Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.2894
Peer-review started: April 24, 2015
First decision: October 14, 2015
Revised: December 16, 2015
Accepted: January 17, 2016
Article in press: January 18, 2016
Published online: March 14, 2016
Processing time: 60 Days and 1.7 Hours
Sentinel lymph node (SLN) navigation surgery is accepted as a standard treatment procedure for malignant melanoma and breast cancer. However, the benefit of reduced lymphadenectomy based on SLN examination remains unclear in cases of gastric cancer. Here, we review previous studies to determine whether SLN navigation surgery is beneficial for gastric cancer patients. Recently, a large-scale prospective study from the Japanese Society of Sentinel Node Navigation Surgery reported that the endoscopic dual tracer method, using a dye and radioisotope for SLN biopsy, was safe and effective when applied to cases of superficial and relatively small gastric cancers. SLN mapping with SLN basin dissection was preferred for early gastric cancer since it is minimally invasive. However, previous studies reported that limited gastrectomy and lymphadenectomy may not improve the patient’s postoperative quality of life (QOL). As a result, the benefit of SLN navigation surgery for gastric cancer patients, in terms of their QOL, is limited. Thus, endoscopic and laparoscopic limited gastrectomy combined with SLN navigation surgery has the potential to become the standard minimally invasive surgery in early gastric cancer.
Core tip: Recently, a large-scale prospective study reported that the endoscopic dual tracer method, using a dye and radioisotope for sentinel lymph node (SLN) biopsy, was safe and effective when applied to cases of superficial and relatively small gastric cancers. However, previous studies reported that limited gastrectomy and lymphadenectomy may not improve the patient’s postoperative quality of life (QOL). As a result, the benefit of SLN navigation surgery for gastric cancer patients, in terms of their QOL, is limited. Thus, endoscopic and laparoscopic limited gastrectomy combined with SLN navigation surgery has the potential to become the standard minimally invasive surgery for early gastric cancer.
- Citation: Tani T, Sonoda H, Tani M. Sentinel lymph node navigation surgery for gastric cancer: Does it really benefit the patient? World J Gastroenterol 2016; 22(10): 2894-2899
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/2894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.2894
The sentinel lymph nodes (SLN) are defined as the first lymph nodes that drain from a primary tumour. The SLN concept was first introduced after reports that metastases of downstream lymph nodes were believed to be absent when no metastasis of the SLN was observed in patients with malignant melanoma (MM)[1]. SLN navigation surgery, based on the SLN concept, has since become accepted as a standard treatment procedure for MM. However, SLN navigation surgery has not been as effective in gastrointestinal (GI) cancers such as colorectal cancer and gastric cancer. In MM and breast cancer, limited lymphadenectomy based on the SLN examination has led to a decrease in postoperative lymph oedema and improvement in the patient’s postoperative quality of life (QOL)[2,3]. However, SLN navigation surgery has not been widely accepted for the treatment of GI cancers worldwide. Furthermore, the benefit of limited lymphadenectomy based on SLN examination remains unclear in cases of GI cancers.
In the present report, we review previous studies in the literature to determine whether SLN navigation surgery is beneficial in gastric cancer patients.
Currently, the application of the SLN concept to gastric cancer is controversial due to the complexity of the lymphatic drainage from gastric tumours. Since 2000, several studies have been performed in cases of gastric cancer using the SLN concept in east Asian countries. The reported detection rates, sensitivity, and accuracy of the SLN examination procedure were 55%-100%, 40%-100%, and 70%-100%, respectively[4]. A meta-analysis of SLN examination with 46 studies, published between 2001 and 2009, which included 2684 gastric cancer patients was published[5]. The authors reported that the detection rate and sensitivity of SLN examination were 97.5% and 87.8%, respectively. The authors cautioned that SLN examination was probably not clinically valid for limited lymphadenectomy because of its low sensitivity and inter-study heterogeneity. They also reported that more than four collected SLNs was only associated with its higher sensitivity[5]. A different meta-analysis was subsequently published including 38 studies and 2128 patients[6]. In this meta-analysis, the detection rate, sensitivity, and accuracy of the SLN examination were concordance with the previous reports[4,5]. T stage, type of tracers, injection procedures, type of surgery (laparoscopic or open), and using immunohistochemistry for diagnosis or not were related to the detection rate and sensitivity. More recently, a multicentre prospective study from the Gastric Cancer Surgical Study Group of the Japanese Clinical Oncology Group (JCOG) was published[7]. This study revealed that the high false negative rates and the necessity of a learning curve, in addition to the pathological examination of only one frozen section, were limitations for detecting SLN metastasis. To overcome these limitations, a large-scale prospective multicentre study was performed by the Japanese Society of Sentinel Node Navigation Surgery[8]. Twelve skilled institutes who experienced a lot of cases (> 30 cases) of SLN mapping took part in the study. cT1-2N0M0 gastric cancers < 4 cm in diameter were included in this trial. Following examination of the identified SLNs during surgery, standard gastrectomy with D2 lymphadenectomy was performed according to the current Japanese gastric cancer treatment guidelines[9]. Eventually, 397 patients were included in the analysis. The detection rate, sensitivity, and accuracy of the SLN examination were 97.5% (387/397), 93% (53/57), and 99% (383/387), respectively. Surprisingly, false-negative rate was only 1% (four patients). Three of these four patients had pathological lymph node metastases only in the lymphatic basin - a lymphatic area including SLNs and downstream lymphatic flow. The other one patient with a metastasis outside the lymphatic basin had a primary tumour with > 4 cm. These results suggest that the ‘‘lymphatic basin’’ based surgery rather than the ‘‘SLN’’ based surgery was recommended for early gastric cancer (EGC) treatment by the expert institutes.
At present, a radioisotope (RI) alone or with a dye are used as tracers for the detection of SLN in gastric cancer.
In the RI method, 99mTc-phytate or 99mTc-tin colloid is commonly applied as a tracer. Since the half-life of 99mTc is approximately 6 h, the 99mTc RI tracer is injected endoscopically into the submucosa around the tumour 1 d before operation, and we measured the radioactivity of lymph nodes using a hand-held gamma probe at surgery. An important advantage of the RI method is the objective measurement of the intensity of radioactivity and the detection of SLN even in thick intraperitoneal adipose tissues. Furthermore, RI tracers remain in the lymph nodes for a relatively long period of time and, therefore, are preferred in laparoscopic surgery. However, RI tracers are expensive and a radioactivity-controlled area is necessary when using radioisotopes. Therefore, the RI method is performed only in a limited number of institutes in Japan and South Korea[4,10-12].
Conversely, the dye method is widely used for the detection of SLN in gastric cancer. Indocyanine green (ICG), sulfan blue, and isosulfan blue are commonly used as a tracer. These were injected into the submucosa by intraoperative endoscopy, or into the subserosa from luminal outside. The enhanced visibility, low cost, safety, and the ability to stain not only the lymph nodes but also the lymphatic route are the reason why the dye method is widely used.
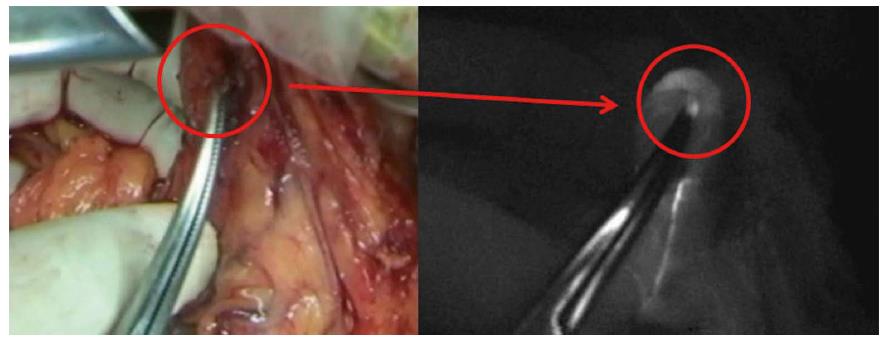
Recently, the double tracer method (i.e., dye and RI) has been recommended for reliable SLN detection in gastric cancer[8]. Moreover, a different type of fluorescence imaging system was developed for SLN navigation surgery[13]. The photodynamic eye (PDE; Hamamatsu Photonics, Hamamatsu, Japan) is able to visualize ICG fluorescence emitted by a light-emitting diode. The PDE visualizes SLNs and lymphatic vessels more clearly than the usual ICG staining method. In our institute, we have performed SLN biopsy using ICG fluorescence based on the established method for breast cancer[14]. We could detect minute SLN metastasis with this method (Figure 1). However, it is necessary to make the operating room pitch-darkness for detecting SLNs while performing SLN mapping using the PDE. However, newly developed ICG fluorescence systems such as the D-light P system (Karl Storz, Tuttlingen, Germany) do not need for switching off the room lights when we try to detect SLN. Moreover, with this system, we can perform the SLN examination even in laparoscopic surgery. Therefore, in the future, this may become the standard method to detect SLN in GI malignancies.
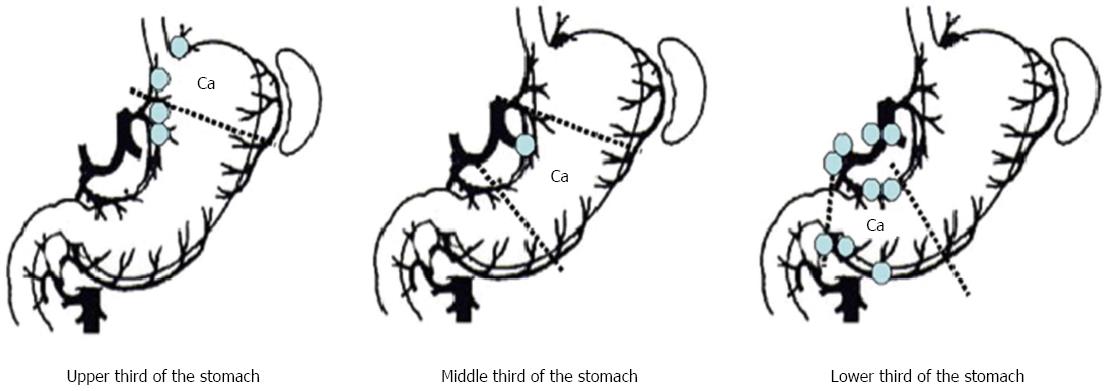
In order to apply the results of SLN examination to surgery, accurate intraoperative diagnosis is required. Intraoperative diagnosis using hematoxylin and eosin (H-E) staining of a frozen section from the lymph node is the standard technique for SLN biopsy. However, the previous JCOG study questioned the reliability of frozen section examination using just one section[7]. In a Japanese multicentre trial, it was also reported that 24% of patients with SLN metastases that were diagnosed using permanent sections could not be identified using H-E staining of frozen sections collected during surgery[8]. Multistep level sections, immunohistochemistry[15,16], reverse transcription polymerase chain reaction (RT-PCR)[17-20], and the one-step nucleic acid amplification assay (OSNA)[21], have all been developed for reduction of the false-negative rate and provide additional tools for diagnosing micrometastases in SLNs. By using both complete serial sectioning and immunohistochemistry, lymph node micrometastases were identified in four out of 35 patients (11%), and in six of the 1028 nodes (0.58%), where metastases were not detected with the permanent section method[15]. Shimizu et al[20] developed a more sensitive real-time RT-PCR method by using cytokeratin 19 (CK19) mRNA, cytokeratin 20 (CK20) mRNA, and carcinoembryonic antigen (CEA) mRNA. They reported that 27% of patients with pathologically negative lymph nodes were positive for RT-PCR. In this study, there were seven cases (6.8%) that SLNs were negative but non-SLNs were positive for RT-PCR. However, three of these seven cases had RT-PCR positive lymph node in the same lymph node station as corresponding SLNs and four of seven cases had RT-PCR positive lymph node in the same lymphatic basin. In our previous study, lymph node micrometastases in pT1 cases were also limited to the perigastric lymph nodes near the primary tumour and the lymph node station along to the left gastric artery according to lymph node mapping (Figure 2)[22]. Moreover, Kumagai et al[23] demonstrated that the sensitivity and specificity of the OSNA assay was higher than conventional H-E examination. Recently, it has been possible to reduce the detection time to 30-40 min[24], and these novel molecular techniques have also raised the sensitivity to detect SLN metastases as part of the intraoperative diagnosis. It is very important to improve the precision of the intraoperative SLN metastases by developing new technologies[25-27]. However, increasing the diagnostic sensitivity leads to increased false-negative rates for such molecular diagnostic techniques. Therefore, the false-negative rates may never become zero even if there are further developments in molecular techniques in the future. Nonetheless, almost all studies investigating micrometastases in SLN examination support the oncological safety of lymphatic basin dissection (LBD)[15,20,28]. Currently, LBD is best method for limited lymphadenectomy in gastric cancer.
If SLN navigation surgery is validated as a treatment, it will enable the omission of extended lymphadenectomy and will limit the area of gastrectomy [pylorus-preserving gastrectomy (PPG)/segmental gastrectomy]. A lot of investigators have reported postoperative objective and subjective outcomes of such limited surgery. These studies have reported that PPG leads to better maintenance of body weight[29], lower incidence of dumping syndrome or early emptying[30], mild regurgitation gastritis[31,32], less disturbed bowel movements[30], better preservation of gallbladder function[33], less post-gastrectomy syndrome[34], and lower incidence of cholecystolithiasis[32], as compared to conventional distal gastrectomy. However, such studies either involved small case series[29,31,33], or were single-centre or retrospective studies[29-33]. There is no published prospective randomised control trial (RCT) comparing the patient’s postoperative QOL between distal gastrectomy and PPG. Moreover, it has been reported that patient’s QOL following laparoscopy-assisted PPG is equivalent to that following laparoscopy-assisted distal gastrectomy[35]. With regard to the extent of lymphadenectomy, an RCT carried out in Taiwan reported that patients with D3 lymphadenectomy showed no significant difference in short- and long-term QOL as compared to patients with D1 lymphadenectomy[36].
In summary, pylorus-preserving and limited lymphadenectomy may not improve the patient’s postoperative QOL following gastrectomy.
The indications for endoscopic treatments such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are limited to cases with (1) mucosal tumours; (2) histologically differentiated adenocarcinomas; (3) tumours < 2 cm in diameter; and (4) no ulcer or no ulcer scar in the lesions. This is because if the primary tumour meets these criteria, lymph node metastasis is considered to be absent[37,38]. Standard gastrectomy is recommended even for cT1 gastric cancer, outside the general criteria for endoscopic treatments, in the recent Japanese Guideline for Gastric Cancer[37]. However, lymph node metastasis is present only in 10%-20% of such patients. Recent technical advances in endoscopic treatments have enabled the en-bloc resection regardless of the size and histological type of the gastric tumour. Theoretically, if all SLNs are histologically negative for cancer metastases, EMR/ESD, instead of gastrectomy, may be appropriate for the curative resection of cT1 EGC, outside the EMR/ESD criteria. A combination of laparoscopic SLN biopsy and EMR/ESD for cT1 EGC is a very attractive treatment option as a novel minimally invasive approach. However, it is still arguable whether EMR/ESD would be sufficient to achieve negative vertical margin. Therefore, surgical or endoscopic full-thickness resection is oncologically safer than EMR/ESD. Recently, laparoscopic full-thickness resection was developed for gastric tumours. However, this procedure may be associated with the iatrogenic dissemination of cancer cells due to the exposure of the gastric mucosa to the peritoneal cavity. Nunobe et al[39] developed a modified method for laparoscopic and endoscopic cooperative surgery (LECS) to avoid such dissemination. More recently, different techniques have been developed, such as the combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (CLEAN-NET)[40], and non-exposed endoscopic wall-inversion surgery (NEWS)[41]. Such techniques theoretically avoid the gastric mucosa from exposing to the peritoneal cavity. These novel techniques with SLN mapping are expected to enable the appropriate treatment of EGC when EMR/ESD is not applicable. Recently, SENORITA trial (NCT01804998) has been conducted in South Korea. This study is a multicenter phase III randomized control trial (RCT) enrolling 580 patients. This trial is the first large scale RCT to confirm the usefulness of SLN navigation surgery for EGC, which is not suitable for EMR/ESD. We need to pay attention to the final result of this study.
The benefit of SLN navigation surgery for gastric cancer patients, in terms of the postoperative QOL, is limited. EMR/ESD and laparoscopic limited gastrectomy combined with SLN navigation surgery have the potential to become the standard minimally invasive surgery for EGC patients.
P- Reviewer: Murata A, Reim D S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
| 1. | Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3238] [Cited by in RCA: 2932] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 2. | de Vries M, Hoekstra HJ, Hoekstra-Weebers JE. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann Surg Oncol. 2009;16:2840-2847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Schulze T, Mucke J, Markwardt J, Schlag PM, Bembenek A. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol. 2006;93:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol. 2013;20:522-532. [PubMed] |
| 5. | Ryu KW, Eom BW, Nam BH, Lee JH, Kook MC, Choi IJ, Kim YW. Is the sentinel node biopsy clinically applicable for limited lymphadenectomy and modified gastric resection in gastric cancer? A meta-analysis of feasibility studies. J Surg Oncol. 2011;104:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Miyashiro I, Hiratsuka M, Sasako M, Sano T, Mizusawa J, Nakamura K, Nashimoto A, Tsuburaya A, Fukushima N. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer. 2014;17:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1896] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 10. | Uenosono Y, Natsugoe S, Higashi H, Ehi K, Miyazono F, Ishigami S, Hokita S, Aikou T. Evaluation of colloid size for sentinel nodes detection using radioisotope in early gastric cancer. Cancer Lett. 2003;200:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Mochiki E, Kuwano H, Kamiyama Y, Aihara R, Nakabayashi T, Katoh H, Asao T, Oriuchi N, Endo K. Sentinel lymph node mapping with technetium-99m colloidal rhenium sulfide in patients with gastric carcinoma. Am J Surg. 2006;191:465-469. [PubMed] |
| 12. | Kim MC, Kim HH, Jung GJ, Lee JH, Choi SR, Kang DY, Roh MS, Jeong JS. Lymphatic mapping and sentinel node biopsy using 99mTc tin colloid in gastric cancer. Ann Surg. 2004;239:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. 2008;25:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Abe H, Mori T, Umeda T, Tanaka M, Kawai Y, Shimizu T, Cho H, Kubota Y, Kurumi Y, Tani T. Indocyanine green fluorescence imaging system for sentinel lymph node biopsies in early breast cancer patients. Surg Today. 2011;41:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Ishii K, Kinami S, Funaki K, Fujita H, Ninomiya I, Fushida S, Fujimura T, Nishimura G, Kayahara M. Detection of sentinel and non-sentinel lymph node micrometastases by complete serial sectioning and immunohistochemical analysis for gastric cancer. J Exp Clin Cancer Res. 2008;27:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Uenosono Y, Natsugoe S, Ehi K, Arigami T, Hokita S, Aikou T. Detection of sentinel nodes and micrometastases using radioisotope navigation and immunohistochemistry in patients with gastric cancer. Br J Surg. 2005;92:886-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Ajisaka H, Miwa K. Micrometastases in sentinel nodes of gastric cancer. Br J Cancer. 2003;89:676-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Takeuchi H, Ueda M, Oyama T, Shimizu Y, Kitagawa Y. Molecular diagnosis and translymphatic chemotherapy targeting sentinel lymph nodes of patients with early gastrointestinal cancers. Digestion. 2010;82:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Arigami T, Natsugoe S, Uenosono Y, Mataki Y, Ehi K, Higashi H, Arima H, Yanagida S, Ishigami S, Hokita S. Evaluation of sentinel node concept in gastric cancer based on lymph node micrometastasis determined by reverse transcription-polymerase chain reaction. Ann Surg. 2006;243:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Shimizu Y, Takeuchi H, Sakakura Y, Saikawa Y, Nakahara T, Mukai M, Kitajima M, Kitagawa Y. Molecular detection of sentinel node micrometastases in patients with clinical N0 gastric carcinoma with real-time multiplex reverse transcription-polymerase chain reaction assay. Ann Surg Oncol. 2012;19:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Yaguchi Y, Sugasawa H, Tsujimoto H, Takata H, Nakabayashi K, Ichikura T, Ono S, Hiraki S, Sakamoto N, Horio T. One-step nucleic acid amplification (OSNA) for the application of sentinel node concept in gastric cancer. Ann Surg Oncol. 2011;18:2289-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Sonoda H, Yamamoto K, Kushima R, Okabe H, Tani T. Detection of lymph node micrometastasis in gastric cancer by MUC2 RT-PCR: usefulness in pT1 cases. J Surg Oncol. 2004;88:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Kumagai K, Yamamoto N, Miyashiro I, Tomita Y, Katai H, Kushima R, Tsuda H, Kitagawa Y, Takeuchi H, Mukai M. Multicenter study evaluating the clinical performance of the OSNA assay for the molecular detection of lymph node metastases in gastric cancer patients. Gastric Cancer. 2014;17:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Yanagita S, Natsugoe S, Uenosono Y, Arigami T, Arima H, Kozono T, Funasako Y, Ehi K, Nakajo A, Ishigami S. Detection of micrometastases in sentinel node navigation surgery for gastric cancer. Surg Oncol. 2008;17:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Soltesz EG, Kim S, Kim SW, Laurence RG, De Grand AM, Parungo CP, Cohn LH, Bawendi MG, Frangioni JV. Sentinel lymph node mapping of the gastrointestinal tract by using invisible light. Ann Surg Oncol. 2006;13:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Koyama T, Tsubota A, Nariai K, Mitsunaga M, Yanaga K, Takahashi H. Novel biomedical imaging approach for detection of sentinel nodes in an experimental model of gastric cancer. Br J Surg. 2007;94:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ojima T, Kinami S, Nakamura K, Oyama K, Inokuchi M, Fujita H, Ninomiya I, Fushida S, Fujimura T, Kitamura S. Advantages of the rapid double-staining method for intraoperative detection of micrometastasis in sentinel lymph nodes. Oncol Rep. 2013;30:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Miyake K, Seshimo A, Kameoka S. Assessment of lymph node micrometastasis in early gastric cancer in relation to sentinel nodes. Gastric Cancer. 2006;9:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Isozaki H, Okajima K, Momura E, Ichinona T, Fujii K, Izumi N, Takeda Y. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 1996;83:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Nakane Y, Akehira K, Inoue K, Iiyama H, Sato M, Masuya Y, Okumura S, Yamamichi K, Hioki K. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Hepatogastroenterology. 2000;47:590-595. [PubMed] |
| 32. | Park do J, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008;32:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 33. | Imada T, Rino Y, Takahashi M, Suzuki M, Tanaka J, Shiozawa M, Kabara K, Hatori S, Ito H, Yamamoto Y. Postoperative functional evaluation of pylorus-preserving gastrectomy for early gastric cancer compared with conventional distal gastrectomy. Surgery. 1998;123:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Fujita J, Takahashi M, Urushihara T, Tanabe K, Kodera Y, Yumiba T, Matsumoto H, Takagane A, Kunisaki C, Nakada K. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2016;19:302-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 35. | Tomikawa M, Korenaga D, Akahoshi T, Kohshi K, Sugimachi K, Nagao Y, Tsutsumi N, Takenaka K, Kakeji Y, Hashizume M. Quality of life after laparoscopy-assisted pylorus-preserving gastrectomy: an evaluation using a questionnaire mailed to the patients. Surg Today. 2012;42:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, Whang-Peng J. Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. Br J Cancer. 2008;98:54-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Japanese Gastric Cancer Association. Gastric cancer treatment guidelines. 4th ed. Tokyo: Kanehara Publishing 2014; . |
| 38. | Sano T, Hollowood A. Early gastric cancer: diagnosis and less invasive treatments. Scand J Surg. 2006;95:249-255. [PubMed] |
| 39. | Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am. 2012;21:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 41. | Goto O, Takeuchi H, Kawakubo H, Sasaki M, Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |