control, Peking University Cancer Hospital, for reviewing the statistical methods used for this study.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, causing more than 500000 deaths each year. Clinical evidence has confirmed the efficacy of radiofrequency ablation (RFA) for the treatment of localized HCC. The promising and optimistic short- and mid-term outcomes for RFA in HCC have been widely reported; however, the studies on the long-term outcomes of a 10-year follow-up are limited. RFA was not the sole first-line treatment in some of the 10-year studies. Additionally, this study showed the long-term outcomes of RFA as a first-line treatment in HCC patients in China in a more heterogeneous population with expanded inclusion criteria that included a larger number of treated tumors > 3 cm and tumors with poorer Child-Pugh class scores.
In this study, the overall 5- and 10-year survival rates for HCC patients with tumors of 3-5 cm were 47.3% and 25.2%, which appears to be fairly good despite tumor size being a prognostic factor for local progression-free survival through univariate analysis. Tumor size did not significantly affect the overall survival in a multivariate analysis. In addition to tumor factors and patient factors, procedural factors were prognostically analyzed in this study. Contrast-enhanced ultrasound application was found to be helpful in improving the overall survival after RFA, and the type of RFA electrodes had an effect on local progression-free survival.
Thus far, only a few studies have covered 10-year outcomes with a sufficient number of HCC patients to draw firm conclusions. This study showed the long-term outcome of RFA in 316 HCC patients, including a more heterogeneous population with expanded inclusion criteria that included a larger number of treated tumors > 3 cm and tumors with a poorer Child-Pugh class scores as a first-line treatment. Follow-up ranged from 12 to 158 mo (median: 72.5 mo) with 5-, 7-, and 10-year overall survival and local progression-free survival rates of 49.7%, 41.1%, and 28.4% and 42.7%, 35.1%, and 19.5%, respectively.
These 10-year survival outcomes after percutaneous RFA of HCC appear to be comparable to survival outcomes after surgical resection. Understanding the prognostic factors, which include tumor characteristics, liver function Child-Pugh classification, and application of contrast ultrasound before RFA, is critical for patient care, and this study is helpful in establishing a treatment protocol for HCC with ultrasound-guided RFA.
RFA - a medical procedure in which part of the electrical conduction system of the heart, tumor or other dysfunctional tissue is ablated using the heat generated from high-frequency alternating current. The radiofrequency waves passing through the electrode increase the temperature within the tumor tissue, which results in tumor destruction. First-line treatment: the initial, preferred, or best treatment for a disease. It is often the therapy that combines the best efficacy with the best safety profile and/or the lowest cost.
This is an interesting paper. This monocentric, large experience shows us that RFA is a good first-line treatment option in HCC patients. The analysis of the prognostic factors is also important. The authors have described their retrospective experience with the use of RFA in a large group of patients with HCC, and with a significant long-term follow-up. This is an important experience in the field of HCC therapy.
P- Reviewer: Colnot S, Grieco A, Hashimoto N S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Liu XM
| 1. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 2. | Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248-S260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 4. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 6. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 7. | Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, Vallone P, Mastro A, Fornage BD, Curley SA. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12:616-628. [PubMed] |
| 8. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [PubMed] |
| 9. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 10. | Peng ZW, Zhang YJ, Chen MS, Lin XJ, Liang HH, Shi M. Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J Surg Oncol. 2010;36:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 12. | Zwiebel WJ. Sonographic diagnosis of hepatic vascular disorders. Semin Ultrasound CT MR. 1995;16:34-48. [PubMed] |
| 13. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, Zhang H, Wu W. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428-436. [PubMed] |
| 15. | Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, Yin SS, Zhang H, Poon RT. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006;17:671-683. [PubMed] |
| 16. | Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, Zhang ZY, Wang S, Chen MH. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J Gastroenterol. 2015;21:1554-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377-S390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Goldberg SN, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan JP. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364-1373. [PubMed] |
| 21. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 22. | Kim SH, Choi SB, Lee JG, Kim SU, Park MS, Kim do Y, Choi JS, Kim KS. Prognostic factors and 10-year survival in patients with hepatocellular carcinoma after curative hepatectomy. J Gastrointest Surg. 2011;15:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Giuliante F, Ardito F, Pinna AD, Sarno G, Giulini SM, Ercolani G, Portolani N, Torzilli G, Donadon M, Aldrighetti L. Liver resection for hepatocellular carcinoma ≤3 cm: results of an Italian multicenter study on 588 patients. J Am Coll Surg. 2012;215:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, Tso WK, Fan ST, Poon RT. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Hsieh CB, Chang HM, Chen TW, Chen CJ, Chan DC, Yu JC, Liu YC, Chang TM, Shen KL. Comparison of transcatheter arterial chemoembolization, laparoscopic radiofrequency ablation, and conservative treatment for decompensated cirrhotic patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:505-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Kim YK, Kim CS, Chung GH, Han YM, Lee SY, Jin GY, Lee JM. Radiofrequency ablation of hepatocellular carcinoma in patients with decompensated cirrhosis: evaluation of therapeutic efficacy and safety. AJR Am J Roentgenol. 2006;186:S261-S268. [PubMed] |
| 28. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 29. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2873] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 30. | Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 31. | Lupo L, Panzera P, Giannelli G, Memeo M, Gentile A, Memeo V. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford). 2007;9:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, Dela Torre A, Cho KC, Contractor D, Korogodsky M. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, Zhang YQ, Lau WY. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology. 2007;244:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Yamamoto K, Imamura H, Matsuyama Y, Hasegawa K, Beck Y, Sugawara Y, Makuuchi M, Kokudo N. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16:2795-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and control of percutaneous treatments with contrast-enhanced ultrasound. Eur Radiol. 2003;13 Suppl 3:N87-N90. [PubMed] |
| 37. | Chen MH, Yang W, Yan K, Dai Y, Wu W, Fan ZH, Callstrom MR, Charboneau JW. The role of contrast-enhanced ultrasound in planning treatment protocols for hepatocellular carcinoma before radiofrequency ablation. Clin Radiol. 2007;62:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |