Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.2931
Peer-review started: June 12, 2015
First decision: November 5, 2015
Revised: December 4, 2015
Accepted: January 9, 2016
Article in press: January 11, 2016
Published online: March 14, 2016
Processing time: 268 Days and 18.9 Hours
AIM: To evaluate the effect of resveratrol, alone and in combination with fenofibrate, on fructose-induced metabolic genes abnormalities in rats.
METHODS: Giving a fructose-enriched diet (FED) to rats for 12 wk was used as a model for inducing hepatic dyslipidemia and insulin resistance. Adult male albino rats (150-200 g) were divided into a control group and a FED group which was subdivided into 4 groups, a control FED, fenofibrate (FENO) (100 mg/kg), resveratrol (RES) (70 mg/kg) and combined treatment (FENO + RES) (half the doses). All treatments were given orally from the 9th week till the end of experimental period. Body weight, oral glucose tolerance test (OGTT), liver index, glucose, insulin, insulin resistance (HOMA), serum and liver triglycerides (TGs), oxidative stress (liver MDA, GSH and SOD), serum AST, ALT, AST/ALT ratio and tumor necrosis factor-α (TNF-α) were measured. Additionally, hepatic gene expression of suppressor of cytokine signaling-3 (SOCS-3), sterol regulatory element binding protein-1c (SREBP-1c), fatty acid synthase (FAS), malonyl CoA decarboxylase (MCD), transforming growth factor-β1 (TGF-β1) and adipose tissue genes expression of leptin and adiponectin were investigated. Liver sections were taken for histopathological examination and steatosis area were determined.
RESULTS: Rats fed FED showed damaged liver, impairment of glucose tolerance, insulin resistance, oxidative stress and dyslipidemia. As for gene expression, there was a change in favor of dyslipidemia and nonalcoholic steatohepatitis (NASH) development. All treatment regimens showed some benefit in reversing the described deviations. Fructose caused deterioration in hepatic gene expression of SOCS-3, SREBP-1c, FAS, MDA and TGF-β1 and in adipose tissue gene expression of leptin and adiponectin. Fructose showed also an increase in body weight, insulin resistance (OGTT, HOMA), serum and liver TGs, hepatic MDA, serum AST, AST/ALT ratio and TNF-α compared to control. All treatments improved SOCS-3, FAS, MCD, TGF-β1 and leptin genes expression while only RES and FENO + RES groups showed an improvement in SREBP-1c expression. Adiponectin gene expression was improved only by RES. A decrease in body weight, HOMA, liver TGs, AST/ALT ratio and TNF-α were observed in all treatment groups. Liver index was increased in FENO and FENO + RES groups. Serum TGs was improved only by FENO treatment. Liver MDA was improved by RES and FENO + RES treatments. FENO + RES group showed an increase in liver GSH content.
CONCLUSION: When resveratrol was given with half the dose of fenofibrate it improved NASH-related fructose-induced disturbances in gene expression similar to a full dose of fenofibrate.
Core tip: The current work may justify the use of lower doses of fenofibrate in combination with resveratrol to protect the liver from fructose induced hepatic steatosis and damage. The synergistic effect may be due to antioxidant, anti-inflammatory and anti-hyperlipidemic effect of resveratrol. As an add-on therapy, resveratrol can augment the beneficial outcome of a lower dose of fenofibrate and reduces its toxic or side effects.
- Citation: Abd El-Haleim EA, Bahgat AK, Saleh S. Resveratrol and fenofibrate ameliorate fructose-induced nonalcoholic steatohepatitis by modulation of genes expression. World J Gastroenterol 2016; 22(10): 2931-2948
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/2931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.2931
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are now the number one cause of liver disease in Western countries, in the Middle East, Far East, Africa, the Caribbean and Latin America[1]. The most reproducible risk factors for NAFLD/NASH are central obesity, insulin resistance, fasting hyperglycemia and hypertriglyceridemia[2].
Induction of NASH seems to be a multi-factorial process. Thus, suppressor of cytokine signaling (SOCS) proteins appears to play a key role in the induction of insulin resistance in obese rodents[3]. Furthermore, studies in obese mice proved that SOCS-3 overexpression was linked to decreased tyrosine phosphorylation of insulin receptor (IR) and insulin receptor substrate (IRS) proteins. Besides, increased expression of SOCS-3 was supplementary to enhanced expression of sterol-regulatory element-binding protein (SREBP)-1c, a transcriptional activator of all lipogenic enzymes[4]. These changes would eventually end up by an increased rate of fatty acid synthesis and would lead to classic fatty liver and increased lipogenesis[5].
Moreover, adipose tissue plays a key role in energy homeostasis, since its metabolic products, adipokines (leptin and adiponectin) exert local, peripheral and central effects. Leptin is thought to participate in NASH development while adiponectin is considered as an anti-inflammatory adipokine[6].
Recent studies ascertained that obesity is a systemic, low-grade inflammation[7] and that enhanced expression of tumor necrosis factor-α (TNF-α) is one of the earliest events after liver injury and that it represents a major trigger of the cytokine response[8]. Currently, the role of oxidative stress in disease progression from steatosis to steatohepatitis and potentially cirrhosis cannot be ignored[9].
On the other hand, it is well known that fructose is a highly lipogenic sugar molecule, which triggers the accumulation of TGs and FFAs into the hepatic tissues as well as in circulating blood, and leads to insulin resistance[10]. Fructose feeding has therefore been historically utilized as a model for studying various aspects of hepatic dyslipidemia and insulin resistance.
As for the management of NASH, treatments other than lifestyle modification by diet and exercise have not been fully established[11]. Nonetheless, the use of antihyperlipidemic agents stems from the association of dyslipidemia with NAFLD[12].
Additionally, several antioxidants were found to be effective in NAFLD treatment[12]. One of these is resveratrol, a polyphenol with known antioxidant and anti-inflammatory effects. Although the antioxidant effects of resveratrol have been proposed to contribute to its beneficial effects, the underlying molecular mechanism is not completely understood. Resveratrol treatment in mice fed a high calorie diet consistently improved various health parameters including glucose homeostasis and survival[13], and has therefore been suggested to act as a calorie restriction mimetic. Moreover, resveratrol decreased NAFLD severity in rats[14].
In the present study, we investigated the effect of resveratrol treatment on fructose-induced NASH in rats and explored the potential molecular mechanisms through which the protective effects of resveratrol may work. We also explored the possible modulatory effects of fenofibrate and resveratrol given separately and in combination (half doses) on fructose-induced NASH. Besides, the expressions of a number of genes known to be critically involved in lipid metabolism were targeted.
Adult male albino rats, weighing 150-200 g, were used in the present study. They were purchased from the animal house of the National Cancer Institute (Cairo, Egypt). The animal protocol was designed to minimize pain or discomfort to the animals. Animals were housed under controlled environmental conditions: constant temperature (25 °C ± 2 °C), humidity (60% ± 10%), and a 12/12-h light/dark cycle. Standard chow diet and water were allowed ad libitum two weeks prior to experimentation. All animals except the normal group were given 10% fructose in drinking water for 12 wk in order to induce NASH[15]. All experiments on laboratory animals were performed in accordance with the protocol approved by Faculty of Pharmacy, Cairo University Research Ethics Committee, Cairo, Egypt. PT number (660).
The tested agents and doses used in the present study were: fenofibrate (100 mg/kg; po) (Eva Pharm)[16] and resveratrol (70 mg/kg po) (Finest Nutrition, Walgreen)[17]. The drugs were suspended in 0.5% carboxymethyl cellulose (CMC).
After a period of adaptation, animals were randomly assigned into a normal control group and a fructose enriched diet group (FED). The FED group was distributed into 4 groups, a control FED group and 3 treated groups, each composed of 8-12 rats. The normal control group was fed a normal chow diet consisting of 66% carbohydrates, 22% protein, 6% fats, 3% fiber and 3% minerals and vitamins mixture and purchased from Alfa Media Trade (Giza, Egypt). The high fructose fed groups received the same diet plus fructose (10%) in drinking water for a period of 12 wk. Treatments were carried out by each drug separately and in combination of half the dose of each, for 4 wk from the 9th week until the end of the 12 wk experimental period. Group I: normal control, II: control FED, III: fenofibrate 100 mg/kg (FENO), IV: resveratrol 70 mg/kg (RES), V: fenofibrate combined with resveratrol 50 mg/kg + 35 mg/kg (FENO + RES).
Body weight was determined during the experimental period (12 wk) at two weeks intervals.
At the end of the treatment period, blood samples were taken from retro-orbital sinus of rats under ether anesthesia, after being fasted for 18 h, to minimize feeding-induced variations in lipid pattern. Blood samples were allowed to clot at room temperature then serum was separated by centrifugation of blood at 3000 rpm for 15 min using a centrifuge (Hettich universal 32A, Germany). Each sample was divided into several aliquots, one for each estimated biochemical parameter, and stored at -20 °C until analysis was performed.
Animals were then sacrificed by cervical dislocation. Livers and epididymal fats were carefully and rapidly excised. The removed livers were washed with cold normal saline and dried on filter papers then weighed for the determination of liver index. Liver index percent was determined (= liver weight / body weight × 100).
Samples of the liver, taken 5 mm away from the edge of the largest hepatic lobe were frozen at -80 °C for the determination of hepatic genes expressions. The other lobes of the liver were homogenized in ice-cold saline, using a homogenizer (Heidolph Diax 900, Germany), to prepare 20% homogenate. The prepared homogenate was divided into several aliquots that were stored at -20 °C until assayed later for estimation of the chosen biochemical parameters. The remaining part of the large hepatic lobe was fixed with 10% formaldehyde for histopathological examination.
Besides, the epididymal fat located above the epididymis was dissected and frozen at -80 °C for estimation of adipose tissue gene expression.
The dead bodies were frozen till incineration.
Liver lipids were extracted according to the method of Bligh and Dyer[18] for the determination of liver triglycerides.
The liver fixed with 10% formaldehyde was dehydrated and embedded in paraffin wax, cut into sections of 7-10 μm thickness, stained with hematoxylin and eosin (HE) and then examined under light microscope. Images were acquired with a Leica ICC50 HD digital camera attached to a Leica motorized light microscope system. Steatosis area was determined using Leica application suite (LAS) image analysis program which automatically detects, measures and evaluates multiple image features.
Blood glucose and oral glucose tolerance test: Two days before the end of the treatment period, rats were fasted for 18 h, with free access to water, to minimize feeding-induced variations in glucose pattern. Glucose level was determined in a blood sample obtained from the tail vein. The blood glucose and oral glucose tolerance test (OGTT) was carried out using glucometer test strips (Fine test, Korea). Rats were given oral glucose (20% solution in a dose 2 g/kg of body weight), and droplets of blood from the tail vein were withdrawn at 0 (prior to glucose administration), 15, 30, 60 and 120 min after glucose load, to evaluate the resulting blood glucose concentrations.
Serum insulin and Insulin resistance: Insulin was determined using DRG® Insulin (Rat) ELISA (EIA-2048) version 8.0, 2013. Insulin resistance was calculated using homeostasis model of assessment (HOMA) = blood glucose (mmol/L) × serum insulin (pmol/L)/155[19].
Serum and liver TGs: Serum and liver TGs were determined according to the method of Bucolo and David[20].
Oxidative stress parameters: Liver homogenate was used for determination of thiobarbituric acid reactive substances (TBARs), measured as MDA[21], reduced glutathione (GSH)[22] and superoxide dismutase (SOD)[23].
Liver function tests: Serum alanine transaminase (ALT) and aspartate aminotransferase (AST) were determined according to the method of Reitman and Frankel[24].
Serum TNF-α: TNF-α was determined using Quantikine® ELISA Rat TNF-α Immunoassay kit. c2012 R&D Systems, Inc.
Hepatic and adipose tissue genes expression: Hepatic genes expressions of SOCS-3, SREBP-1c, fatty acid synthase (FAS), malonyl CoA decarboxylase (MCD), TGF-β1, and adipose tissue genes expressions of leptin and adiponectin were measured using real time-polymerase chain reaction (RT-PCR).
Total RNA was isolated from the liver samples stored for determination of hepatic genes expressions using Thermo Scientific GeneJET RNA Purification Kit following the manufacturer’s protocol. Total RNA was purified using the supplied purification column, after which it was reverse transcribed using Thermo Scientific RevertAid First Strand cDNA Synthesis Kit. RT-PCR analysis was performed using the SYBR Green procedure and Stratagene Mx3000P instrument. Expression of mRNA values was normalized relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard.
The following primer pairs were employed (Primer-Blast program was used to assist in the design of primers) (Table 1).
| Primer name | Forward primer | Reverse primer |
| GAPDH | 5'-ACAAGATGGTGAAGGTCGGTGTGA-3' | 5'-TTGAACTTGCCGTGGGTAGAGTCA-3' |
| SOCS-3 | 5'-CTGGCCGCCGCCTCGTCTCGG-3' | 5'-ACGGCACTCCAGTAGAATCCG-3' |
| SREBP1c | 5'-GGAGCCATGGATTGCACATT-3' | 5'-GCTTCCAGAGAGGAGCCCAG-3' |
| FAS | 5'-AGAGGCTGTTCTCAAGGAAGG-3' | 5'-AGGGTACATCCCAGAGGAAGT-3' |
| MCD | 5'-CGGCACCTTCCTCATAAAGC-3' | 5'-GGGTATAGGTGACAGGCTGGA-3' |
| Leptin | 5'-TTCAAGCTGTGCCTATCCACAAAG-3' | 5'-TGAAGCCCGGGAATGAAGTC-3' |
| Adiponectin | 5'-GGAAACTTGTGCAGGTTGGATG -3' | 5'-GGGTCACCCTTAGGACCAAGAA-3' |
| TGF-β1 | 5'-TGAGTGGCTGTCTTTTGACG-3' | 5'-ACTTCCAACCCAGGTCCTTC-3' |
At the end of a RT-PCR running with SYBR Green chemistry, the relative quantification was determined according to the method of Pfaffl[25]. The RT-PCR results were analyzed with applied biosystem software.
Data are expressed as means ± SE. Comparisons between means were carried out using one way analysis of variance test followed by Tukey Kramer multiple comparison’s test. For all statistical tests, the level of significance was fixed at P < 0.05.
GraphPad Prism® software package, version 6 (GraphPad Software, Inc., United States) was used to carry out all statistical tests.
The statistical methods of this study were reviewed by Dr. Nelly Alieldin, professor of biostatistics and cancer epidemiology, National Cancer Institute, Egypt.
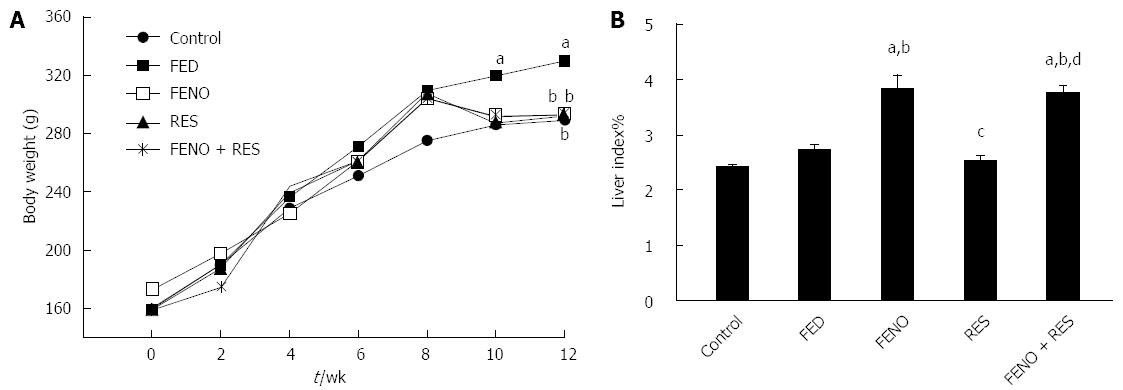
As presented in Figure 1A, there was a gradual gain in body weight in all groups although the extent was variable. Comparing body weights on week 12, fructose-fed rats reached a body weight of 330.0 g compared to 289.8 g in control rats, indicating a 10% more weight gain in the FED group. This rise in body weight was virtually normalized by all treatment regimens.
Figure 1B shows that there was no change in liver index in the FED group compared to control and that treatment with FENO alone or FENO + RES significantly increased liver index% nearly to the same extent i.e., 3.83% and 3.75% respectively.
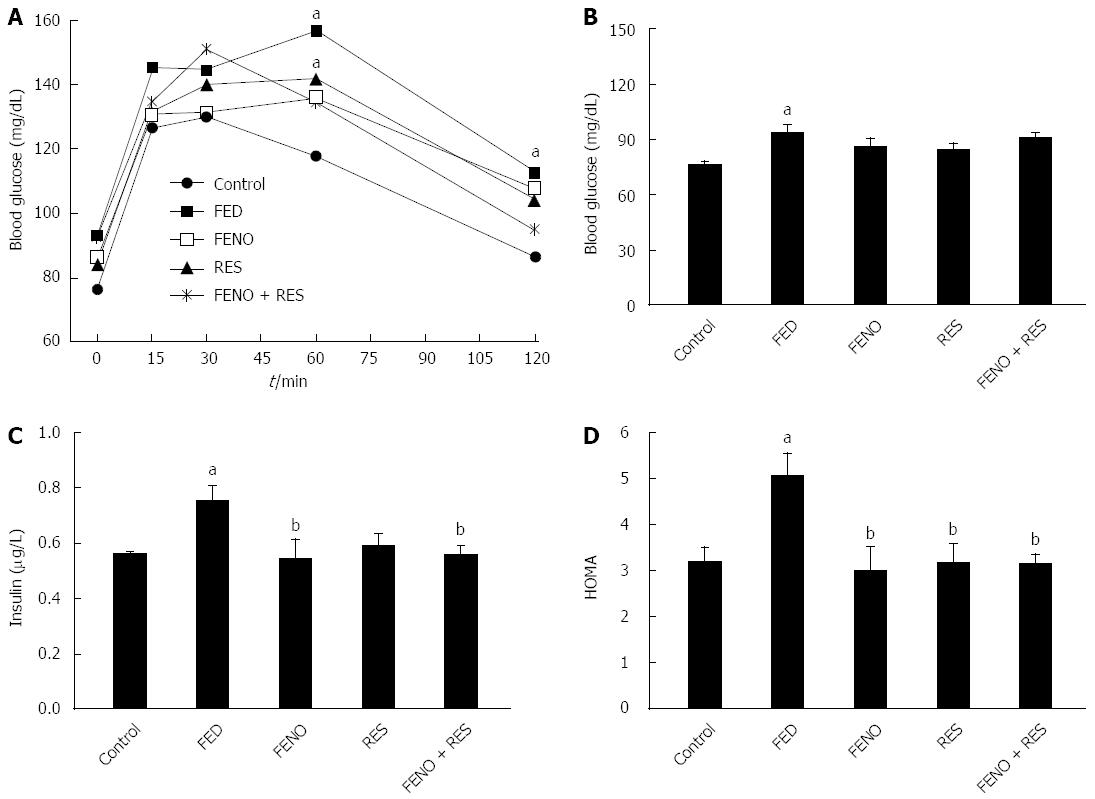
Figure 2A depicts the impairment of glucose tolerance in the fructose-fed group as compared to control. Thus, in the control group ingestion of glucose caused a marked steady rise in blood sugar level that reached a peak of 126.7 mg/dL at 15 min; it was maintained at 30 min (130.0 mg/dL) and became nearly normal 2 h after glucose loading. In FED rats, blood glucose peaked at 15 min, there was another peak at 60 min, and was still 26% higher than normal after 2 h. Moreover, the peak blood glucose level in the FED group was higher (156.9 mg/dL) and delayed. Glucose tolerance was improved but not normalized by all treatments.
Beside the alterations in OGTT, feeding rats with fructose caused an increase in blood glucose level to 93.00 mg/dL compared to control (76.25 mg/dL), an increase in serum insulin level to 0.753 μg/L compared to control (0.560 μg/L) leading to a 58% rise in HOMA index. Treatment did not affect blood sugar level but it caused a recovery of both insulin level and HOMA index. It was noticed that the improvement produced by a full dose of FENO was equivalent to that of half the dose given with RES. Figure 2B, C and D illustrate the changes in glucose, insulin and HOMA respectively.
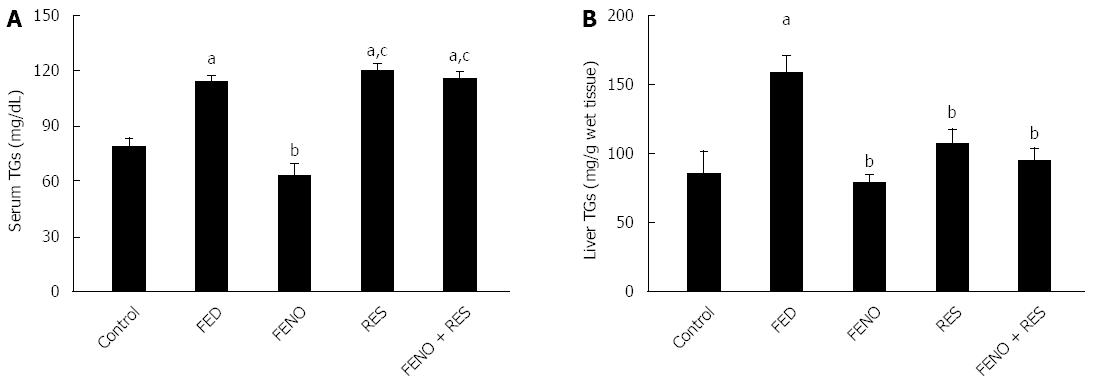
The changes in serum and liver triglycerides are illustrated in Figure 3A and B respectively. Fructose fed rats showed a rise of 44.5% in serum TGs and 86.3% in hepatic triglycerides content as compared to the control group. FENO, being a well-documented antihyperlipidemic drug, was effective in preventing the accumulation of both serum and liver TGs. RES, on the other hand was much less effective in this respect.
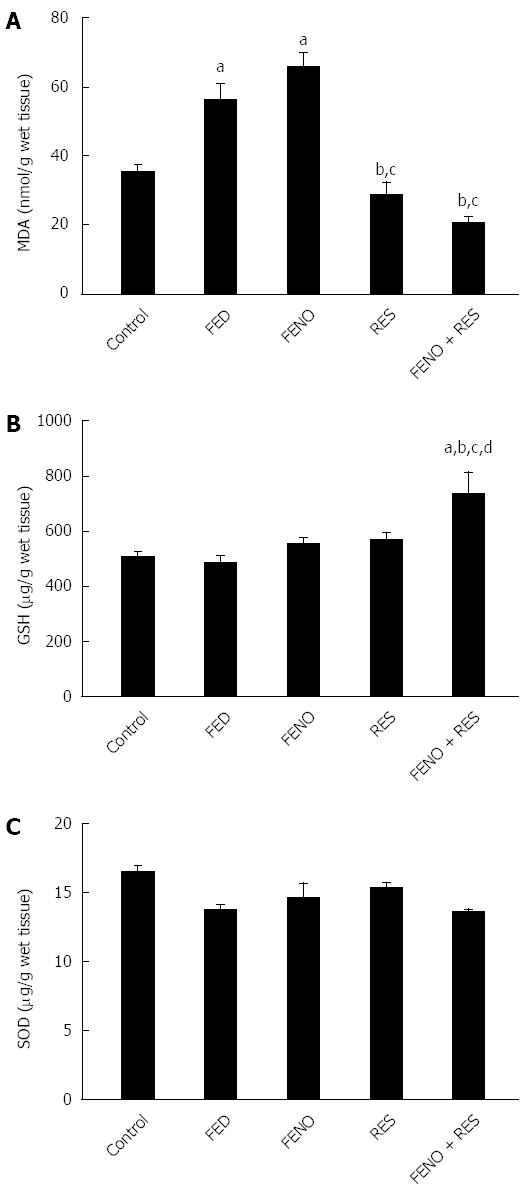
Figure 4 shows the changes in the redox balance in the liver. Fructose feeding significantly elevated liver MDA by 59% without affecting both liver GSH content and SOD activity. Being an antioxidant free radical scavenger, RES alone, or in combination FENO + RES prevented the upsurge of MDA. Moreover, the effect of the drug combination on GSH superseded that of either treatment alone.
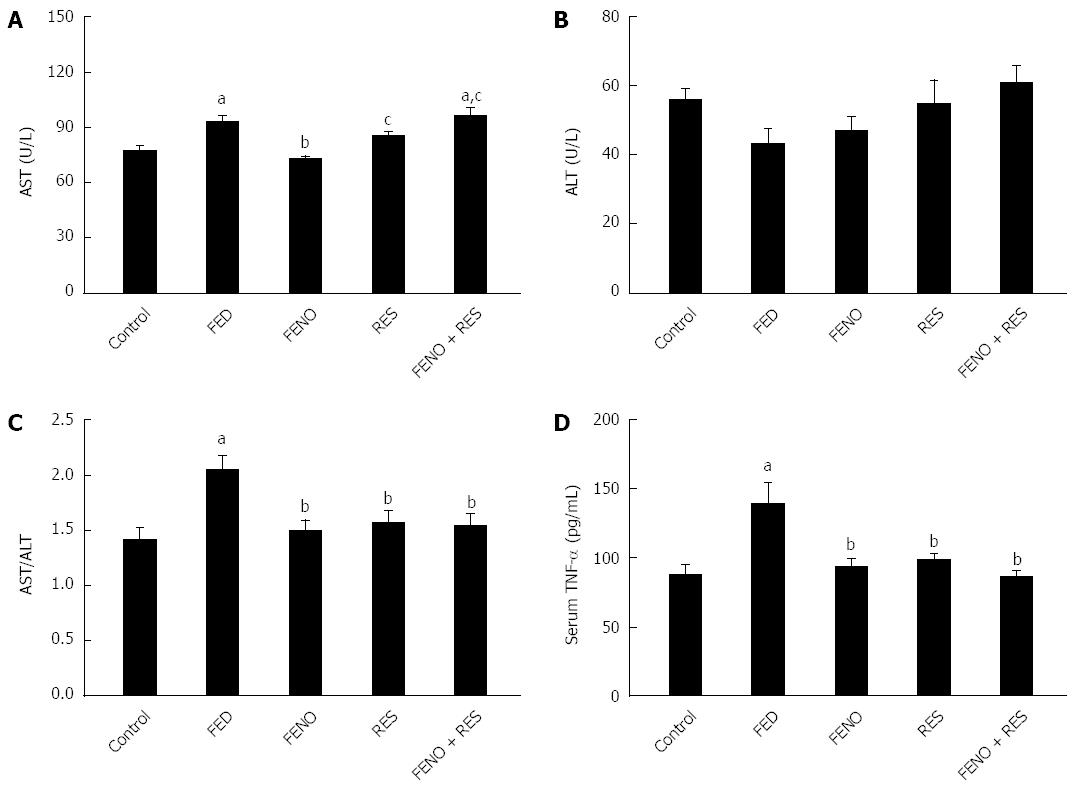
As shown in Figure 5A-C, feeding fructose amplified the activity of AST without affecting ALT; there was a 2 fold increase in the AST/ALT ratio. Additionally, Serum TNF-α (Figure 5D) was augmented (1.6 fold) compared to control. All treatments opposed the injurious effect of FED and normalized both AST/ALT ratio and serum TNF-α level. Interestingly, RES enhanced the effect of half the dose of FENO in the above-mentioned parameters such that it was equivalent to the effect of a full dose.
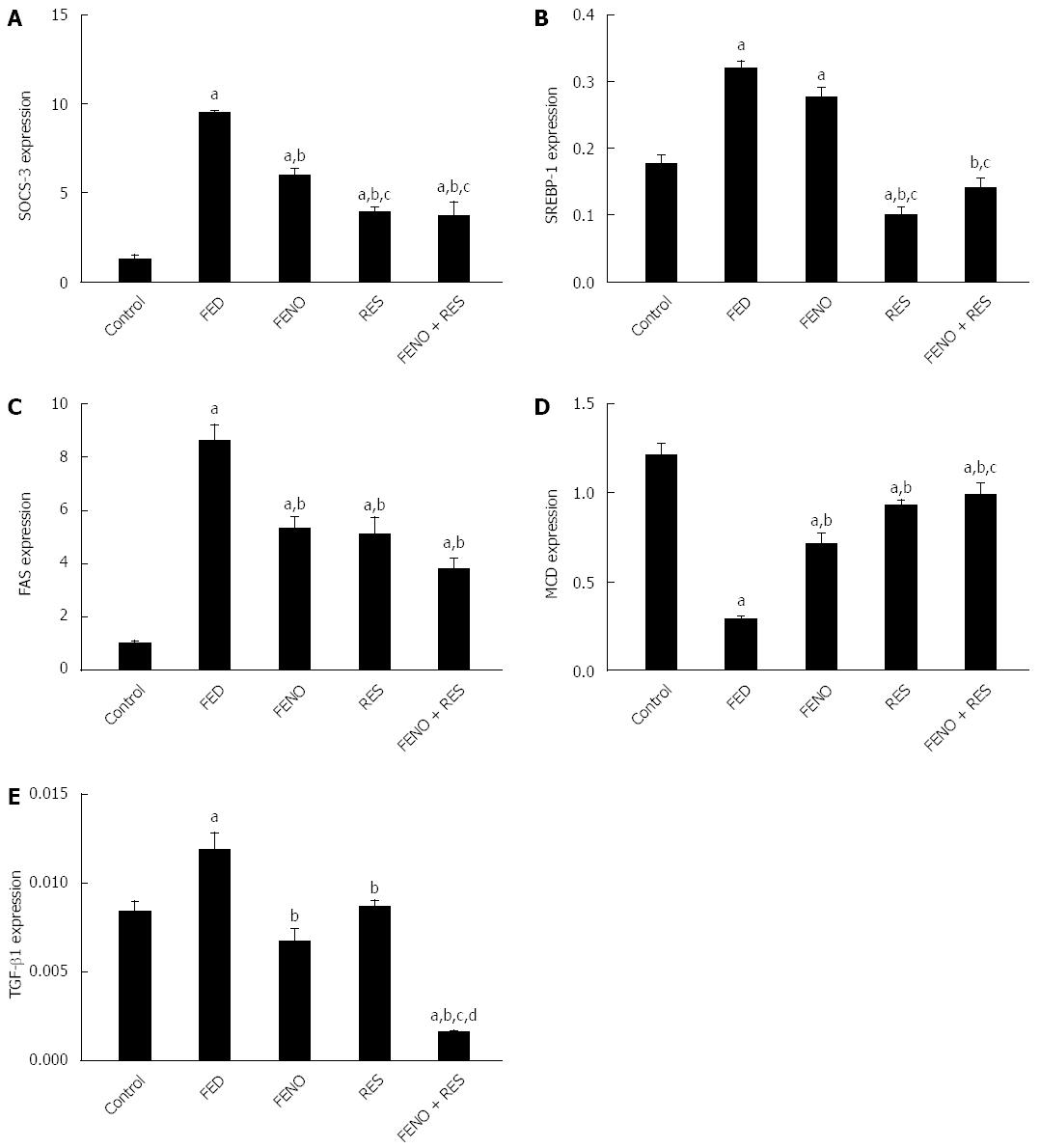
The expressions of SOCS-3, SREBP-1c, FAS, MCD and TGF-β1 in the control group were 1.287, 0.177, 1.04, 1.21 and 0.0084 respectively. Fructose feeding displayed profound changes in these signaling pathways. Thus, there was about 7-fold increase in SOCS-3 gene expression, 2-fold increase in SREBP-1c, 8-fold increase in FAS, whereas MCD genes expressions revealed a 4-fold decline compared to control group.
Treatment of the rats given FED with FENO, RES and FENO + RES trimmed down SOCS-3 gene expression to 5.958, 3.933 and 3.707 respectively compared to FED (9.500). Again in this context, the decrease in SOCS-3 gene expression by half the dose of FENO when combined with RES was 38% more compared to FENO indicating that insulin sensitivity was better in the combination (Figure 6A).
Although FENO treatment did not modify SREBP-1c gene expression, RES alone or combined with FENO caused moderation by 68.7% and 56% respectively.
The hepatic expression of the FAS gene, responsible for de novo lipogenesis, was reduced by FENO, RES and FENO+RES treatments to 5.34, 5.14 and 3.78 respectively compared to FED (8.60).
Furthermore, MCD gene expression in liver was increased in FENO, RES and FENO + RES treatment groups by 2.5, 3.0 and 3.5-fold respectively compared to FED group. The improvement afforded by FENO + RES combination was significantly better (38.6%) compared to FENO alone (Figure 6B, C and D).
TGF-β gene expression was upregulated in fructose fed rats (1.4-fold) as compared to the control counterpart and was normalized by treatment with either FENO or RES, while FENO + RES group showed 86.6% suppression in TGF-β gene expression compared to the FED group. Again, RES enhanced the effect of FENO such that the amelioration offered by the combination of half doses exceeded that of the full FENO dose as shown in Figure 6E.
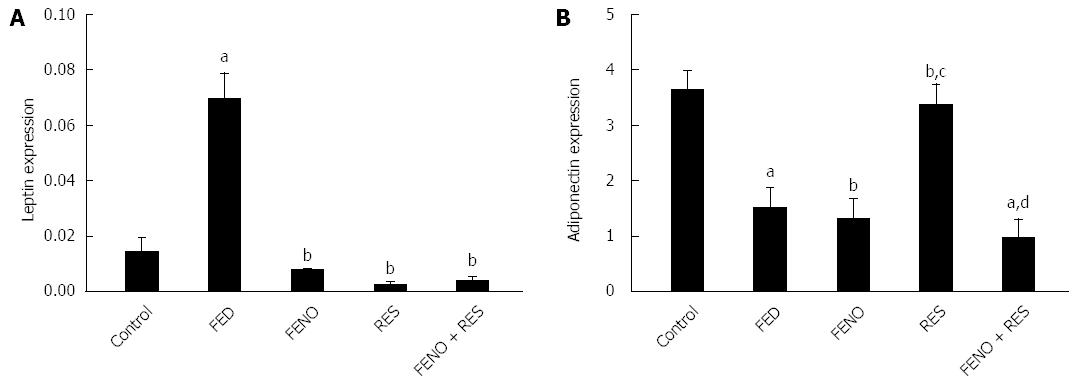
Figure 7 represents the alterations in the expression of leptin and adiponectin genes in adipose tissue. It is obvious that feeding rats with fructose produced an almost 5-fold augmentation in leptin gene expression and 2-fold suppression in adiponectin expression. The upregulation of leptin gene expression was successfully normalized by all treatments. Furthermore, adiponectin gene expression was only adjusted by RES treatment alone.
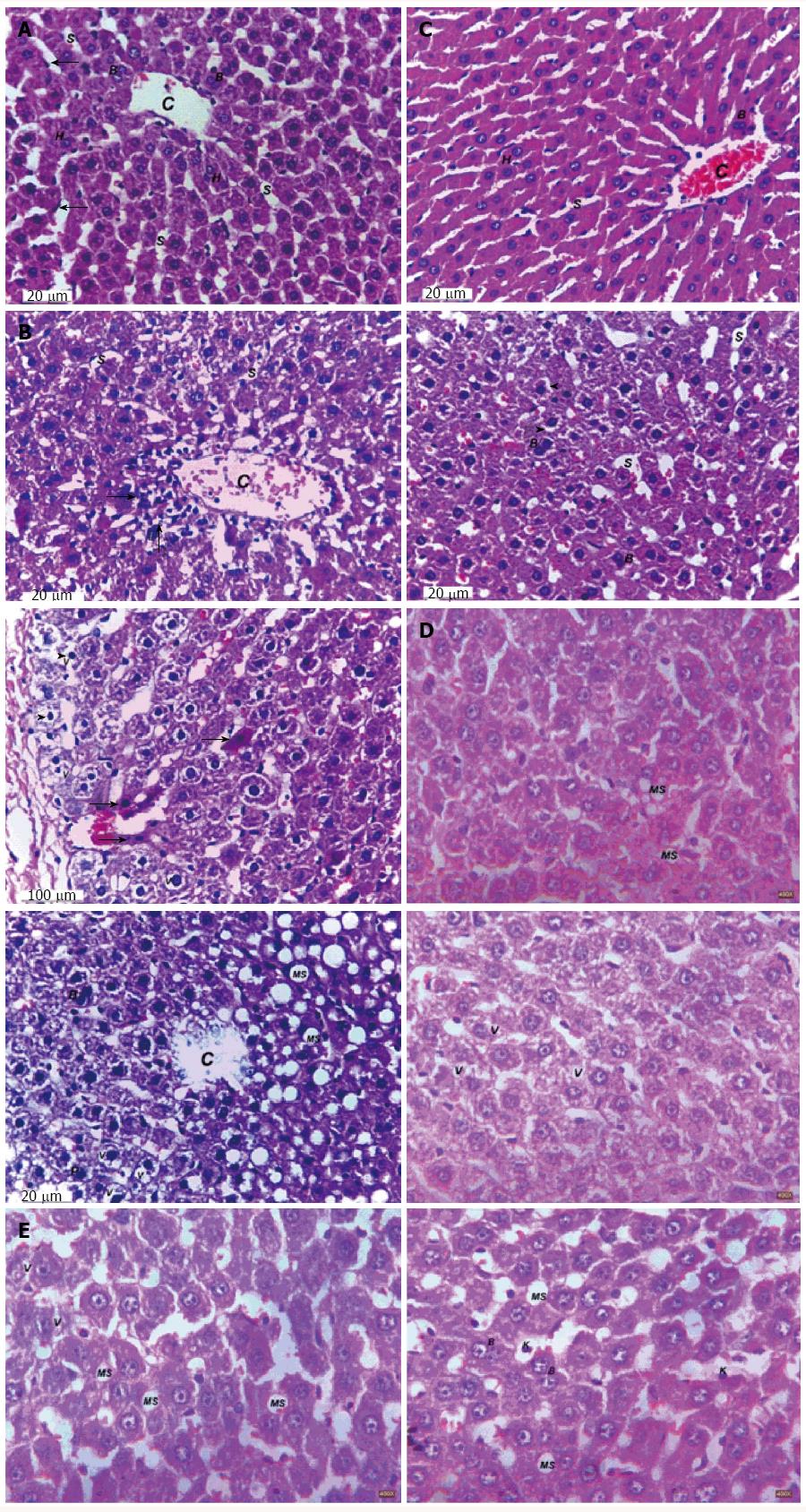
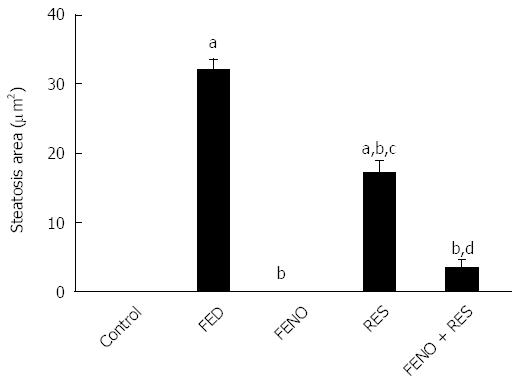
Representative photomicrographs of liver sections are described in Figure 8 and the steatosis area is represented in Figure 9. Feeding rats with 10% fructose in drinking water for 12 wk showed marked steatosis reaching 32.02 μm2/image. Histopathological examination revealed cellular infiltration, cytoplasmic vacuolations, increased Kupffer cells’ number, pyknosis and apoptosis of hepatic nuclei. Treatment with FENO completely prevented the induction of steatosis and preserved normal liver histology with irregular blood sinusoids and some degree of cytoplasmic vacuolations in hepatic cells. Notably, RES and FENO + RES treatments caused a marked improvement in the general structure of the liver tissue and markedly decreased the average steatosis area to 46% and 89% respectively and prevented most of the histopathological abnormalities observed in the FED group. The only abnormality seen was macrovesicular steatosis together with hepatic cell vacuolations, both of which are less than observed in the FED group.
Several studies emphasized that consumption of high fructose diets led to obesity, fatty liver, hypertriglyceridemia and insulin resistance[26,27]. One of the contributing factors is the fast hepatic uptake of fructose from the portal circulation compared to glucose and its bypassing the phosphofructokinase step in glycolysis. In the liver, fructose increases de novo synthesis of fatty acids[28] and increases inflammation[29], both contribute to increased hepatic lipogenesis.
In the present study fructose accelerated the accumulation of fat in the liver of rats, as evidenced by histological analysis and hepatic triglyceride content. In addition to hepatic fat accumulation, fructose induced hyperglycemia, hyperinsulinemia and insulin resistance. These results are in harmony with other previous studies[10,30].
One mechanism of fructose-induced insulin resistance is the defective transduction of insulin signaling[31]. Decreased number of IR in liver and skeletal muscle could provide an alternative explanation[32].
Several studies found that fructose-induced insulin resistance correlated with serum concentration of triglycerides[33,34]. Although insulin resistance is, at least partly, responsible for hypertriglyceridemia the possibility that hypertriglyceridemia itself may contribute to insulin resistance should not be ruled out[33]. As a consequence of increased circulatory TGs, the current study showed an accumulation of TGs in the liver. This is an important cause of NASH and may be the major risk factor for the observed glucose intolerance, hyperglycemia and insulin resistance in the FED rats. The primary source of hepatic TGs synthesis are the adipose tissue derived FFAs[35]. Although an excessive availability of plasma fatty acids is an important determinant of steatosis, lipogenesis is also considered as an important contributing factor. In fact, prolonged consumption of fructose also increases de novo lipogenesis and this contributes to fat accumulation in the liver[26,36].
Another important factor which plays an important role in NASH development by high fructose diet is fructose-induced oxidative stress[30]. This was demonstrated in this study by increased hepatic MDA content, reflecting enhanced lipid peroxidation. The fructose-induced oxidative stress could be attributed to accumulation of advanced glycation end-products[37,38].
In parallel with these abnormalities, the AST/ALT ratio was elevated. Most causes of liver cell injury are associated with a release of AST and ALT from damaged hepatocytes into the blood. The magnitude of AST and ALT elevations vary depending on the cause of the hepatocellular injury[39,40].
The role of fructose in the development of hepatic oxidative stress and predisposition to necroinflammation and fibrogenesis has been emphasized[41]. Indeed we found a significant increase in the gene expression of hepatic fibrogenic marker TGF-β1 and this result was in agreement with that of Sivakumar and Anuradha[42]. Moreover, fructose-induced increase in leptin gene expression could be another cause of augmented TGF-β1 expression. This is because a positive correlation exists between the expression of TGF-β1 in the liver and insulin resistance, obesity and steatosis[43]. Clinically, TGF-β1 plasma concentration is elevated in NASH patients compared to patients with hepatic steatosis and healthy subjects, suggesting that this cytokine is involved in fibrogenesis in NASH[44].
The possible molecular mechanism responsible for all these detrimental effects of fructose may be explained by the changes observed in hepatic SOCS-3, SREBP-1c, FAS and MCD genes expression in addition to adipose tissue leptin and adiponectin genes expression.
Regarding the SOCS-3 gene, its enhanced expression would result in decreased tyrosine phosphorylation of IRS proteins and consequently attenuation of insulin action. Another contributing role of SOCS-3 arises from its ability to increase fatty acid synthesis by up-regulation of SREBP-1c expression, presumably through suppression of STAT3 phosphorylation and persistent hyperinsulinemia[4].
As for the increased SREBP-1c gene expression, this leads to increased lipid synthesis[5]. Beside SREBP-1c, FAS gene expression was increased. These results are in accordance with another study that reported the upregulation of FAS and SREBP-1c protein expression in high-fructose-fed rats[45]. Increased FAS expression, which is responsible for fatty acid synthesis, together with decreased MCD expression, which is responsible for fatty acid oxidation, would collectively explain the increased fat accumulation in the liver currently noticed in the FED rats. Overproduction of fatty acids results in further insulin resistance, creating a vicious cycle. The overexpression of SOCS proteins may be a critical step in this vicious cycle[4].
Aside from hyperinsulinemia and insulin resistance, fructose feeding caused an increase in leptin gene expression in epididymal adipose tissue. This could possibly be mediated by elevation in serum TNF-α. Previous studies pointed to a direct correlation between TNF-α and leptin levels in mice[46], hamsters[47] and in diabetic patients[48]. Likewise, leptin gene expression was increased in diet-induced obese mice, in mice with genetic obesity (db/db)[49] and in obese patients[50].
Leptin plays an important role in limiting TGs accumulation in liver and skeletal muscle through direct activation of AMPK[51] and consequently increases fatty acid β-oxidation. However, leptin resistance was found to be associated with reduction of leptin-mediated JAK-STAT signaling, and induction of SOCS-3[52]. Therefore, the observed hypertriglyceridemia and hepatic steatosis induced by fructose in the present study would have resulted from a reduction in the hepatic catabolism of fatty acids driven by a state of leptin resistance.
There was an increase in leptin gene expression together with a significant reduction in epididymal adipose tissue adiponectin gene expression. In turn this would lead to insulin resistance[53] through accumulation of hepatic TGs and down-regulation of insulin signalling.
It is now known that adipose tissue has a pivotal role in obesity, insulin resistance, NASH and other metabolic abnormalities and that obesity is associated with insulin resistance[54], inflammation[55], macrophages accumulation[56] and expression of TNF-α. This explains the rise in TNF-α in rats given high fructose diet. The possible cause of serum TNF-α increase is the rise observed in leptin gene expression as this confirms the finding of a dramatic increase in serum TNF-α level after leptin injection[57].
Fenofibrate has been used for many years to treat dyslipidemias and has also recently been shown to have anti-inflammatory effects. In view of the lipid lowering properties of fenofibrate, we attempted to study its efficacy in controlling the metabolic derangements in rats fed fructose to induce NASH. Our study showed that fenofibrate, besides decreasing body weight, it restored liver histology to normal, prevented hepatic steatosis and maintained irregular blood sinusoids and some degree of cytoplasmic vacuolations in hepatic cells and this was reflected by improvement in liver function tests. These effects are in harmony with those reported by many investigators[58,59].
The histological improvement observed by fenofibrate was accompanied by restoration of insulin sensitivity and reduction in hepatic and serum TGs. Other investigators demonstrated that, PPAR-α activators improve insulin sensitivity in rodents with genetic and/or high fat diet-induced insulin resistance[60], in rats with NASH induced by high fat and low methionine and choline diet[61] and in patients with metabolic syndrome[62]. This was attributed to limitation of lipid accumulation in liver and muscles[63] and increasing fatty acid β-oxidation in hepatocytes mitochondria[64].
The current investigation showed that, fenofibrate increased liver index. Being a PPAR-α agonist, it may cause hepatomegaly in rodents but not in humans[65].
The current study offers possible molecular mechanisms responsible for fenofibrate beneficial effects. Genes expressions were remodeled either in the liver or adipose tissue where the drug caused a decrease in hepatic genes expressions of SOCS-3 and FAS and increased MCD without affecting that of SREBP-1c.
Enhanced liver MCD expression would result in decreased malonyl CoA and, subsequently, decreased free fatty acid and TGs deposition in liver. This confirms Lee et al[66] finding that, PPAR-α activates rat hepatic MCD transcription. In support, the highly elevated malonyl-CoA levels in the skeletal muscle and liver of pre-diabetic, high fat fed-rat were significantly reduced by fenofibrate[67].
An unexpected finding was the suppression of FAS expression by FENO although SREBP was not decreased to any significant extent. This might be by virtue of the activation of AMPK as reported by Chen et al[68] who showed that, the lipid lowering effects of fenofibrate may be exerted through a PPAR-α/AMPK dependent pathway leading to increased fatty acid β-oxidation. AMPK activation by fenofibrate would also suppress FAS expression.
Because fibrogenesis is an integral part of steatohepatitis, another devastating effect that was ameliorated by fenofibrate is the elevated expression of hepatic TGF-β, a fibrogenic marker. The reduction in TGF-β expression was associated with attenuated obesity, reduced adipose leptin expression, increased serum TNF-α and hepatic SOCS-3 expression, factors that precipitate fibrosis.
In adipose tissue, fenofibrate caused an improvement in leptin sensitivity as it decreased adipose tissue leptin gene expression. This was associated with decreased SOCS-3 gene expression. This adds support to the finding that, SOCS-3 insufficiency enhances leptin sensitivity[69]. Several studies showed that, PPAR-α agonists decrease leptin levels in obesity induced animal models and in type 2 diabetic patients with hypertriglyceridemia[70].
Adiponectin is implicated to be an anti-inflammatory adipokine and TNF-α antagonist. Accordingly, its expression is related to obesity, insulin resistance and TNF-α. However, in the current model, fenofibrate caused no change in adipose tissue adiponectin gene expression. This effect was in harmony with the study showing that, fenofibrate treatment in high fat fed rats failed to decrease adiponectin plasma level or gene expression in adipose tissue[71]. Similarly, in obese women with type 2 diabetes, serum adiponectin concentration was not significantly affected by fenofibrate treatment[72]. One of the explanations afforded is that PPAR-α activation, besides reducing adiposity of body, it increases adiponectin receptor-1 expression in adipose tissue and this could enhance adiponectin effects although no change can be detected in circulating adiponectin levels[73].
The effect of fenofibrate on oxidative stress is a matter of controversy, depending on the condition in which it is used. In the present study 4 wk treatment with fenofibrate did not alter the oxidative stress parameters in the liver. This was consistent with the results of El-Sheikh and Rifaai who reported that, PPAR-α could not ameliorate cyclophosphamide-induced oxidative stress[74]. However, some studies have shown a detrimental effect of long term fenofibrate use[75]. Others have shown a beneficial effect in streptozotocin-induced diabetic rats[76].
Resveratrol is a natural dietary polyphenol which has been shown to combine antioxidant, anti-inflammatory and antihyperlipidemic effects[14,17]. In the present study, when resveratrol was given to rats fed high fructose diet it prevented the gain in body weight and modulated the severity of liver damage seen microscopically and relieved the development of NASH. It ameliorated insulin resistance via reducing hepatic lipid deposition, prevented lipid peroxidation and ROS formation and inhibited inflammation by suppressing the production of TNF-α.
The first observation with resveratrol was a desirable decrease in body weight. It has been reported that resveratrol supplementation decreases body weight[77] and it has an anti-obesity potential[78]. The decrease in body weight could also be attributed to the enhanced insulin sensitivity and decreased accumulation of body-fat mediated in part by adipokines changes as resveratrol reduced leptin and increased adiponectin mRNA levels. This is in harmony with a number of previous studies showing the importance of leptin adiponectin balance[79,80]. In contrast, other studies denied beneficial effects of resveratrol on body weight and obesity[13,81]. The discrepancy could be due to the duration of study, dosage of resveratrol, and age of animals.
In the histopathological examination, the only abnormality seen was macrovesicular steatosis together with hepatic cell vacuolations, both of which are less than that observed in the FED group. Several investigators have demonstrated a similar hepatoprotective effect of resveratrol against steatosis in mice[81] or rats[82] fed high fat diet.
The histological improvement of resveratrol was extended to involve an improvement in insulin sensitivity. This lends credit to previous studies showing improved insulin sensitivity in NAFLD rats[83] fed high fat diet and in obese humans[84]. The enhanced insulin sensitivity was reflected herein by decreased hepatic TGs deposition. This was in accordance with other investigators who demonstrated a reduction in hepatic fat deposition in experimental NAFLD models[14,83].
Another important property of resveratrol is having anti-inflammatory activity. This was shown in the present study by preventing the rise in serum TNF-α. Anti-inflammatory activity was also indicated by inhibition of inflammation in mice fed high fat diet[80], in NAFLD rats[14] and decreasing IL-6 and TNF-α mRNA and reduced the Kupffer cells number induced in the injured liver of mice after bile duct ligation[85]. In obese humans, resveratrol decreased expression levels of genes of inflammatory pathways, and plasma levels of several inflammatory markers[84].
Being an effective antioxidant, resveratrol could also inhibit the progression of steatosis or steatohepatitis. In the fructose fed rats it decreased hepatic MDA content. Resveratrol has been shown to prevent free radicals and inflammatory cytokines-induced hepatic damage by scavenging ROS and decreasing lipid peroxidation[86]. Resveratrol administration reduces oxidative stress in obese rats[87,88] and in hyperlipidemic rats[17]. Other studies supposed that, the intake of resveratrol containing preparations and polyphenols from muscadine grapes suppress or prevent oxidative stress induced by high fat high carbohydrate meal[89].
The observed resveratrol-induced improvement in NASH related parameters and histopathology was associated with a significant hepatoprotective effect revealed by the reduction in AST/ALT ratio. Other study showed that, resveratrol significantly reduced both ALT, AST serum levels in cholestatic liver injury[85].
On the molecular basis, resveratrol decreased hepatic SOCS-3 gene expression, therefore preventing insulin and leptin resistance. Similar findings were reported clinically in normal, healthy subjects in the postprandial state where resveratrol suppressed oxidative and inflammatory stress responses to in high fat high carbohydrate meal[89].
Additional findings reported in this study were the down-regulation of the expressions of the lipogenic genes; SREBP-1c and FAS. This was also reported in an earlier study[90]. Resveratrol may improve fructose-induced metabolic abnormalities by activating AMPK pathway[83]. Because resveratrol activates AMPK, one would expect that, AMPK activation in the liver shuts down anabolic processes like cholesterol and TG biosynthesis by reducing the activities of SREBP-1c and FAS. AMPK activation also promotes catabolic processes. This would lead to a reduction in lipid synthesis and higher fatty acid oxidation rates and finally, prevention of liver steatosis. Furthermore, resveratrol positively activates SIRT-1 through activation of AMPK[91]. In turn, SIRT-1 activation gives protection against insulin resistance[92] and results in SREBP-1c inhibition.
The expression of hepatic MCD gene was upregulated by resveratrol leading to suppression of lipogenesis and enhanced β-oxidation.
As for adipose tissue, resveratrol attenuated the expression of leptin gene, indicating an enhancement of leptin sensitivity and at the same time it increased adiponectin expression. Both of these actions would, in turn, lead to activation of AMPK[51,93]. The reduction in adipose leptin gene expression was associated with decreased hepatic SOCS-3 expression with consequent enhancement of leptin sensitivity[69].
The reduction observed in oxidative stress, serum TNF-α and leptin expression in addition to stimulation of adiponectin expression was reflected in reduced TGF-β expression in liver by resveratrol treatment. As an anti-inflammatory, resveratrol decreased TGF-β1 mRNA expression. It has been reported that resveratrol decreases NF-κB which controls transcription of TGF-β induced by CCl4[94].
There is an interplay between obesity, leptin, adiponectin, TNF-α and TGF-β.
Fenofibrate is an important lipid-regulating drug and could ameliorate NASH as shown in the present study. However, its use for long-term and in high-dose could induce the possibility of liver function damage[95,96]. In order to regulate blood lipid effectively, protect against NASH and avoid the occurrence of drug-induced liver injury, we investigated the effect of combining RES and half the dose of FENO. This will provide a definite experimental basis for the clinical use of this combination. The combination of half dose fenofibrate with half dose resveratrol showed a protection that was equivalent to the effect of the full FENO dose. Thus, there was a sort of additive or synergistic effect. The histopathological abnormalities observed in the FED group were prevented; the only abnormality seen was macrovesicular steatosis together with hepatic cell vacuolations, both of which are less than that observed in the FED group. This was accompanied by a correction of insulin resistance and a reduction in the deposition of TGs in the liver. Both effects could be, at least, additive.
One side effect of fenofibrate reported in the present investigation was increased lipid peroxidation. However, the combination improved the oxidative stress state as evidenced by a significant decrease in MDA as compared to fenofibrate alone and the decrease was even comparable to the full dose of resveratrol. In addition the combination produced a synergistic effect on liver GSH level.
Moreover, the effect of combination on serum TNF-α and liver function tests could be additive.
The decrease in hepatic SOCS-3 gene expression by fenofibrate was substantiated when combined with resveratrol. There was a significant reduction in SREBP-1c expression as compared to fenofibrate alone possibly due to enhanced adiponectin expression. FAS gene expression in liver dropped off and this could be an additive effect. Besides, the combination exerted a favorable synergistic effect on MCD expression.
The reduction in adipose leptin gene expression, which was additive, was associated with a reduction in hepatic SOCS-3 expression. Fenofibrate has no effect on the reduced adiponectin expression caused by fructose, but it increased adiponectin receptors number, while resveratrol enhanced adiponectin expression. So, combining the two drugs produced a favorable effect on insulin sensitivity and TGs accumulation.
Furthermore, the combination exerted a beneficial synergistic effect on the fibrogenic marker, TGF-β possibly due to decreased leptin gene expression caused by both treatments in addition to enhanced adiponectin expression driven by resveratrol treatment.
However, the anti-hypertriglyceridemic effect of fenofibrate, in the combination, was attenuated, possibly due to lowering the dose of fenofibrate to one half.
In conclusion, the current work may justify the use of lower doses of fenofibrate in combination with resveratrol to protect the liver from fructose induced hepatic steatosis and damage. The synergistic effect may be due to antioxidant, anti-inflammatory and anti-hyperlipidemic effect of resveratrol. As an add-on therapy, resveratrol can augment the beneficial outcome of a lower dose of fenofibrate and reduces its toxic or side effects.
The authors would like to thank Dr. Sherif Zaky Professor of histology, Faculty of Medicine, Cairo University, Cairo, Egypt, for his generous assistance and valuable information that helped in reporting the histopathological changes. We would like to thank the members of Biotechnology Unit, Faculty of Agriculture, Cairo University Research Park (FA-CURP) for their assistance in the achievement of the real time-PCR experiments. We are also grateful to Dr. Nelly Alieldin, Professor of biostatistics and cancer epidemiology, National Cancer Institute, Egypt for revising the statistical methods of this study.
Obesity, insulin resistance, hypertriglyceridemia and oxidative stress all contribute to the development of steatosis and its progression to nonalcoholic steatohepatitis (NASH). Fenofibrate that corrects dyslipidemia and resveratrol, an antioxidant, are possible candidates for NASH therapy. This study aimed to investigate the potential molecular mechanisms through which these two agents may work and the benefit from their combined use in lower doses.
There is still no approved drug for the treatment of NASH. However potential targets for NASH therapy are insulin resistance, inflammatory signaling and fibrosis, both of the latter two effects are promoted by oxidative stress. PPARα agonists as fenofibrate inhibits the development of steatosis, improves insulin resistance, and reduces hepatic inflammation, while resveratrol gives favorable results through both its antioxidant effect and non-antioxidant properties on genes expressions, downregulation of the hepatic inflammatory pathways, and antifibrogenesis effects. Novel therapies with insulin-sensitizing effects but also anti-inflammatory and antifibrotic actions are under research and development.
Several studies have tested either fenofibrate or resveratrol individually in NASH, however neither of them alone can be an ideal therapy for NASH as fenofibrate, although it prevented steatosis and improved insulin resistance, it poses the risk of developing liver function damage which is both dose and time-dependent while resveratrol, although it blunts many of the underlying disturbances characteristic of NASH, its use individually is insufficient. Combining both, in half dosesm has given better results than the full dose of fenofibrate alone in terms of hepatoprotection and correction of the underlying genes expressions responsible for insulin resistance as SOCS3, lipogenesis as SREBP-1C and fibrogenesis as TGFβ-1 that have been altered in NASH.
The combination, in the reduced doses, provides a promising experimental evidence for better hepatoprotection, and amelioration of the underlying insulin resistance, hepatic inflammation, and fibrogenesis in fructose-induced NASH and would thus establish the basis for further clinical investigation.
“Non-alcoholic fatty liver disease” is the accumulation of fat in liver due to causes other than excessive alcohol use (also called steatosis). NASH “non-alcoholic steatohepatitis” is the accumulation of fat in liver that is associated with inflammation and often accompanied by fibrosis. Fenofibrate is an antihyperlipidemic drug. Resveratrol is an antioxidant polyphenol extracted from grape.
The paper shows that the use of lower doses of fenofibrate in combination with resveratrol to protect the liver from fructose induced hepatic steatosis and damage.
P- Reviewer: Ding MX, Julie NL S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 3. | Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944-47949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 316] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Ueki K, Kadowaki T, Kahn CR. Role of suppressors of cytokine signaling SOCS-1 and SOCS-3 in hepatic steatosis and the metabolic syndrome. Hepatol Res. 2005;33:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737-12742. [PubMed] |
| 6. | Tsochatzis E, Papatheodoridis GV, Archimandritis AJ. The evolving role of leptin and adiponectin in chronic liver diseases. Am J Gastroenterol. 2006;101:2629-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S-465S. [PubMed] |
| 8. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 685] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 9. | García-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernández-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825-834. [PubMed] |
| 10. | Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond). 2005;2:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 554] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1659] [Article Influence: 118.5] [Reference Citation Analysis (2)] |
| 12. | Lam BP, Younossi ZM. Treatment regimens for non-alcoholic fatty liver disease. Ann Hepatol. 2009;8 Suppl 1:S51-S59. [PubMed] |
| 13. | Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3166] [Cited by in RCA: 3244] [Article Influence: 170.7] [Reference Citation Analysis (0)] |
| 14. | Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911-922. [PubMed] |
| 16. | Chen YJ, Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J Pharmacol Exp Ther. 2008;324:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Zhu L, Luo X, Jin Z. Effect of Resveratrol on Serum and Liver Lipid Profile and Antioxidant Activity in Hyperlipidemia Rats. Asian-Aust J Anim Sci. 2008;21:890-895. |
| 18. | Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911-917. [PubMed] |
| 19. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] |
| 20. | Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476-482. [PubMed] |
| 21. | Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271-278. [PubMed] |
| 22. | Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882-888. [PubMed] |
| 23. | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-474. [PubMed] |
| 24. | Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56-63. [PubMed] |
| 25. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [PubMed] |
| 26. | Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1205] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 27. | Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 840] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 28. | Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S-765S. [PubMed] |
| 29. | Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Armutcu F, Kanter M, Gurel A, Unalacak M. Excessive Dietary Fructose is Responsible for Lipid Peroxidation and Steatosis in the Rat Liver Tissues. Turkiye Klinikleri J Med Sci. 2007;27:164-169. |
| 31. | Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345-32353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 985] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 32. | Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens. 2003;16:973-978. [PubMed] |
| 33. | Lee MK, Miles PD, Khoursheed M, Gao KM, Moossa AR, Olefsky JM. Metabolic effects of troglitazone on fructose-induced insulin resistance in the rat. Diabetes. 1994;43:1435-1439. [PubMed] |
| 34. | Jalal R, Bagheri SM, Moghimi A, Rasuli MB. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr. 2007;41:218-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299:E685-E694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 37. | Levi B, Werman MJ. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J Nutr. 1998;128:1442-1449. [PubMed] |
| 38. | Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 39. | Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 40. | Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Alisi A, Manco M, Pezzullo M, Nobili V. Fructose at the center of necroinflammation and fibrosis in nonalcoholic steatohepatitis. Hepatology. 2011;53:372-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Sivakumar AS, Anuradha CV. Effect of galangin supplementation on oxidative damage and inflammatory changes in fructose-fed rat liver. Chem Biol Interact. 2011;193:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Kabel AM, Abd Elmaaboud MA, Albarraq AA. Ameliorative potential of omega 3 fatty acids and HMG-CoA reductase inhibitors on experimentally-induced non-alcoholic steatohepatitis. Prostaglandins Leukot Essent Fatty Acids. 2015;96:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667-1672. [PubMed] |
| 45. | Ren LP, Song GY, Hu ZJ, Zhang M, Peng L, Chen SC, Wei L, Li F, Sun W. The chemical chaperon 4-phenylbutyric acid ameliorates hepatic steatosis through inhibition of de novo lipogenesis in high-fructose-fed rats. Int J Mol Med. 2013;32:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Berkowitz DE, Brown D, Lee KM, Emala C, Palmer D, An Y, Breslow M. Endotoxin-induced alteration in the expression of leptin and beta3-adrenergic receptor in adipose tissue. Am J Physiol. 1998;274:E992-E997. [PubMed] |
| 47. | Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 615] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 48. | Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997;82:3408-3413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Wrann CD, Rosen ED. New insights into adipocyte-specific leptin gene expression. Adipocyte. 2012;1:168-172. [PubMed] |
| 50. | Lönnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1:950-953. [PubMed] |
| 51. | Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1554] [Cited by in RCA: 1462] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 52. | Münzberg H, Myers MG. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 365] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 53. | Yadav H, Jain S, Yadav M, Sinha PR, Prasad GB, Marotta F. Epigenomic derangement of hepatic glucose metabolism by feeding of high fructose diet and its prevention by Rosiglitazone in rats. Dig Liver Dis. 2009;41:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1350] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 55. | Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep. 2011;11:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 3626] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 57. | Ikejima K, Honda H, Yoshikawa M, Hirose M, Kitamura T, Takei Y, Sato N. Leptin augments inflammatory and profibrogenic responses in the murine liver induced by hepatotoxic chemicals. Hepatology. 2001;34:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Li W, Wang D, Song G, Zuo C, Qiao X, Qin S. The effect of combination therapy of allicin and fenofibrate on high fat diet-induced vascular endothelium dysfunction and liver damage in rats. Lipids Health Dis. 2010;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282:E1180-E1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Guerre-Millo M, Gervois P, Raspé E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638-16642. [PubMed] |
| 61. | Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008;82:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Ueno H, Saitoh Y, Mizuta M, Shiiya T, Noma K, Mashiba S, Kojima S, Nakazato M. Fenofibrate ameliorates insulin resistance, hypertension and novel oxidative stress markers in patients with metabolic syndrome. Obes Res Clin Pract. 2011;5:e267-e360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Lee HJ, Choi SS, Park MK, An YJ, Seo SY, Kim MC, Hong SH, Hwang TH, Kang DY, Garber AJ. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002;296:293-299. [PubMed] |
| 64. | Oosterveer MH, Grefhorst A, van Dijk TH, Havinga R, Staels B, Kuipers F, Groen AK, Reijngoud DJ. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis, and elongation in mice. J Biol Chem. 2009;284:34036-34044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. The PPAR alpha-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicol Sci. 2008;101:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J. 2004;378:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Zhao Z, Lee YJ, Kim SK, Kim HJ, Shim WS, Ahn CW, Lee HC, Cha BS, Ma ZA. Rosiglitazone and fenofibrate improve insulin sensitivity of pre-diabetic OLETF rats by reducing malonyl-CoA levels in the liver and skeletal muscle. Life Sci. 2009;84:688-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Chen WL, Chen YL, Chiang YM, Wang SG, Lee HM. Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARα/AMPK/FoxO1/ATGL pathway. Biochem Pharmacol. 2012;84:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 70. | Damci T, Tatliagac S, Osar Z, Ilkova H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur J Intern Med. 2003;14:357-360. [PubMed] |
| 71. | Larsen PJ, Jensen PB, Sørensen RV, Larsen LK, Vrang N, Wulff EM, Wassermann K. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003;52:2249-2259. [PubMed] |
| 72. | Anderlová K, Dolezalová R, Housová J, Bosanská L, Haluzíková D, Kremen J, Skrha J, Haluzík M. Influence of PPAR-alpha agonist fenofibrate on insulin sensitivity and selected adipose tissue-derived hormones in obese women with type 2 diabetes. Physiol Res. 2007;56:579-586. [PubMed] |
| 73. | Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358-3370. [PubMed] |
| 74. | El-Sheikh AA, Rifaai RA. Peroxisome Proliferator Activator Receptor (PPAR)- γ Ligand, but Not PPAR- α, Ameliorates Cyclophosphamide-Induced Oxidative Stress and Inflammation in Rat Liver. PPAR Res. 2014;2014:626319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Curran CR, Miller N. The impact of corporate culture on nurse retention. Nurs Clin North Am. 1990;25:537-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Olukman M, Sezer ED, Ulker S, Sözmen EY, Cınar GM. Fenofibrate treatment enhances antioxidant status and attenuates endothelial dysfunction in streptozotocin-induced diabetic rats. Exp Diabetes Res. 2010;2010:828531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2983] [Cited by in RCA: 3178] [Article Influence: 167.3] [Reference Citation Analysis (1)] |
| 78. | Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, Sun Y, Zhang Z, Zhu W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem. 2012;23:1100-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Eseberri I, Lasa A, Churruca I, Portillo MP. Resveratrol metabolites modify adipokine expression and secretion in 3T3-L1 pre-adipocytes and mature adipocytes. PLoS One. 2013;8:e63918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 81. | Tauriainen E, Luostarinen M, Martonen E, Finckenberg P, Kovalainen M, Huotari A, Herzig KH, Lecklin A, Mervaala E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and Fatty liver formation. J Nutr Metab. 2011;2011:525094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Shang J, Chen LL, Xiao FX. [Resveratrol improves high-fat induced nonalcoholic fatty liver in rats]. Zhonghua Ganzangbing Zazhi. 2008;16:616-619. [PubMed] |
| 83. | Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 84. | Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 986] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 85. | Chan CC, Cheng LY, Lin CL, Huang YH, Lin HC, Lee FY. The protective role of natural phytoalexin resveratrol on inflammation, fibrosis and regeneration in cholestatic liver injury. Mol Nutr Food Res. 2011;55:1841-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017-1026. [PubMed] |
| 87. | Gómez-Zorita S, Fernández-Quintela A, Macarulla MT, Aguirre L, Hijona E, Bujanda L, Milagro F, Martínez JA, Portillo MP. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr. 2012;107:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 88. | Franco JG, Lisboa PC, Lima NS, Amaral TA, Peixoto-Silva N, Resende AC, Oliveira E, Passos MC, Moura EG. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J Nutr Biochem. 2013;24:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Ghanim H, Sia CL, Korzeniewski K, Lohano T, Abuaysheh S, Marumganti A, Chaudhuri A, Dandona P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 90. | Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008;22:1367-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 91. | Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 692] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 92. | Rutanen J, Yaluri N, Modi S, Pihlajamäki J, Vänttinen M, Itkonen P, Kainulainen S, Yamamoto H, Lagouge M, Sinclair DA. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59:829-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3051] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 94. | Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2008;28:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 95. | Jiao HL, Ye P, Zhao BL. Protective effects of green tea polyphenols on human HepG2 cells against oxidative damage of fenofibrate. Free Radic Biol Med. 2003;35:1121-1128. [PubMed] |
| 96. | Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol. 2009;37:521-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |