Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.2960
Peer-review started: March 27, 2015
First decision: May 18, 2015
Revised: August 12, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: March 14, 2016
Processing time: 345 Days and 1.4 Hours
AIM: To search for a new chronic pancreatitis model in mice suitable for investigating the pathophysiological processes leading to pancreatic fibrosis.
METHODS: The mice were randomly divided into 2 groups (n = 50), control group and model group. The mice in model group were given ethanol (10%) in drinking water after injection of dibutyltin dichloride (DBTC) (8 mg/kg BW) in tail vein. The mice in control group were injected with only solvent into tail vein (60% ethanol, 20% glycerine and 20% normal saline) and drank common water. At days 1, 7, 14, 28, and 56 after application of DBTC or solvent, 10 mice in one group were killed at each time point respectively. Blood was obtained by inferior vena cava puncture. The activity of amylase, concentration of bilirubin and hyaluronic acid in serum were assayed. The pancreas was taken to observe the pancreatic morphology by HE staining, and to characterize the pancreatic fibrosis by Masson staining. The expression of F4/80, CD3 and fibronectin (FN) were assayed by immuno-histochemistry or Immunofluorescence technique. Collagen type I (COL1A1) in pancreas were detected by Western blot. The expression of matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) mRNA in the pancreas was assessed by real time PCR.
RESULTS: DBTC induced an acute edematous pancreatitis within 1 d. The dilated acini, scattered acinar cell necrosis, and inflammatory cells were found at day 7. Extensive infiltration with inflammatory cells following deposition of connective tissue was observed at day 14. At day 28, level of pancreatic fibrosis was aggravated. The pancreatic tissue was replaced by an extended interstitial fibrosis at the end of 2 mo. There was significant difference in the level of amylase, bilirubin and hyaluronic acid in serum between control group and model group (P < 0.05). The level of COL1A1 and FN in pancreas increased. The expression of MMP-1 mRNA in pancreas decreased, but TIMP-1 mRNA increased at model group.
CONCLUSION: DBTC joint Ethanol drinking can induce chronic pancreatitis in accordance with the pathophysiological modification of human. DBTC joint Ethanol-induced pancreatitis in mice is an effective and handy experimental method. The model is suitable to study the mechanism of pancreatic fibrosis in chronic pancreatitis.
Core tip: It was assured that the model with chronic fibrotic lesions in pancreas was induced by single intravenous injection of dibutyltin dichloride (DBTC) and additional daily ethanol ingestion. But until now, all experiments about the animal model induced by DBTC just have been performed in rat. However, there are some differences in the structure of bile ducts between rat and mouse. As we known, rat has no gall bladder, but mouse’s gall bladder structure is similar to humans’. So we adopted DBTC injection joint long-term consumption of ethanol to observe whether chronic pancreatitis could be induced in mice and the related pathophysiology.
- Citation: Zhang H, Liu B, Xu XF, Jiang TT, Zhang XQ, Shi YL, Chen Y, Liu F, Gu J, Zhu LJ, Wu N. Pathophysiology of chronic pancreatitis induced by dibutyltin dichloride joint ethanol in mice. World J Gastroenterol 2016; 22(10): 2960-2970
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/2960.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.2960
Chronic pancreatitis (CP) is an intractable disease of the pancreas, which typically presents with attacks of severe upper abdominal pain. CP is characterized by an irreversible, irregular fibrosis and chronic continuing inflammation, and permanent loss of exocrine and endocrine functions[1,2]. The distribution of extracellular matrix (ECM) components has been shown in pancreas, indicating an increased deposition of disorganized matrix components in chronic pancreatitis[3,4]. The mechanisms by which this fibrotic process occurs are not fully understood.
A variety of etiologic factors have been described for the development of human chronic pancreatitis. In recent years, biliary tract diseases and alcoholism have been regarded as the leading causes of chronic pancreatitis[5-7]. In Sparmann’s experiment[8], the rats were given a single intravenous application of dibutyltin dichloride (DBTC) (8 mg/kg body weight) to induce pancreatitis. After 24 h of DBTC treatment, they found pancreatic edema and distention of the biliopancreatic duct. The microscopic result showed administration of DBTC induced destruction of duct epithelial cells and that the clotting by necrotic cells caused an obstruction of the biliopancreatic duct.
Chronic ingestion of alcohol is well-known for the pathogenesis of human chronic pancreatitis,but the ingestion of alcohol alone over a long time in animals does not induce chronic pancreatitis[9,10] and our primary experiment. In Merkord’s study[11], they found an additional intake of ethanol (15% in drinking water ad libitum) increased the toxic effects of DBTC on pancreas and led to a stronger fibrosis in pancreas. In rats, it was assured that the model with chronic fibrotic lesions in pancreas was induced by single intravenous injection of DBTC and additional daily ethanol ingestion. But in mouse, the model of chronic pancreatitis was usually induced with cerulein for a long and repetition injection[12,13]. It must cost a good deal to induce the model. As a good method, DBTC/ethanol not only mimics the main causes of human chronic pancreatitis, but also is effective, economic, and handy. However, as we known, there are some differences in the structure of bile ducts between rat and mouse[14]. So it is not clear whether DBTC/ethanol can induce the model of chronic pancreatitis in mice and its pathophysiology is similar to human.
In the present experiment, the influence of DBTC joint ethanol on pancreatic lesion in mice was investigated. By assaying the pathophysiological change of pancreas, we look forward to search for a new chronic pancreatitis model in mouse suitable for investigating the pathophysiological processes leading to pancreatic fibrosis.
KM mice (20-25 g) obtained from Experimental Animal Center of 4th Medical University of PLA. (Grade SPF IICertificate No. 2005-005) were fasted but free access to water for 12 h. All experiments were performed in accordance with the animal care and handling guidelines of the Committee on Animal Care of Shaanxi Province. The antibodies against F4/80, CD3, COL1A1 and FN were purchased from Santa Cruz Biotechnology Inc. or Boster Biotechnology Inc. The secondary antibodies and kits for immunohistochemistry or immunofluorescence were provided by Boster Biotechnology Inc., China. DBTC was purchased from Sigma-Aldrich Co (Lot.205494). The Kits for amylase bilirubin and hyaluronic acid obtained from Biotechnology Company. Q-PCR kit and primers were provided by Transgen Biotech Co, China. All other chemicals were purchased from local source at the highest purity available.
The mice were randomly divided into 2 groups, namely control group and model group (n = 50). The mice in model group were injected DBTC solution (DBTC was solved in 60% ethanol, 20% glycerine and 20% normal saline, the concentration was 3.2 μg/μL, 25 μL/10 g BW) into tail vein with a insulin syringe at a dose of 8 mg/kg of body weight[8]. The time of injection must exceed over 1 min. After injection of DBTC, the mice were given ethanol (10%) in drinking water. The mice in control group were injected only solvent (60% ethanol, 20% glycerine and 20% normal saline) into tail vein and drunk common water.
The mice were sacrificed at days 1, 7, 14, 28, and 56 after application of DBTC or solvent (n = 10 at each time point). Blood samples were obtained by inferior vena cava puncture and centrifuged at 4 °C, and then serum was stored at -80 °C for amylase, bilirubin and hyaluronic acid analysis. The activity of amylase, concentration of bilirubin and hyaluronic acid in serum were assayed respectively. The pancreas was taken to observe the pancreatic morphology by H&E staining, and to characterize the pancreatic fibrosis by Masson staining. The expression of F4/80, CD3 and FN were assayed by immuno-histochemistry or Immunofluorescence technique. COL1A1 in pancreas was detected by Western blot. The expression of matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) mRNA in the pancreas was assessed by real time PCR.
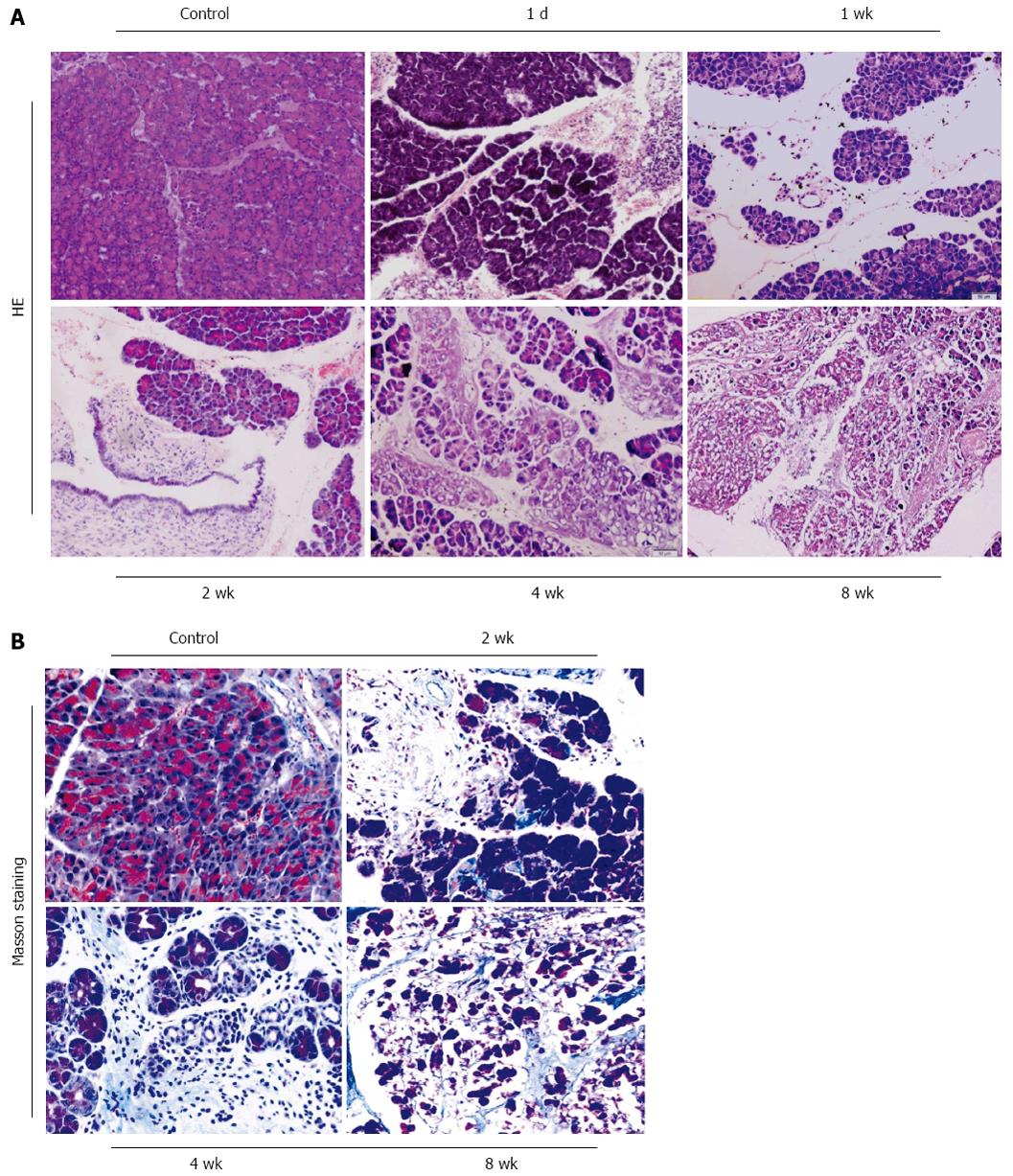
For morphological analyses, at days 1, 7, 14, 28, and 56, pancreas were removed, immediately immersed in 4% neutral phosphate-buffered paraformaldehyde for 12 h, embedded in paraffin, and sectioned (5 μm). The sections of the specimens were stained with HE or Masson staining, then observed the morphological changes and fibrosis in pancreas under a light microscope.
Immunohistochemical or Immunofluorescence analyses were performed according to standard protocols. For Immunohistochemistry, antibodies were given in the following concentrations. 1:300 dilution of F4/80 antibody (Santa Cruz Biotechnology Inc. sc-71088) or CD3 antibody (Biosynthesis Biotechnology Inc. bs-10498R) was diluted in blocking solution in a humid chamber overnight at 4 °C; 1:500 dilution of a biotinylated secondary antibody for 40 min; the bound peroxidase was visualized by reaction for 2-5 min in a solution containing 50 mg of 3,3-diaminobenzidine (DAB); counterstained with hematoxylin, dehydrated, and mounted. For Immunofluorescence, 1:300 dilution of FN antibody (Boster Biotechnology Inc. BA-1772) was incubated in a humid chamber overnight 4 °C in the dark; 1:500 dilution of FITC labeled secondary antibody for 30 min; counterstained with DAPI (1:10000) for 30 s, dehydrated and mounted coverslip with anti-fade mounting medium. The slides for F4/80 or CD3 were examined under a light microscope. Bright-field images were acquired using a Zeiss Axio1 imager with an AxioCam HRc (Zeiss, Germany). For FN, the slides were examined under a fluorescent microscope. Dark-field images were acquired using a Olympus imager (Olympus, Japan).
For western blotting analysis, cellular proteins were prepared from pancreas by standard methods. Protein concentration were determined (Bio-Rad Protein Assay) and adjusted to 4 μg/μL. The samples were boiled at 95 °C for 5 min before loading, and then subjected to SDS-polyacrylamide gel electrophoresis and blotted to PVDF membranes. The membranes were probed with the following antibodies: anti- COL1A1 (Santa Cruz Biotechnology Inc. sc-25974). A low-molecular weight protein marker was used to determine the size of detected bands in the western blot.
RNA was extracted from pancreatic tissue (RNeasy minicolumns; Transgen, China) and transcribed into complementary DNA using Oligo (dT) Primer and SuperScript II reverse transcriptase (both Transgen, China). For quantitative polymerase chain reaction (Q-PCR), 100 ng complementary DNA was used for each reaction and amplified. Primers, number of cycle, and annealing temperature are given in Table 1. Reverse-transcription polymerase chain reaction was performed using a SYBR Green reaction mix. The assay was performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). All experiments were performed in triplicates, and expression levels were normalized to β-actin and calculated using the 2-ΔΔCt method. Primer Sequences were shown in Table 1.
| Gene | Cycles | Tm (°C) | Forward primer | Reverse primer |
| MMP-1 | 40 | 60 | 5-GTG AAT GGC AAG GAG ATG ATG G-3 | 5-ACG AGG ATT GTT GTG AGT AAT GG-3 |
| TIMP-1 | 40 | 60 | 5-CAT CTC TGG CAT CTG GCA TCC-3 | 5-CGC TGG TAT AAG GTG GTC TCG-3 |
| β-actin | 40 | 60 | 5-ACC ACA CCT TCT ACA ATG AG-3 | 5-ACG ACC AGA GGC ATA CAG-3 |
Data were expressed with means ± SD and compared by the non paired Student t test and one-way analysis of variance.
We invited an expert in Biomedical Statistics to evaluate the statistical method in our manuscript. The statement of statistical analyses was shown in the Materials and Methods section. We have explained the bio-statistical methods from our experimental data in the Figure legend.
One day after DBTC injection, an acute edematous pancreatitis was induced. One week after model, acute pancreatitis had developed with mild infiltration of neutrophilic granulocytes, acinar cell edema and necrosis. At day 14, the pancreatic duct showed extreme dilatation, edema and a partial necrosis of the surface epithelium, infiltration of lymphocytes and macrophages following deposition of connective tissue in pancreas was observed. At 4 wk to 8 wk, level of pancreatic fibrosis was aggravated. The pancreatic tissue was characterized by the lost of acinar cells and islets, replacement of an extended interstitial fibrosis at the end of 2 mo (Figure 1A, × 100). Collagen deposition increased significantly and was found by Masson staining in the model group compared with the control group (Figure 1B, × 200). In model group, the mortality rate was about 30.4% and 8 wk after administration of DBTC and ethanol, 71.4% (10/14) of the survival mice showed periductal and interstitial fibrosis as well as an infiltration of inflammatory cells in the pancreas.
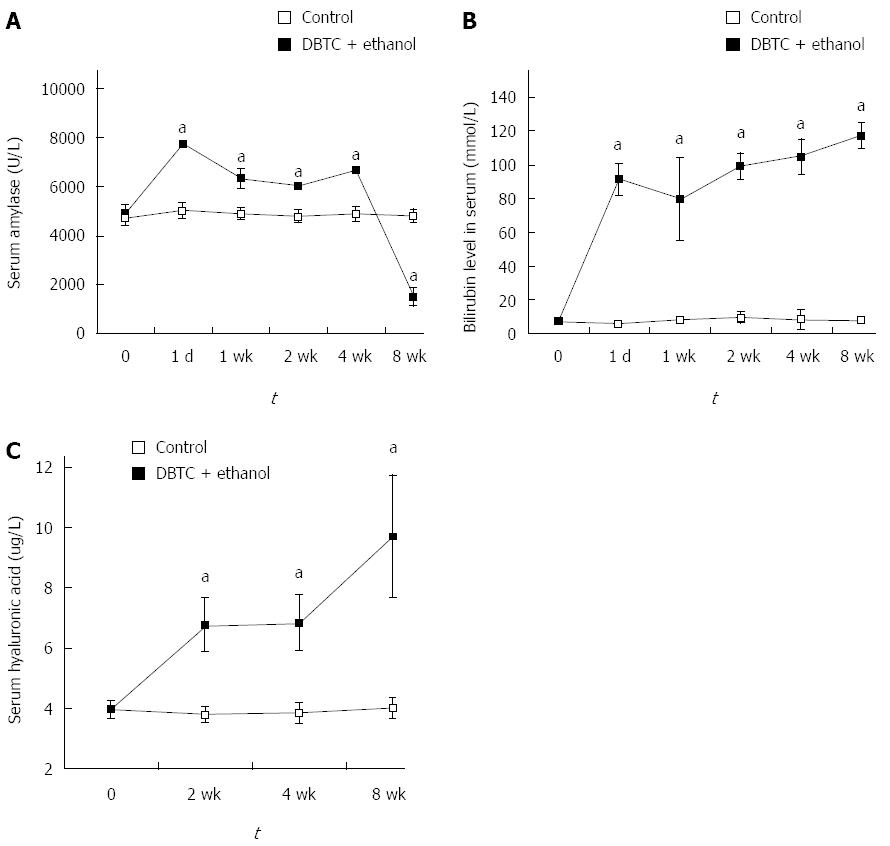
The activity of amylase was increased after 24 h. At 1 wk, the activity of amylase less decreased, but still did not return to normal value and remained the elevated level until 4 wk. At 8 wk after DBTC and ethanol application, with the development of pancreatic fibrosis and loss of pancreatic tissue, the activity of amylase declined sharply (Figure 2A). Bilirubin level in serum was also increased from 1 d to 8 wk. This finding indicated that the pathogenetic mechanism of the model included liver injury and obstruction of biliopancreatic duct (Figure 2B). The serum concentration of ECM component hyaluronic acid was found to be increased at 2 wk, 4 wk and went up more at 8 wk after DBTC and ethanol application (Figure 2C). There were significant differences in the level of amylase, bilirubin and hyaluronic acid in serum between control group and model group (P < 0.01).
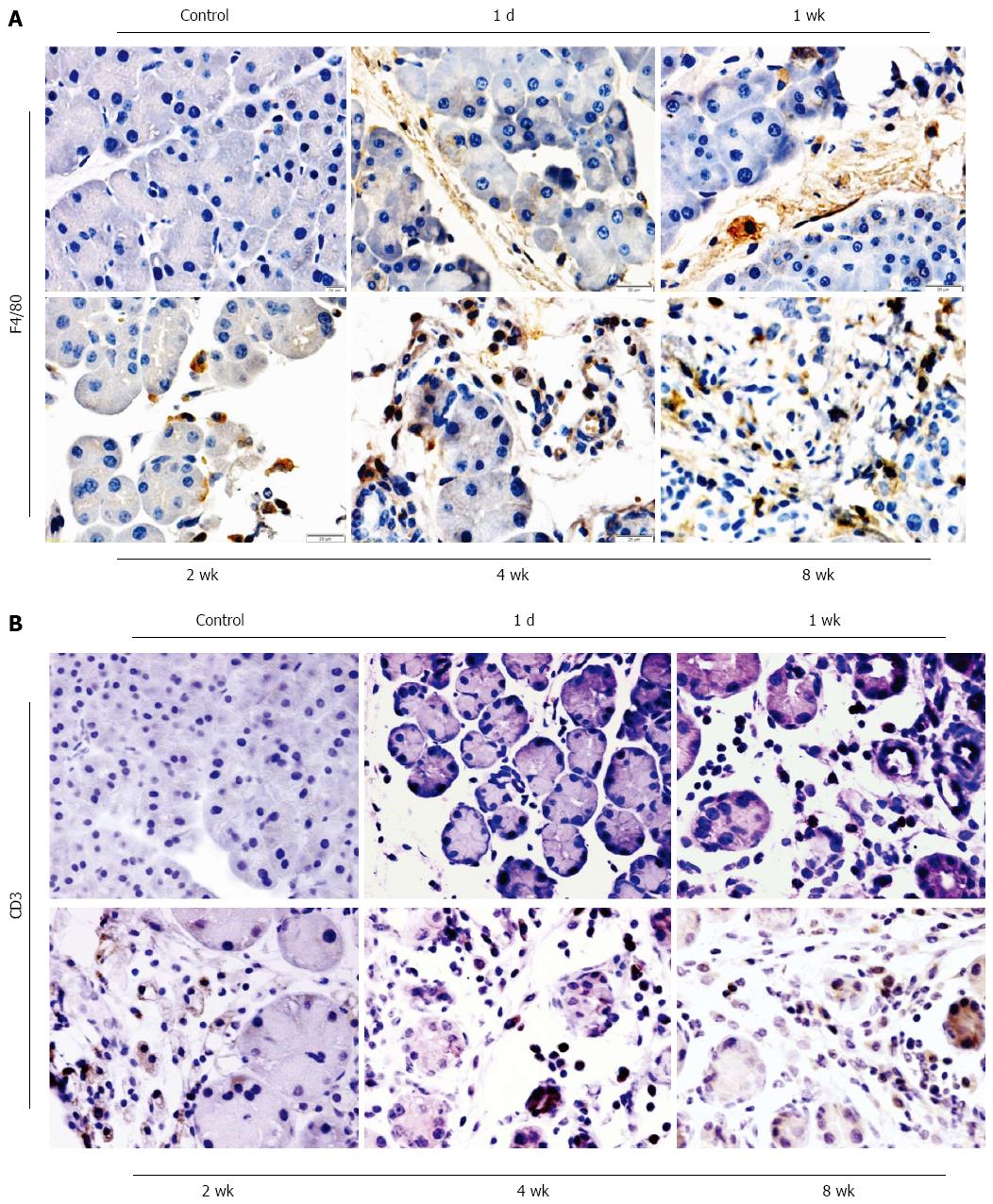
The infiltration of inflammatory cells in the pancreas of mice with pancreatitis induced by DBTC and ethanol were shown in Figure 3. One day after DBTC application, the leukocytes infiltrating in pancreas were identified as polymorphonuclear leukocytes and a few macrophages marked with F4/80 (Figure 3A). The T-lymphocyte marker CD3 could not be detected on the first day after DBTC application (Figure 3B). But 7 d after DBTC and ethanol application, the number of infiltrating T-lymphocyte increased, the granulocytic infiltration decreased. After 2 wk, during the following several weeks, there was a marked increase of macrophages and T lymphocytes. The positive ratio of macrophage and T lymphocytes reached the highest level at 4 wk, and sustained the high level at 8 wk. The result showed that macrophages and T-lymphocytes were the predominant population of inflammatory cells during the development of chronic pancreatitis.
The expression of fibronectin (FN) and collagen type I (COL1A1) in the mouse pancreas were assessed by Immunofluorescence and Western blot analysis respectively.
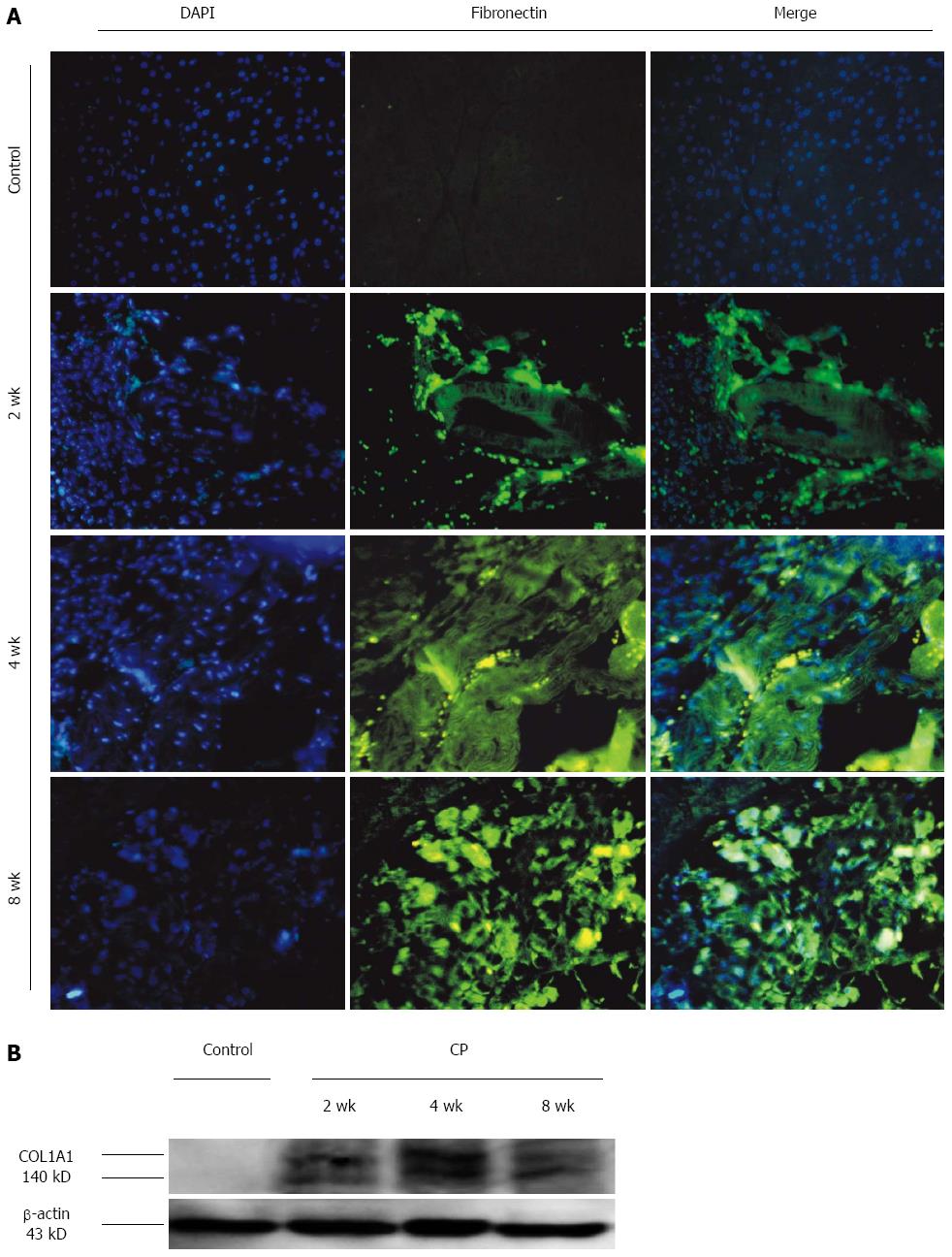
As shown in Figure 4A, there was no FN expression in the pancreas of control group. DBTC and ethanol administration led to an obvious expression of FN in pancreas. The time course of the induction showed a weak level on 1 d and 1 wk. Subsequently, the FN expression increased markedly at 2 wk and remained elevated level during the observation period.
In Figure 4B, the expression of COL1A1 was shown during the DBTC pancreatitis. The expression level of COL1A1 was corrected with β-actin. There was no COL1A1 expression in the control group. The relative expression of COL1A1 was elevated at 2 wk and remained elevated level during the observation period.
These findings were in agreement with the morphological alterations in the pancreas. The single intravenous application of DBTC and continuous ethanol drinking could induce a typical morphological modification from acute interstitial pancreatitis with pancreas edema, acinar cells necrosis, to chronic pancreatitis with persistent inflammation and permanent fibrosis in pancreas of mouse.
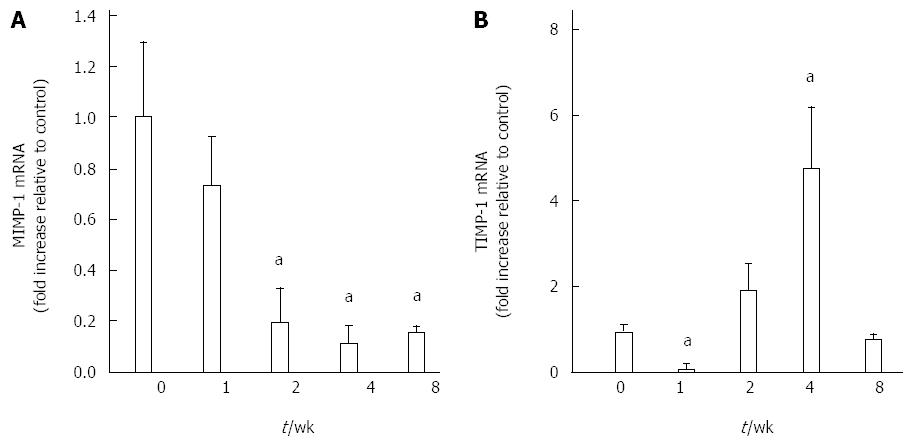
As shown in Figure 5, the expression of MMP-1mRNA and TIMP-1 mRNA in the pancreas was assessed by real-time PCR. Decreased MMP-1 levels were detected in pancreas samples obtained from mice with CP induced by DBTC and ethanol. Compared with the control group, the MMP-1 expression level decreased at 1 wk and went down more at 2 wk and sustained the low level at 4 as well as 8 wk in model group (P < 0.01).
Figure 5B showed the TIMP-1 mRNA expression level. One week after DBTC and ethanol application, TIMP-1 mRNA decreased significantly (P < 0.05). But it suddenly returned to a higher level at 2 wk and went up more at 4 wk (P < 0.05). At 8 wk, the TIMP-1 mRNA level went back to near normal.
Chronic Pancreatitis (CP) is a common disease which caused by biliary disorders or long-term alcohol drinking and showed specific pathological manifestations including localized or diffuse chronic inflammation in pancreatic parenchyma, and other irreversible damage in pancreatic acini and islet cell[15,16]. The shrink or disappear of pancreatic parenchyma causes significant clinical manifestations which is characterized by different degrees of disorder for pancreatic exocrine and endocrine function[17,18]. The patient’s life quality will be influenced seriously. Although the etiology of chronic pancreatitis is various, the eventual pathological change keeps in agreement. The sustained progress in chronic inflammation leads to atrophy in pancreatic parenchyma gradually and be replaced by fibrous tissue eventually. Progressive pancreatic fibrosis has been looked as the main pathological features.
According to the common clinical etiology,some experiment mimicked the inducer to make chronic pancreatitis in animals[19,20]. DBTC is a fat-soluble substances and excreted from liver, gallbladder to pancreatic duct after intravenous injection[11,21]. By drainage, pancreatic duct epithelial cells are damaged and cell necrosis appear. Damaged epithelium cells will be gathered, then caused the obstruction of pancreatic duct[8]. It mimics the pathologic progress of biliary tract diseases which is accompanied by pancreas and bile duct obstruction. In rats[22,23], some experiments showed administration of DBTC induced destruction of duct epithelial cells and that the clotting by necrotic cells caused an obstruction of the biliopancreatic duct and occurred chronic pancreatitis. At the same time adding ethanol into drinking water aggravated the toxic effects of DBTC on pancreas and lead to a stronger pancreatic fibrosis[24]. It was assured that the model with chronic fibrotic lesions in pancreas was induced by single intravenous injection of DBTC and additional daily ethanol ingestion. But until now,all experiments about the animal model induced by DBTC just have been performed in rat[8,11,22,23]. Because of the differences in the structure of pancreas and bile ducts between rat and mouse, it is not clear whether DBTC/ethanol can induce the model of chronic pancreatitis in mice. As we known, rat has no gall bladder, but mouse’s gall bladder structure is similar to human’s. So we adopted DBTC injection joint long-term consumption of ethanol to observe whether chronic pancreatitis could be induced in mice.
Our results showed that acute pancreatitis had developed with mild infiltration of neutrophilic granulocytes, acinar cell edema and necrosis at 1 wk. At 2 wk, we observed extreme dilatation of pancreatic duct, infiltration of lymphocytes and macrophages, as well as deposition of fiber. With the development of injury, pancreatic tissue was lost and replaced by irregular extended interstitial fibrosis at the end of 2 mo. The expression of FN and COL1A1 in pancreas, serum concentration of ECM component hyaluronic acid increased markedly at 2 wk and remained elevated level during the observation period. At the early stage, acute pancreatic injury incurred the overdue activation of amylase in serum. But at 8w, with the development of pancreatic fibrosis and loss of pancreatic tissue, the activity of amylase declined sharply. It implied that pancreatic exocrine was destroyed seriously. The morphological alterations in the pancreas and biological parameters suggested that the single intravenous application of DBTC and continuous ethanol drinking could induce a typical morphological modification from acute interstitial pancreatitis with pancreas edema, acinar cells necrosis, to chronic pancreatitis with persistent inflammation and permanent fibrosis in pancreas of mouse.
During the whole observation period, bilirubin level in serum was increased. The result indicated that the pathogenetic mechanism of the model included liver injury and obstruction of common biliopancreatic duct. Combined with the morphological alteration in extreme dilatation and epithelia cells necrosis of pancreatic duct, it was assured that DBTC induced destruction of duct epithelial cells and the clotting by necrotic cells caused an obstruction of the biliopancreatic duct and chronic pancreatitis.
Matrix metalloproteinases (MMPs) are a group of peptide enzyme depended on the zinc ions. Its main function is to degrade the ECM composition including collagen or fiber connection protein[25]. Tissue inhibitor of metalloproteinases (TIMPs) are the most important family of molecules involved in regulation of extracellular MMP activity. TIMPs are produced by a wide variety of cells and often cosecreted with MMPs, providing local autoregulation of MMP activity. The activity of MMP can be inhibited by TIMPs[26]. There are 14 kinds of MMPs and 4 kinds of TIMPs have been found. The most important representative is MMP-1 and TIMP-1 respectively. MMP-1specifically degrades type I collagen, which is a major component of the extracellular matrix, as well as other types of collagens. Recent studies considered that it was accompanied with MMP-1 down-expression and TIMP-1 over-expression during the progress of fibrosis in liver or pancreas[27-29]. In this experiment, we induced chronic pancreatic fibrosis and assessed the level of MMP-1 mRNA and TIMP-1 mRNA in the mouse pancreas. MMP-1 expression level decreased at 1 wk and went down more at 2 wk, sustained the low level at 4 and 8 wk in model group. One week after DBTC and ethanol application, TIMP-1 mRNA decreased significantly. But it suddenly returned to a higher level at 2 wk and went up more at 4 wk. At 8 wk, the TIMP-1 mRNA level went back to near normal. As we known, the function of MMP-1 plays an important role in degrading the ECM composition[30]. Its low level leads to the low ability for degrading the ECM. TIMP-1 over-expression inhibited the activity of MMP-1, further weakened the capability of MMP-1 degrading type I collagen and caused the deposition of ECM in pancreas[31], namely pancreatic fibrosis.
It is worthwhile to note that the technician should master skillful technique for tail vein injection, and injection speed of DBTC must be slowly. As we known, DBTC was dissolved in ethanol and glycerin, so if DBTC solution was leaked into subcutaneous tissue of tail, the far end of tail would be necrosis and fall off. If the injection speed of DBTC was too fast, the sudden death would occur because of pulmonary embolism. In our experiment, the mortality rate was about 30% in model group. Four and eight weeks after administration of DBTC and ethanol, 71.4% of the survival mice showed periductal and interstitial fibrosis as well as an infiltration of inflammatory cells in the pancreas. Our results suggested that single intravenous application of DBTC and continuous ethanol drinking can induce chronic pancreatitis in accordance with the pathophysiological modification of human. DBTC joint Ethanol-induced pancreatitis in mice is an effective and handy experimental method. The model is suitable to study the mechanism of pancreatic fibrosis in chronic pancreatitis.
Biliary tract diseases and alcoholism have been regarded as the leading causes of chronic pancreatitis. It was assured that the model with chronic fibrotic lesions in pancreas was induced by single intravenous injection of dibutyltin dichloride (DBTC) and additional daily ethanol ingestion in rats.
DBTC is a toxic substance and excreted from biliary and pancreatic duct. It can induced destruction of duct epithelial cells and caused an obstructive chronic pancreatitis. Ethanol aggravated the toxic effects of DBTC on pancreas and lead to a stronger pancreatic lesion.
There are some differences in the structure of bile ducts between rat and mouse. So it is not clear whether DBTC/ethanol can induce the model of chronic pancreatitis in mice. By assaying the pathophysiological change of pancreas, the authors look forward to search for a new chronic pancreatitis model in mice to investigate the pathophysiological processes leading to pancreatic fibrosis.
Single intravenous application of DBTC and continuous ethanol drinking can induce chronic pancreatitis in accordance with the pathophysiological modification of human. DBTC joint Ethanol-induced pancreatitis in mice is an effective and handy experimental method. The model is suitable to study the mechanism of pancreatic fibrosis in chronic pancreatitis.
DBTC is a fat-soluble substance and excreted from liver, gallbladder to pancreatic duct after intravenous injection. By drainage, pancreatic duct epithelial cells were damaged and cell necrosis appeared. Damaged epithelium cells gathered, then caused the obstruction of pancreatic duct. It mimics the pathologic progress of biliary tract diseases which is accompanied by pancreas and bile duct obstruction.
The authors describe a mouse model of pancreatic fibrosis induced by a single DBTC injection associated with ad libitum alcohol consumption. This model has been used to experimental studies in rats but not in mice. They reproduce progressive histological and biochemical pancreatic changes after acute pancreatitis. The methods are well described and suitable to characterize the pathological events in the pancreas. The results are well presented and the original aim is accomplished: a chronic pancreatitis model in mice is described. The model permits to investigate the progression of acute pancreatitis towards a chronic process.
P- Reviewer: Berger Z, Kaplan M S- Editor: Kong JX L- Editor: A E- Editor: Liu XM
| 1. | Milosavljevic T, Kostic Milosavljevic M, Krstic M, Jovanovic I. Classification of chronic pancreatitis. Dig Dis. 2010;28:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon. 2014;60:530-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Shimizu K. Mechanisms of pancreatic fibrosis and applications to the treatment of chronic pancreatitis. J Gastroenterol. 2008;43:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Brock C, Nielsen LM, Lelic D, Drewes AM. Pathophysiology of chronic pancreatitis. World J Gastroenterol. 2013;19:7231-7240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Ansari D, Andersson E, Andersson B, Andersson R. Chronic pancreatitis: potential future interventions. Scand J Gastroenterol. 2010;45:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Camara SN, Ramdany S, Zhao G, Gou SM, Xiong JX, Yang ZY, Yin T, Yang M, Balde OT, Barry AB. Etiology, pathology, management and prognosis of chronic pancreatitis in Chinese population: A retrospective study. J Huazhong Univ Sci Technolog Med Sci. 2015;35:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Sparmann G, Merkord J, Jäschke A, Nizze H, Jonas L, Löhr M, Liebe S, Emmrich J. Pancreatic fibrosis in experimental pancreatitis induced by dibutyltin dichloride. Gastroenterology. 1997;112:1664-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Li J, Guo M, Hu B, Liu R, Wang R, Tang C. Does chronic ethanol intake cause chronic pancreatitis?: evidence and mechanism. Pancreas. 2008;37:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Perides G, Tao X, West N, Sharma A, Steer ML. A mouse model of ethanol dependent pancreatic fibrosis. Gut. 2005;54:1461-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Merkord J, Weber H, Sparmann G, Jonas L, Hennighausen G. The course of pancreatic fibrosis induced by dibutyltin dichloride (DBTC). Ann N Y Acad Sci. 1999;880:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Neuschwander-Tetri BA, Burton FR, Presti ME, Britton RS, Janney CG, Garvin PR, Brunt EM, Galvin NJ, Poulos JE. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Treiber M, Neuhöfer P, Anetsberger E, Einwächter H, Lesina M, Rickmann M, Liang S, Kehl T, Nakhai H, Schmid RM. Myeloid, but not pancreatic, RelA/p65 is required for fibrosis in a mouse model of chronic pancreatitis. Gastroenterology. 2011;141:1473-1485, 1485.e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Tian XY, Sun JF. Placket of pancreatic ducts and collection of pancreas secretions in rodent animals. Acta laboratorium animalis scientia sinica. 2005;8:255-258. [DOI] [Full Text] |
| 15. | DiMagno EP. A short, eclectic history of exocrine pancreatic insufficiency and chronic pancreatitis. Gastroenterology. 1993;104:1255-1262. [PubMed] |
| 16. | Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19:7276-7281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (3)] |
| 17. | D’Haese JG, Ceyhan GO, Demir IE, Layer P, Uhl W, Löhr M, Rychlik R, Pirilis K, Zöllner Y, Gradl B. Pancreatic enzyme replacement therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis: a 1-year disease management study on symptom control and quality of life. Pancreas. 2014;43:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev. 2012;28:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 20. | Aghdassi AA, Mayerle J, Christochowitz S, Weiss FU, Sendler M, Lerch MM. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Merkord J, Weber H, Kröning G, Hennighausen G. Repeated administration of a mild acute toxic dose of di-n-butyltin dichloride at intervals of 3 weeks induces severe lesions in pancreas and liver of rats. Hum Exp Toxicol. 2001;20:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Vera-Portocarrero LP, Lu Y, Westlund KN. Nociception in persistent pancreatitis in rats: effects of morphine and neuropeptide alterations. Anesthesiology. 2003;98:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Liu XQ, Yang ZL, Liang S. DBTC Induced SD Rat Model of Chronic Pancreatitis and Determination of Serum AMS and TNF-αLevels. Practical Preventive Medicine. 2007;14:682-685. [DOI] [Full Text] |
| 24. | Merkord J, Weber H, Jonas L, Nizze H, Hennighausen G. The influence of ethanol on long-term effects of dibutyltin dichloride (DBTC) in pancreas and liver of rats. Hum Exp Toxicol. 1998;17:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Arakaki PA, Marques MR, Santos MC. MMP-1 polymorphism and its relationship to pathological processes. J Biosci. 2009;34:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119-17123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 408] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 27. | Tahara H, Sato K, Yamazaki Y, Ohyama T, Horiguchi N, Hashizume H, Kakizaki S, Takagi H, Ozaki I, Arai H. Transforming growth factor-α activates pancreatic stellate cells and may be involved in matrix metalloproteinase-1 upregulation. Lab Invest. 2013;93:720-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Zhang LJ, Chen YX, Chen ZX, Huang YH, Yu JP, Wang XZ. Effect of interleukin-10 and platelet-derived growth factor on expressions of matrix metalloproteinases-2 and tissue inhibitor of metalloproteinases-1 in rat fibrotic liver and cultured hepatic stellate cells. World J Gastroenterol. 2004;10:2574-2579. [PubMed] |
| 29. | Sri Manjari K, Nallari P, Balakrishna N, Vidyasagar A, Prabhakar B, Jyothy A, Venkateshwari A. Influence of matrix metalloproteinase-1 gene -1607 (1G/2G) (rs1799750) promoter polymorphism on circulating levels of MMP-1 in chronic pancreatitis. Biochem Genet. 2013;51:644-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Li L, Bachem MG, Zhou S, Sun Z, Chen J, Siech M, Bimmler D, Graf R. Pancreatitis-associated protein inhibits human pancreatic stellate cell MMP-1 and -2, TIMP-1 and -2 secretion and RECK expression. Pancreatology. 2009;9:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Qin T, Liu CJ, Zhang HW, Pan YF, Tang Q, Liu JK, Wang YZ, Hu MX, Xue F. Effect of the IkBα mutant gene delivery to mesenchymal stem cells on rat chronic pancreatitis. Genet Mol Res. 2014;13:371-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |