Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.2949
Peer-review started: September 16, 2015
First decision: October 14, 2015
Revised: October 28, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: March 14, 2016
Processing time: 171 Days and 1.9 Hours
AIM: To evaluate the effect of artesunate (AS) supplementation on bacterial translocation (BT) and gut microbiota in a rat model of liver cirrhosis.
METHODS: Fifty-four male Sprague-Dawley rats were randomly divided into a normal control group (N), a liver cirrhosis group (M) and a liver cirrhosis group intervened with AS (MA). Each group was sampled at 4, 6 and 8 wk. Liver cirrhosis was induced by injection of carbon tetrachloride (CCl4), intragastric administration of 10% ethanol, and feeding a high fat diet. Rats in the MA group were intragastrically administered with AS (25 mg/kg body weight, once daily). Injuries of the liver and intestinal mucosa were assessed by hematoxylin-eosin or Masson’s trichrome staining. Liver index was calculated as a ratio of the organ weight (g) to body weight (g). The gut microbiota was examined by automated ribosomal intergenic-spacer analysis of fecal DNA. BT was assessed by standard microbiological techniques in the blood, mesenteric lymph nodes (MLNs), liver, spleen, and kidney.
RESULTS: Compared to group N, the body weight was reduced significantly in groups M and MA due to the development of liver cirrhosis over the period of 8 wk. The body weight was higher in group MA than in group M. The liver indices were significantly elevated at 4, 6 and 8 wk in groups M and MA compared to group N. AS supplementation partially decreased the liver indices in group MA. Marked histopathologic changes in the liver and small intestinal mucosa in group M were observed, which were alleviated in group MA. Levels of pro-inflammatory interleukin-6 and tumor necrosis factor-α were significantly elevated at 8 wk in ileal homogenates in group M compared to group N, which were decreased after AS supplementation in group MA. The dysbiosis of gut microbiota indicated by the mean diversity (Shannon index) and mean similarity (Sorenson index) was severe as the liver cirrhosis developed, and AS supplementation had an apparent intervention effect on the dysbiosis of gut microbiota at 4 wk. The occurrence of BT was increased in the liver of group M compared to that of group N. AS supplementation reduced BT in group MA at 8 wk. BT also occurred in the MLNs, spleen, and kidney, which was reduced by AS supplementation. BT was not detected in the blood in any group.
CONCLUSION: Dysbiosis of gut microbiota, injury of intestinal mucosal barrier and BT occurred as liver cirrhosis progressed, which might enhance inflammation and aggravate liver injury. AS may have other non-antimalarial effects that modulate gut microbiota, inhibit BT and alleviate inflammation, resulting in a reduction in CCl4, alcohol and high fat-caused damages to the liver and intestine.
Core tip: Artesunate (AS) is antimalarial yet potentially anti-inflammatory. We evaluated the effect of AS supplementation on bacterial translocation (BT) and the gut microbiota in a rat model of liver cirrhosis. Dysbiosis of gut microbiota, injury of intestinal mucosal barrier and BT occurred as liver cirrhosis progressed, which might enhance inflammation and aggravate liver injury. AS may have other non-antimalaria effects that modulate gut microbiota, inhibit BT and alleviate inflammation, leading to a reduction in carbon tetrachloride, alcohol and high fat-caused damages to the liver and intestine.
- Citation: Chen YX, Lai LN, Zhang HY, Bi YH, Meng L, Li XJ, Tian XX, Wang LM, Fan YM, Zhao ZF, Han DW, Ji C. Effect of artesunate supplementation on bacterial translocation and dysbiosis of gut microbiota in rats with liver cirrhosis. World J Gastroenterol 2016; 22(10): 2949-2959
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/2949.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.2949
There are a wide variety of micro-organisms in the human gut, which are harmless and have an important role in human nutrition and health, promoting nutrient supply, preventing pathogen colonization, and shaping and maintaining normal mucosa immunity[1]. Under certain circumstances, these normally harmless bacteria pose an imminent threat when intestinal bacteria translocate to extra-intestinal organs[2-5], that is, bacterial translocation (BT). BT is defined as the migration of intestinal flora and/or their toxins into mesenteric lymph nodes (MLNs) and/or other extra-intestinal organs, which can lead to the development of many diseases, such as sepsis, multiple organ dysfunctions and even multiple organ failure[6]. In patients with cirrhosis, BT is the main cause of spontaneous infection, and its incidence is up to 30% in patients with decompensated cirrhosis, which can cause high mortality[7]. Antibacterial prophylaxis is effective in preventing infectious complications. However, increasing antimicrobial resistance makes the patients at high risk, thus non-antibiotic pathways are desperately needed to decrease the risk of bacterial infections by reducing pathological BT as we enter the post-antibiotic era[8].
Phytomedicines, such as artesunate (AS), are one of several non-antibiotic approaches to the treatment and prevention of infection[9]. AS, a water-soluble hemisuccinate derivative of artemisinin extracted from the Chinese herb Artemisia annua, is a safe and effective antimalarial drug[10]. It has been reported that AS could also decrease serum endotoxin levels in sepsis mice[11], and have a significant effect on endotoxin-induced uveitis in rats[12]. AS exerts an anti-inflammatory role, for example, decreasing the expression and secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8[13].
However, it is uncertain whether AS is effective in preventing BT. In this study, we investigated the effect of AS on BT and composition of intestinal bacteria in rats with liver cirrhosis.
Sprague-Dawley (SD) rats (specific pathogen-free grade), weighing 180-220 g, were obtained from the Laboratory Animal Center of Academy of Military Medical Sciences (Peking, China). All animals were cared carefully during the entire period of this study, under a protocol that was in accordance with institutional guidelines for animal research and was approved by the Ethical and Research Committee of Changzhi Medical College (Changzhi, China).
After two weeks of acclimatization, 54 male SD rats were randomly divided into a liver cirrhosis model group (M), a normal control group (N) and a group (MA) of cirrhosis plus AS (JK Scientific, China) intervention. Each group was divided into three subgroups (n = 6) for testing at 4, 6 and 8 wk, which reflected different stages of hepatic cirrhosis development. Liver cirrhosis (M and MA) was induced by multiple pathogenic factors (including subcutaneous injection with carbon tetrachloride oil solution, intragastric administration of 10% ethanol and feeding a mixture of maize flour, lard, and cholesterol) as described previously[14]. Animals in group MA were intragastrically administered with AS (25 mg/kg body weight, once daily). The animals of group N were fed a standard diet, subcutaneously injected with equal volume of oil solution, and intragastrically administered with water. Animals were sacrificed and sampled at 4, 6 and 8 wk.
At every stage of cirrhosis, animal anatomy was performed after 12 h of food deprivation. Animals were weighed and then anesthetized with 3% sodium pentobarbital (0.1 mL/100 g·w), and the skin was disinfected with alcohol. Blood was collected from the abdominal aorta, and the whole liver was removed and weighed. Tissue samples of the MLNs, liver, spleen, kidney and ileum were dissected and removed. The cecal content was collected and stored at -80 °C for microbiota analysis. All operations were performed under strict sterile conditions.
MLN, liver, spleen and kidney specimens were homogenized in sterile saline (1 mL per 0.1 g) using a glass homogenizer, and 100 μL of suspension or blood was cultured on agar plates for 24 h under aerobic conditions and for 72 h under anaerobic conditions, both at 37 °C. Growth of bacteria was considered as evidence of BT. The translocation ratio (TR) was calculated as a ratio of the total number of positive cultures of blood, MLNs and organs to the total number of cultures of blood, MLNs and organs tested in each group. Bacteria were identified based on colony characteristics, morphological features, and biochemical reactions[15].
Samples from the ileal tissue and the liver were fixed in 10% formalin and embedded in paraffin. Thereafter, 5 μm thick sections were prepared and stained with hematoxylin and eosin (HE), and Masson’s trichrome staining was performed in the liver sections. All sections were studied under light microscopy in a blind fashion.
Liver index was calculated as a ratio (%) of the organ weight (g) to body weight (g).
The tissue homogenates of the liver and ileal tissue were prepared using sonication. The concentrations of proteins were quantified using a BCA protein assay kit (Beyond, China). The levels of IL-6 and TNF-α were determined using enzyme-linked immunosorbent assay (ELISA) kits (RD, United States). The levels of IL-6 and TNF-α were expressed as ng/g protein.
Bacterial genomic DNA was extracted from the cecal content using a QIAamp DNA Stool Mini Kit (QIAGEN, Germany), according to the manufacturer’s instructions. The extracted DNA was stored at -20 °C for further analyses.
The ribosomal intergenic DNA was amplified by PCR using a primer set of ITS-F (5’-GTCGTAACAAGGTAGCCGTA-3’) and ITS-R (5’-GCCAAGGCATCCAC-3’) as described[16]. A fraction (5 μL) of the 50 μL reaction mixture was analyzed with the ABI PRISM 3100 Genetic Analyzer. To identify the DNA amplicons, the rest PCR products were separated on agarose gels, and the major DNA bands were excised, cloned into pMD-19T (Takara, China) and sequenced. Identification of the resulting DNA sequences was conducted by BLAST (http://www.ncbi.nlm.nih.gov/). ARISA was performed by Beijing Protein Innovation.
For ARISA data, the electropherograms were digitized, and the results were exported to Microsoft Excel for further analysis. For diversity analysis, the Shannon indices were calculated in every cecal sample, which took into account the number of species present and the relative content. The similarity values were calculated as the normalized similarity between group pairs using the Sorenson index. Data are presented as mean ± SD, and evaluated using analysis of variance and multiple comparisons between groups with SPSS19.0 software. Differences were considered significant when P-values were less than 0.05.
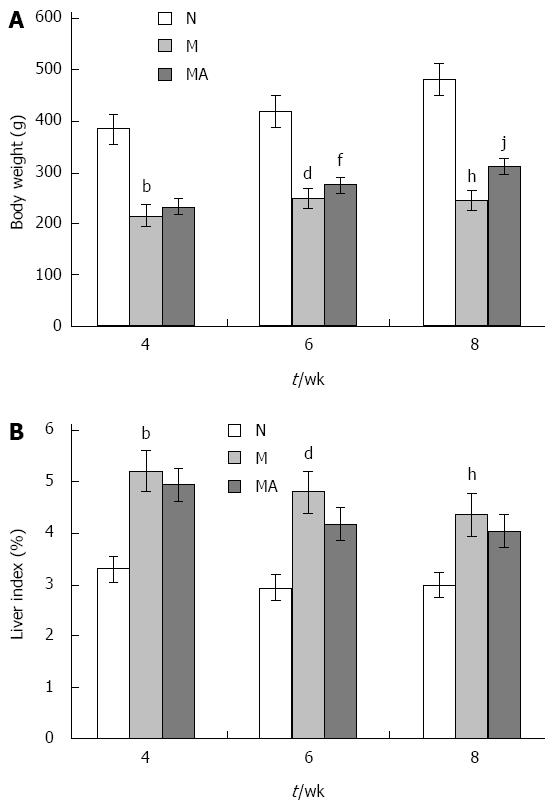
The rats in group N grew well, covered with supple and shiny fur. In group M, the rats had significant less food intake and activity, and were covered with dried and messy fur. The ill manifestation observed in the rats of group M improved obviously in the rats of group MA. No obvious increase in body weight was seen in the rats of group M, but in the rats of group MA there was a sustained body weight increase similar to that of the rats in group N. However, due to the development of liver cirrhosis, the body weight improvement by the AS supplementation was partial as the average body weight in group MA was still lower than that in group N (Figure 1A). Compared to the rats in group N, the liver indices of rats in group M were significantly elevated at 4, 6 and 8 wk. And the liver indices of the rats in group MA were decreased at 4, 6 and 8 wk compared with the indices of the rats in group M (Figure 1B).
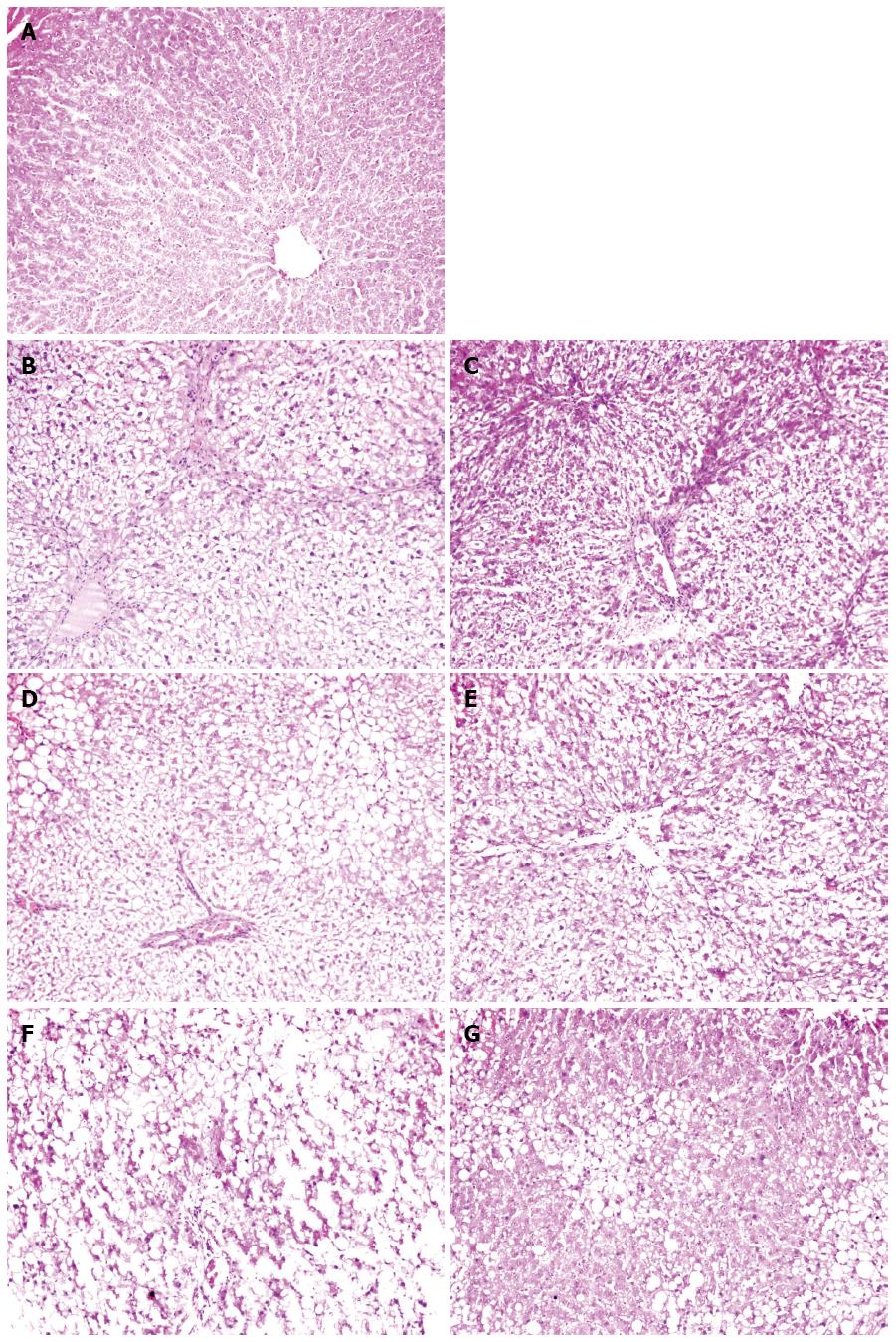
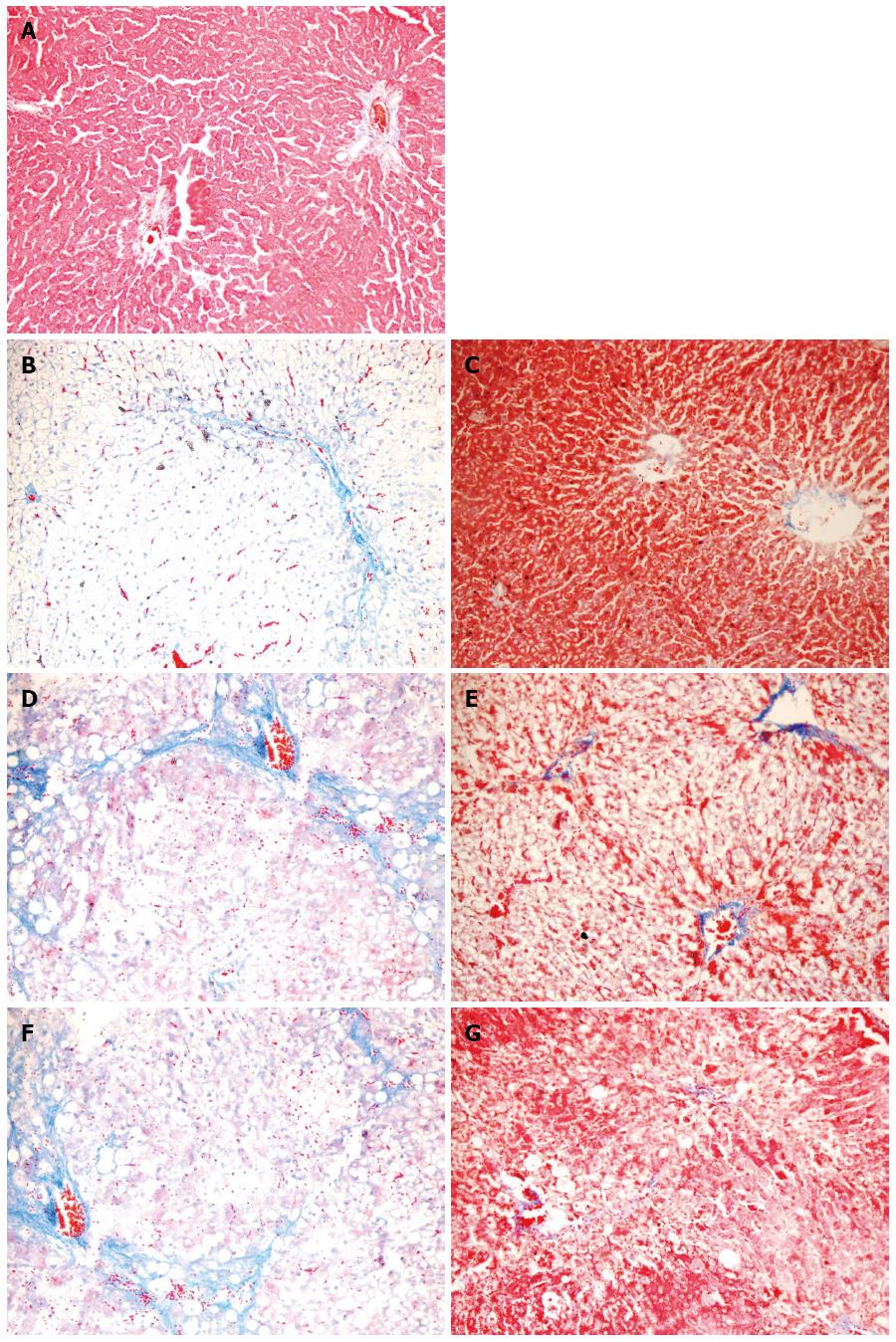
The liver architecture of group N was normal with intact lobule structure and with sinusoids and cord located in order around central veins (Figures 2 and 3A). Derangement of hepatic cord and central lobular necrosis with infiltration of inflammatory cells, cytoplasm rarefaction in liver cells with balloon-like alteration and steatosis, and increased fiber amount in interlobular area were found in the rats of group M at 4 wk (Figures 2 and 3B). Furthermore, more typical balloon-like alteration and steatosis, fiber network formation, further aggravated infiltration of inflammatory cells were observed in the rats of group M at 6 wk (Figures 2 and 3D). False lobule formation, secondary degeneration and necrosis in liver cells with infiltration of inflammatory cells were observed in the rats of group M at 8 wk (Figures 2 and 3F). Compared with group M, hepatic steatosis and fibrosis were obviously reduced in group MA at 4 wk (Figures 2 and 3C), 6 wk (Figures 2 and 3E) and 8 wk (Figures 2 and 3G). AS appeared to have a protective effect on liver injury induced by the multiple pathogenic factors.
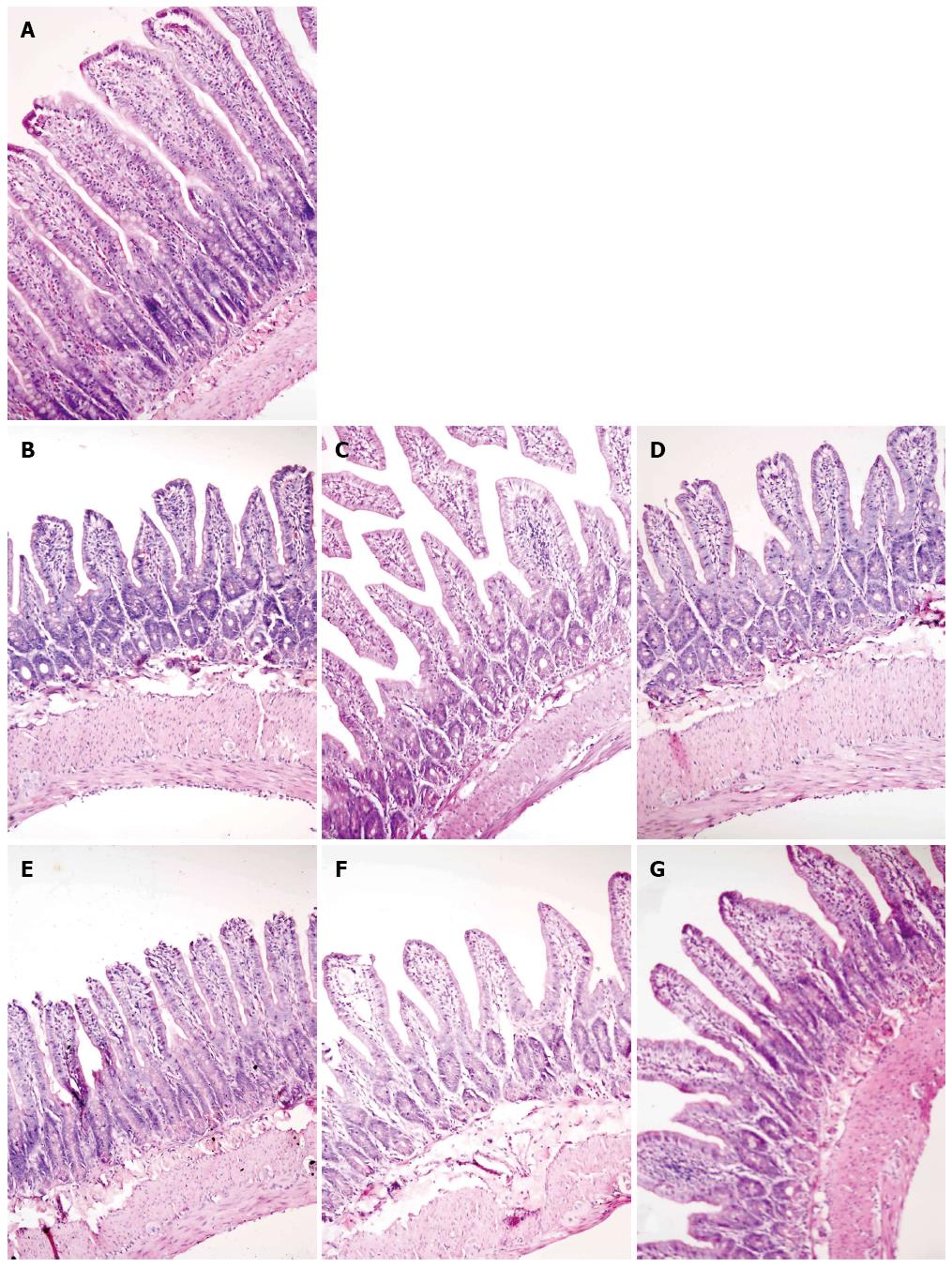
The villous height was homogenously distributed, and the intestinal glands were in order in the rats of group N (Figure 4A). In the rats of group M, ileal histopathological changes were consistent with the hepatic histopathological changes. As the liver injury progressed in the rats of group M, the villi became progressively sparse, short and irregular, the lamina propria in villous roots was gradually thinning, the gland lumen was more enlarged, and the gland cells were much shorter compared to those of the rats in group N (Figure 4B-D). Compared with the rats in group M, the intestinal villi of the rats in group MA were more uniform and longer, and injuries of glands were less at 4 wk (Figure 4E), 6 wk (Figure 4F) and 8 wk (Figure 4G).
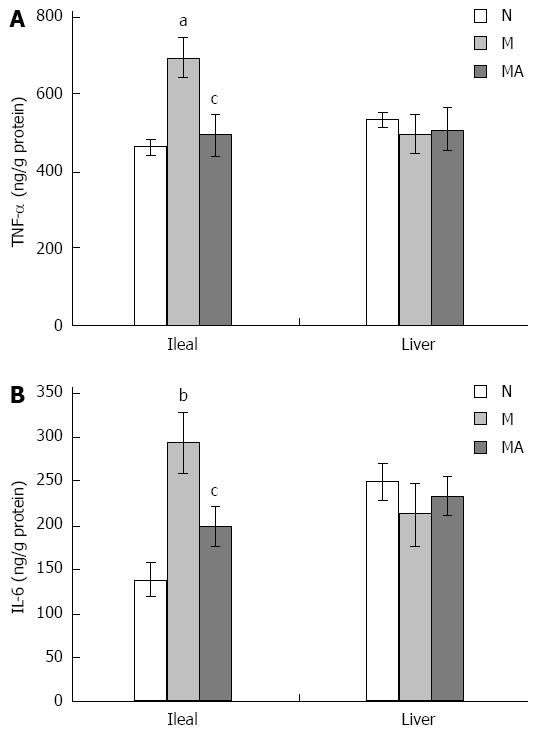
Compared to the rats in group N, levels of pro-inflammatory IL-6 and TNF-α in the ileal homogenates of the rats in group M were significantly elevated at 8 wk. In the liver homogenates of the rats in group M, the levels of IL-6 and TNF-α were elevated at 4 and 6 wk but not at 8 wk. Compared to group M, AS supplementation decreased the levels of IL-6 and TNF-α in the liver homogenates of rats in group MA at 4 and 6 wk. Also compared to group M, the levels of IL-6 and TNF-α in the ileal homogenates of the rats in group MA at 8 wk were significantly decreased by AS supplementation (Figure 5A and B).
Table 1 shows the mean diversity (Shannon index) of each group and the mean similarity (Sorenson index) between two groups in microbiota composition. In group N, the diversity was significantly lower at 8 wk than at 4 wk and 6 wk, although the characteristics of the liver and ileum of the animals were normal. The similarity between 8 wk and 6 wk was lower than that between 6 wk and 4 wk.
| Time points | N | M | MA | |||
| Shannon index | Sorenson index | Shannon index | Sorenson index | Shannon index | Sorenson index | |
| 4 wk | 2.98 ± 0.00608 | 2.44 ± 0.0606b | 0.571 vs N at 4 wk | 2.59 ± 0.0566g | 0.600 vs M at 4 wk | |
| 6 wk | 2.94 ± 0.0284 | 0.686 vs N at 4 wk | 2.44 ± 0.0103d | 0.686 vs N at 6 wk | 2.39 ± 0.0417 | 0.615 vs M at 6 wk |
| 8 wk | 2.32 ± 0.103b,d | 0.581 vs N at 6 wk | 2.63 ± 0.123e | 0.462 vs N at 8 wk | 2.66 ± 0.119 | 0.875 vs M at 8 wk |
Compared with group N, the microbiota diversity of group M was significantly reduced at 4 and 6 wk, but increased at 8 wk. The similarity was lower in group M than in group N, which implies that the dysbiosis of gut microbiota became much severe during the development of cirrhosis. The PCR products of gut bacteria that were prominent in group N and dramatically diminished in group M, were sequenced and identified as Lactobacillus, Eubacterium and Desulfotomaculum. The PCR products of gut bacteria that were prominent in group M but diminished in group N were also sequenced and identified as Clostridium or Desulfosporosinus.
Compared to group M, the diversity in group MA was significantly increased at 4 wk while the similarity was reduced at 6 and 8 wk. There was no obvious change in diversity between group MA and group M at 6 and 8 wk. The PCR products of gut bacteria, which were diminished in group M and dramatically reappeared in group MA at 4 wk, were also sequenced and identified as Lactobacillus, indicating that AS had some intervention effects on the dysbiosis of gut microbiota at early stage of cirrhosis development.
No bacterium was detected in the blood of rats in all groups. A few bacteria translocated to MLNs in rats of group N. However, the occurrence of BT and the numbers of translocated bacteria were increased in MLNs, liver, spleen and kidney of the rats in group M compared to those in group N. TR was significantly higher in group M than in group N at 6 and 8 wk. AS supplementation decreased the occurrence of BT during the development of liver cirrhosis, and TR in group MA was significantly lower than that of group M at 8 wk (Table 2).
A few Escherichia coli (E. coli) and Proteu were found in MLN culture in group N. At 4 wk, Enterococcus faecalis, E. coli, coagulase negative staphylococci and Pseudomonas aeruginosa, mainly Enterococcus faecalis, were found in groups M and MA, and one or two types of bacterium was noted on the positive cultured plates. At 6 wk, Enterococcus faecalis, E. coli, and coagulase negative staphylococci were found in groups M and MA; three species of bacterium in most of positive cultured plates of MLNs and kidney were found simultaneously, and one or two species in other organs. More translocated bacterial species were found at 8 wk than at 4 and 6 wk, which included E. coli, Enterococcus faecalis, coagulase negative staphylococci, Candida albicans, and Clostridium difficile in groups M and MA. In addition, there were 2-5 species of translocated bacterium in MLNs and 1-3 species in other organs in group M, however, there were 2-3 species in MLNs and 1-2 species in other organs in group MA at 8 wk.
In patients with advanced liver cirrhosis, intestinal bacterial overgrowth and BT often exist, causing spontaneous bacterial peritonitis and endotoxemia[17-19]. It has been reported that the multiple pathogenic factors-induced advanced liver disease such as cirrhosis is associated with gastrointestinal function disorder, intestinal bacteria overgrowth, intestinal mucosal barrier injury, suppressed immune function, BT and intestinal endotoxemia (IETM). BT and IETM further exert harmful biologic effects that aggravate liver injury and promote liver failure[20]. BT in MLNs is closely related to the overgrowth of intestinal bacteria and the enhanced permeability of enteric mucosa during development of liver cirrhosis[21-25]. In this study, multiple pathogenic factors-induced liver injury in rats was associated with gastrointestinal microbiota dysbiosis, in which Lactobacillus, Eubacterium and Desulfotomaculum dramatically diminished, Clostridium or Desulfosporosinus appeared which was not detected in the normal rats. This is significant because Lactobacillus and Eubacterium belong to the genera that produce short chain fatty acids (SCFAs) and are dominant in the healthy people[26]. As an important energy source for intestinal epithelial cells, SCFAs improve gut barrier function by promoting cell differentiation, producing mucin and antimicrobial peptide, and upregulating expression of tight junction proteins[27,28]. Furthermore, SCFAs have anti-inflammatory effects that inhibit activation of the transcription factor NF-κB, and consequently reduce formation of pro-inflammatory cytokines[29,30]. Clostridium is enriched in patients with liver cirrhosis and has been reported to cause opportunistic infection[26]. Clostridium difficile was among the species of translocated bacterium in our results. Increased numbers of bacterial flora with diminished diversity in a susceptible genotype has been shown in animal models to contribute to the pathogenesis of Crohn’s disease[31,32]. Lactobacillus, Eubacterium and Clostridium are the bacteria known to have bile salt hydrolase activity or 7α-dehydroxylation ability, and changes of these bacteria would impair bile acid metabolism, thus causing liver damages[33]. Desulfotomaculum and Desulfosporosinus are sulfate-reducing bacteria, which may play a role in gut function.
In rats with liver cirrhosis induced by the multiple pathogenic factors, dysbiosis of gut microbiota occurred, Lactobacillus and Eubacterium were reduced, enteric mucosa was damaged, levels of pro-inflammatory IL-6 and TNF-α were elevated, and numbers of translocated bacteria were increased from the intestine to MLNs, liver, spleen and kidney. All these events could produce massive biologic effects, aggravating liver injury and promoting liver failure.
It has been reported that among the intestinal microbiota, Gram-negative enteric bacilli, such as E. coli, Proteus and Enterobacter, translocate at high levels to the MLNs in gnotobiotic mice, whereas Gram-positive bacteria translocate at intermediate levels and obligatory anaerobe at only very low levels[34]. In Peyer’ patches- and MLN-competent mice, BT in MLNs was noted in 100% of tested mice, but BT in extra-intestinal organs was rare (25%); on the other hand, in Peyer’ patches- and MLN-deficient mice, BT in extra-intestinal organs was noted in 91% of tested mice[35], further demonstrating that MLNs exert an important barrier in the intestine through inhibiting BT. In the present study, BT occurred mainly in MLNs at 4 wk, the occurrence of BT in MLNs and kidney was significantly increased at 6 wk, and the incidences of BT in MLNs, liver and kidney were also significantly increased at 8 wk. The species of translocated bacteria were identified as E. coli, Enterococcus faecalis, coagulase negative staphylococci, Proteu, Pseudomonas aeruginosa, Candida albicans, and Clostridium difficile, which are common spontaneous pathogens in patients with cirrhosis. However, no bacteria were detected in cultured blood in this study. However, this observation dose not exclude the possibility of BT to blood. Teltschik et al[36] reported no bacterial growth in blood culture in the model of CCl4-induced liver cirrhosis, however, bacterial DNA was present in blood in the model of cirrhosis[37], implying that BT may occur in blood of patients with liver cirrhosis.
It has been shown that AS could effectively suppress rat liver fibrosis by CCl4[38] or by immune agents (bovine serum albumin)[39], and there were no remarkable effect of AS on normal rats[40]. In the present study, the body weight of rats intervened with AS in group MA increased gradually, and this increase was more apparent as the experimental time extended. The liver indices of rats in group MA were lower than those in group M to some extent. Meanwhile, the reduced liver histopathological changes indicate that AS may have an effective intervention on the development of cirrhosis.
Previously, it has been reported that AS can increase the susceptibility to β-lactam antibiotics[41]. Here, the results showed that the numbers and species of translocated bacteria declined, and the TR in group MA was significantly lower than that of group M at 8 wk. The damage to the intestinal mucosa caused by liver injury was partially improved, and the levels of pro-inflammatory IL-6 and TNF-α in group MA were decreased in the ileal homogenates. In group MA, the diversity of gut microbiota was significantly increased, and Lactobacillus that were diminished in group M reappeared at 4 wk. All these results suggest that AS could reduce BT occurrence and have some effects on the dysbiosis of gut microbiota during the early development of cirrhosis. We hypothesize that AS may play a role in maintaining the normal intestinal flora, through antagonizing function of inflammatory factors from liver damage and preventing BT. The underlying molecular events need to be investigated further.
In summary, BT, dysbiosis of gut microbiota and injury of intestinal mucosal barrier may occur and become much severe as the liver cirrhosis progresses. The key role of BT and dysbiosis of gut microbiota in liver cirrhosis might be to promote infection-caused inflammation that further aggravates liver injury. Some non-antimalaria effects of AS could intervene on dysbiosis of gut microbiota, alleviate inflammation and inhibit BT, which collectively reduce the multiple pathogenic factors (e.g., CCl4, alcohol and high fat) induced liver cirrhosis.
In patients with advanced liver cirrhosis, intestinal bacteria overgrowth and bacterial translocation (BT) often exist, causing spontaneous bacterial peritonitis and endotoxemia. Antibacterial prophylaxis is effective in preventing infectious complications. However, increasing antimicrobial resistance makes the patients at high risk, thus non-antibiotic pathways are desperately needed to decrease the risk of bacterial infections by reducing pathological BT as we enter the post-antibiotic era.
Artesunate (AS), extracted from the Chinese herb Artemisia annua, is a safe and effective antimalarial drug. It has been reported that AS could also decrease serum endotoxin levels in sepsis mice, and have an anti-inflammatory role. However, it is uncertain whether AS is effective in preventing BT.
This is the first study investigating the effect of AS on BT and composition of intestinal bacteria in rats with liver cirrhosis.
BT, dysbiosis of gut microbiota and injury of intestinal mucosal barrier may occur and become much severe as the liver cirrhosis progresses. The key role of BT and dysbiosis of gut microbiota in liver cirrhosis may be to interfere with a number of biological process that further aggravate liver injury. AS could intervene on dysbiosis of gut microbiota, alleviate inflammation and inhibit BT, which collectively reduce the multiple pathogenic factors induced liver cirrhosis.
Gut microbiota, a wide variety of micro-organisms in the human gut, are harmless and have an important role in human nutrition and health, promoting nutrient supply, preventing pathogen colonization, and shaping and maintaining normal mucosa immunity. BT is defined as the migration of intestinal flora and/or their toxins into mesenteric lymph nodes and/or other extra-intestinal organs.
The authors employed a model of cirrhosis in rats receiving both carbon tetrachloride and alcohol. This is a well-tested model. They describe the rationale for the investigation of the effect of arsenate and show a beneficial effect both on body weight, histopathology, dysbiosis and BT.
P- Reviewer: Malnick SDH, Morales-Gonzalez J S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Chen LW, Chang WJ, Chen PH, Hsu CM. Commensal microflora induce host defense and decrease bacterial translocation in burn mice through toll-like receptor 4. J Biomed Sci. 2010;17:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Lawrance IC. Microbiota and management of inflammatory bowel disease. J Gastroenterol Hepatol. 2012;27:1137-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Fujimoto T, Imaeda H, Takahashi K, Kasumi E, Bamba S, Fujiyama Y, Andoh A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J Gastroenterol Hepatol. 2013;28:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3922] [Cited by in RCA: 3462] [Article Influence: 216.4] [Reference Citation Analysis (0)] |
| 7. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542-2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Carson CF, Riley TV. Non-antibiotic therapies for infectious diseases. Commun Dis Intell Q Rep. 2003;27 Suppl:S143-S146. [PubMed] |
| 10. | Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 867] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 11. | Li B, Zhang R, Li J, Zhang L, Ding G, Luo P, He S, Dong Y, Jiang W, Lu Y. Antimalarial artesunate protects sepsis model mice against heat-killed Escherichia coli challenge by decreasing TLR4, TLR9 mRNA expressions and transcription factor NF-kappa B activation. Int Immunopharmacol. 2008;8:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Wang XQ, Liu HL, Wang GB, Wu PF, Yan T, Xie J, Tang Y, Sun LK, Li C. Effect of artesunate on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52:916-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, Yang X, Lian F, Sun L. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford). 2007;46:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Zhang HY, Han DW, Zhao ZF, Liu MS, Wu YJ, Chen XM, Ji C. Multiple pathogenic factor-induced complications of cirrhosis in rats: a new model of hepatopulmonary syndrome with intestinal endotoxemia. World J Gastroenterol. 2007;13:3500-3507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Chen YX, Zhang HY, Meng L, Li XJ, Lai LN, Tian XX, Wang LM. Bacterial translocation in rats with hepatic cirrhosis and the interventional effect of betaine. Weishengwuxue Tongbao. 2014;41:1629-1636. |
| 16. | Cardinale M, Brusetti L, Quatrini P, Borin S, Puglia AM, Rizzi A, Zanardini E, Sorlini C, Corselli C, Daffonchio D. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl Environ Microbiol. 2004;70:6147-6156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 17. | Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, Riba J, Cabré E, Santos J, Luque T, Ausina V. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X, Zhao HZ. Experimental study on the role of endotoxin in the development of hepatopulmonary syndrome. World J Gastroenterol. 2005;11:567-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 23. | Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Macnaughtan J, Jalan R. Clinical and pathophysiological consequences of alterations in the microbiome in cirrhosis. Am J Gastroenterol. 2015;110:1399-410; quiz 1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Steib CJ, Schewe J, Gerbes AL. Infection as a Trigger for Portal Hypertension. Dig Dis. 2015;33:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1536] [Article Influence: 139.6] [Reference Citation Analysis (40)] |
| 27. | Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One. 2009;4:e6759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1339] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 29. | Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 973] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 30. | Lührs H, Gerke T, Schauber J, Dusel G, Melcher R, Scheppach W, Menzel T. Cytokine-activated degradation of inhibitory kappaB protein alpha is inhibited by the short-chain fatty acid butyrate. Int J Colorectal Dis. 2001;16:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 32. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1277] [Article Influence: 67.2] [Reference Citation Analysis (2)] |
| 33. | Pereira-Fantini PM, Lapthorne S, Joyce SA, Dellios NL, Wilson G, Fouhy F, Thomas SL, Scurr M, Hill C, Gahan CG. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J Hepatol. 2014;61:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988;157:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Suzuki H, Okada Y. Bacterial translocation in alymphoplasia (aly/aly) mice. Folia Biol (Krakow). 2014;62:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Guarner C, González-Navajas JM, Sánchez E, Soriando G, Francés R, Chiva M, Zapater P, Benlloch S, Muñoz C, Pascual S. The detection of bacterial DNA in blood of rats with CCl4-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006;44:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Fang BW, Lai LN, Lin YJ, Ma M, Zhen SL, Lin XZ, Cui ZQ, Gao WZ, Zhang CL, Lou JS. Effects of Artesunate on rats liver fibrosis due to CCl4 poisoned. Zhongguo Yaolixue Tongbao. 2005;21:762-763. |
| 39. | Lai LN, Fang BW. Effect of Artesunate on hepatic fibrosis induced by bovine serum albumin in rats. Zhongyao Yaoli Yu Linchang. 2006;22:35-37. |
| 40. | Lai L, Chen Y, Tian X, Li X, Zhang X, Lei J, Bi Y, Fang B, Song X. Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-κB signaling pathway in rats. Eur J Pharmacol. 2015;765:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Jiang W, Li B, Zheng X, Liu X, Pan X, Qing R, Cen Y, Zheng J, Zhou H. Artesunate has its enhancement on antibacterial activity of β-lactams via increasing the antibiotic accumulation within methicillin-resistant Staphylococcus aureus (MRSA). J Antibiot (Tokyo). 2013;66:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |