Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.446
Peer-review started: July 30, 2015
First decision: August 31, 2015
Revised: September 11, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: January 7, 2016
Processing time: 155 Days and 12 Hours
AIM: To systematically review the data on distinctive aspects of peptic ulcer disease (PUD), Dieulafoy’s lesion (DL), and Mallory-Weiss syndrome (MWS) in patients with advanced alcoholic liver disease (aALD), including alcoholic hepatitis or alcoholic cirrhosis.
METHODS: Computerized literature search performed via PubMed using the following medical subject heading terms and keywords: “alcoholic liver disease”, “alcoholic hepatitis”,“ alcoholic cirrhosis”, “cirrhosis”, “liver disease”, “upper gastrointestinal bleeding”, “non-variceal upper gastrointestinal bleeding”, “PUD”, ‘‘DL’’, ‘‘Mallory-Weiss tear”, and “MWS’’.
RESULTS: While the majority of acute gastrointestinal (GI) bleeding with aALD is related to portal hypertension, about 30%-40% of acute GI bleeding in patients with aALD is unrelated to portal hypertension. Such bleeding constitutes an important complication of aALD because of its frequency, severity, and associated mortality. Patients with cirrhosis have a markedly increased risk of PUD, which further increases with the progression of cirrhosis. Patients with cirrhosis or aALD and peptic ulcer bleeding (PUB) have worse clinical outcomes than other patients with PUB, including uncontrolled bleeding, rebleeding, and mortality. Alcohol consumption, nonsteroidal anti-inflammatory drug use, and portal hypertension may have a pathogenic role in the development of PUD in patients with aALD. Limited data suggest that Helicobacter pylori does not play a significant role in the pathogenesis of PUD in most cirrhotic patients. The frequency of bleeding from DL appears to be increased in patients with aALD. DL may be associated with an especially high mortality in these patients. MWS is strongly associated with heavy alcohol consumption from binge drinking or chronic alcoholism, and is associated with aALD. Patients with aALD have more severe MWS bleeding and are more likely to rebleed when compared to non-cirrhotics. Pre-endoscopic management of acute GI bleeding in patients with aALD unrelated to portal hypertension is similar to the management of aALD patients with GI bleeding from portal hypertension, because clinical distinction before endoscopy is difficult. Most patients require intensive care unit admission and attention to avoid over-transfusion, to correct electrolyte abnormalities and coagulopathies, and to administer antibiotic prophylaxis. Alcoholics should receive thiamine and be closely monitored for symptoms of alcohol withdrawal. Prompt endoscopy, after initial resuscitation, is essential to diagnose and appropriately treat these patients. Generally, the same endoscopic hemostatic techniques are used in patients bleeding from PUD, DL, or MWS in patients with aALD as in the general population.
CONCLUSION: Nonvariceal upper GI bleeding in patients with aALD has clinically important differences from that in the general population without aALD, including: more frequent and more severe bleeding from PUD, DL, or MWS.
Core tip: Alcoholism is highly prevalent worldwide and can cause advanced-alcoholic-liver-disease (aALD) from alcoholic hepatitis or cirrhosis. This work systematically reviews the literature on acute-upper-gastrointestinal-bleeding not directly related to portal hypertension in patients with aALD. Such patients have markedly increased risks of peptic ulcers, and worse outcomes from peptic ulcer bleeding than other patients, including refractory bleeding, rebleeding, and mortality. Such patients apparently have increased frequency and mortality of bleeding from Dieulafoy lesions. Such patients have more frequent, more severe, and more rebleeding from Mallory-Weiss-syndrome than non-cirrhotics. Prompt endoscopy, after resuscitation, is essential to diagnose and appropriately treat these patients, using endoscopic therapy when necessary.
- Citation: Nojkov B, Cappell MS. Distinctive aspects of peptic ulcer disease, Dieulafoy's lesion, and Mallory-Weiss syndrome in patients with advanced alcoholic liver disease or cirrhosis. World J Gastroenterol 2016; 22(1): 446-466
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/446.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.446
Alcohol consumption remains highly prevalent worldwide despite its association with more than 60 diseases as well as accidental injuries[1]. For example, about two-thirds of adult Americans consume some alcohol[2], and up to one-sixth are alcoholics[3]. Although the burden of alcohol-related disease is generally highest in developed countries, the burden is also large and likely increasing in developing countries[4].
Chronic excessive alcohol consumption can cause liver injury, ranging from alcoholic fatty liver disease to alcoholic hepatitis to alcoholic cirrhosis[5]. These entities can occur simultaneously in one individual (e.g., alcoholic hepatitis with alcoholic cirrhosis)[6]. Alcoholic cirrhosis, the least common but most severe form of alcoholic liver disease, occurs in approximately 15% of individuals chronically abusing alcohol[7]. Alcoholic cirrhosis is associated with numerous life-threatening complications, including variceal bleeding, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome, and hepatocellular carcinoma[8].
Alcoholic hepatitis and alcoholic cirrhosis are herein denoted as advanced alcoholic liver disease (aALD). Upper gastrointestinal (UGI) bleeding in patients with aALD is clinically important due to its frequency, severity, and high mortality[9,10]. UGI bleeding accounts for up to 25% of the mortality from cirrhosis[11], and up to 10% of the mortality from alcoholic hepatitis[12]. Table 1 describes etiologies of UGI bleeding associated with aALD or cirrhosis, but excludes etiologies not believed to be associated with cirrhosis, such as reflux esophagitis, duodenitis, GI tumors, and angiodysplasia[13-31]. UGI bleeding related to cirrhosis or aALD includes etiologies directly related vs unrelated to portal hypertension. Etiologies directly related to portal hypertension include esophageal varices[32], gastric varices[15], duodenal varices[16], gastric portal hypertensive gastropathy[33], and gastric antral vascular ectasia (GAVE)[34]. Also, cirrhotic patients rarely develop lower GI bleeding related to portal hypertension from rectal varices[35]. Variceal bleeding has been frequently reviewed because it represents the etiology of UGI bleeding in about 70% of cases in cirrhotic patients, occurs in 25%-40% of cirrhotics, and constitutes a major cause of their mortality[36].
| Bleeding lesion | Endoscopic appearance | Pathophysiology | Nonsurgical treatment | Ref. |
| Etiologies related to portal hypertension | ||||
| Esophageal varices | Serpiginous, bluish-grey, vessels protruding from the mucosa into the lumen that typically are largest just above the gastroesophageal junction | Varices provide a conduit for venous blood blocked from traversing through the liver because of cirrhosis to return to the heart | Endoscopy: variceal banding, variceal sclerotherapy | Garcia-Tsao et al[13], Beppu et al[14] |
| Angiography: TIPS | ||||
| Other: balloon tamponade, octreotide | ||||
| Gastric varices | Serpiginous, bluish-grey, vessels protruding from the mucosa into the lumen that are most commonly located in the gastric cardia, fundus or body | Varices provide a conduit for venous blood blocked from traversing through the liver because of cirrhosis to return to the heart | Angiography: TIPS Other: balloon tamponade, octreotide Endoscopy: cyanoacrylate injection therapy | Garcia-Pagán et al[15] |
| Formation of gastric varices may be promoted by endoscopic obliteration of esophageal varices | ||||
| Duodenal varices | Resemble esophageal varices in endoscopic appearance, but are located within duodenum | Rare site of varices which may be promoted by prior esophageal variceal banding or sclerotherapy and prior duodenal surgery | Endoscopy: variceal banding or sclerotherapy | Copelan et al[16], Matsui et al[17] |
| Angiography: | ||||
| TIPS, angiographic occlusion therapy by embolization or balloon occlusion | ||||
| Portal hypertensive gastropathy | Mosaic or snake-skin appearance of gastric mucosa, especially of the gastric fundus and proximal gastric body, due to dilated, ectatic, superficial mucosal vessels | Network of microcirculation that drains venous blood blocked from passing through the cirrhotic liver to return to the left atrium | TIPS | Patwardhan et al[18], Thuluvath et al[19] |
| Etiologies possibly related to portal hypertension | ||||
| Gastric antral vascular ectasia | Intensely erythematous streaks on longitudinal folds oriented towards the pylorus in the antrum | May be related to stretch of antral vessels from duodenal bulb prolapse. Vascular engorgement from portal hypertension or from hormonal abnormalities (e.g., hyperestrogenemia with cirrhosis) may also contribute to lesion pathogenesis | Endoscopic therapy: APC, thermocoagulation, electrocoagulation, or radiofrequency ablation. | McGorisk et al[20], Payen et al[21] |
| Etiologies possibly related to alcoholism or advanced liver disease | ||||
| Peptic ulcer disease | Focal ulcer: (depressed) crater covered by mucopurulent material | Major causes in general population include H. pylori infection or NSAID use. Idiopathic PUD is increasingly noted. Gastric infections or gastric malignancy may mimic PUD. Pathogenesis of PUD in ALD and cirrhosis discussed in text | Endoscopic therapy: discussed in text | Siringo et al[22], Vergara et al[23], Kamalaporn et al[24], D’Amico et al[25] |
| Mallory- Weiss tear | Longitudinally oriented erythematous tear or crack in the mucosa that straddles the gastroesophageal junction | Laceration due to mucosal trauma from retching or vomiting. Frequently associated with binge drinking or chronic alcoholism | Endoscopic therapy: discussed in text | Paquet et al[26], Schuman et al[27], Jensen et al[28] |
| Dieulafoy’s lesion | Elevated, pigmented spot that projects into the lumen from the mucosal surface without surrounding ulceration | Caliber-persistent artery near mucosal surface can erupt through thin overlying mucosal cells and cause bleeding | Endoscopic therapy: injection therapy, band ligation, electrocoagulation, APC | Cappell[29], Jeon et al[30], Nojkov et al[31] |
| Angiography: local embolization | ||||
| Surgery: wedge resection | ||||
The relative frequency of acute UGI bleeding, not directly related to varices or portal hypertension, is estimated at 30%-40% of UGI bleeding in cirrhotic patients[10,37-40]. Peptic ulcer disease (PUD), Dieulafoy’s lesion (DL), and Mallory-Weiss syndrome (MWS) are strongly associated with alcoholic cirrhosis or cirrhosis in general[41,42]. These bleeding etiologies may be related to alcohol because alcohol can damage the gastric mucosal barrier, stimulate gastric acid secretion, and cause nausea and vomiting that induces MWS[43]. This review comprehensively and critically analyzes the association between PUD, DL, and MWS and aALD or cirrhosis; demonstrates underappreciated, major differences in the pathophysiology and natural history of these bleeding etiologies in cirrhotic patients compared to non-cirrhotic patients; and discusses the clinical consequences in terms of treatment and prognosis.
Computerized search of the literature was performed via PubMed using the following medical subject heading (MeSH) terms and keywords: “alcoholic liver disease”, “alcoholic hepatitis”,“ alcoholic cirrhosis”, “cirrhosis”, “liver disease”, “upper gastrointestinal bleeding”, “non-variceal upper gastrointestinal bleeding”, “PUD”, ‘‘DL’’, ‘‘Mallory-Weiss tear”, and “MWS’’. Of about 1200 articles initially identified by computerized review, about 825 articles were eliminated as not relevant to the subject of this paper after briefly reviewing the articles, including thoroughly reviewing the abstracts. The remaining 375 articles were thoroughly reviewed, and 165 articles were selected for incorporation and citation in this systematic review. The following criteria were applied for study inclusion: publication in peer-reviewed journals, studies published since 1980, reporting outcomes for non-variceal upper GI bleeding in patients with underlying liver disease, and reporting the etiology of liver disease. Criteria for prioritizing publications for review inclusion included: well-designed, prospective trials; recent studies; large study populations; and study emphasis on aALD. However, data from retrospective series, reviews from internationally recognized authorities, and even case reports were included when prospective trials were unavailable. The authors thank Jadranka Stojanovska, M.D., M.S., Assistant Professor of Radiology, University of Michigan Health System, Ann Arbor, Michigan, for her expert biostatistical review of the manuscript.
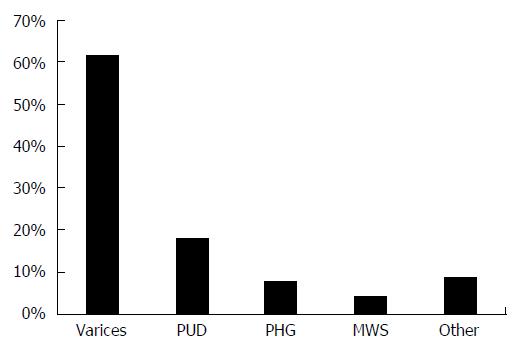
The estimated distribution of common etiologies of acute upper GI bleeding, unrelated to portal hypertension, in patients with cirrhosis are shown in Figure 1[39,44-47].
Epidemiology: Patients with cirrhosis have a significantly increased prevalence of PUD, with a reported point prevalence of 10% or higher[22-24,48,49]. For example, among the 324 of 368 consecutive cirrhotic patients who underwent endoscopic screening for esophageal varices, 11.7% were diagnosed with PUD[22]. The annual incidence of developing new PUD was 4.3% during endoscopic follow-up among 140 of these cirrhotic patients, an annual incidence that is much higher than that of PUD in the general population of < 0.5%[22]. In a prospective study of 130 patients with cirrhosis undergoing esophagogastroduodenoscopy (EGD) for variceal screening, 50 patients (39%) had PUD[24]. There were no significant differences in age, sex, or history of smoking tobacco between patients with vs without PUD. In a prospective South Korean study, PUD was found in 24.3% of the 288 patients with cirrhosis[50].
Advanced cirrhosis (Child-Pugh class B or C) is particularly associated with PUD[24]. For example, among 324 patients undergoing EGD, patients with advanced cirrhosis had a significantly higher frequency of PUD than patients with early cirrhosis (P = 0.04)[22].
PUD is the most common cause of nonvariceal UGI bleeding in patients with cirrhosis[25,46,51]. Among 160 patients with non-variceal UGI bleeding and cirrhosis (mostly from alcohol), 81 patients (50.6%) had PUD, and 53.1% had high-risk endoscopic stigmata of recent hemorrhage (SRH) at the ulcer base[52]. The reported prevalence of acute UGI bleeding from PUD (PUB) in patients with chronic liver disease ranges from 1.6% to 25%[22,47,53-55]. This wide variability likely reflects the diverse patient populations from different countries and different socioeconomic groups among various studies, and study of patients at different stages of liver disease. A recent, nationwide database study from Taiwan, conducted over 7 years, showed that PUB was significantly more prevalent among 4013 patients with nonalcoholic cirrhosis (4.8%), compared to either 8013 patients with chronic hepatitis without cirrhosis (1.6%) or 7793 controls without liver disease (1.6%)[53]. The three patient groups were matched for age, sex, comorbidities, medications, and annual income. This difference remained statistically significant even after adjusting for confounding factors (HR = 4.22; 95%CI: 3.37-5.29, P < 0.001)[53]. Advanced age, male sex, diabetes mellitus, chronic renal disease, prior variceal bleeding, and use of nonsteroidal anti-inflammatory drugs (NSAIDs) were risk factors for PUB in the cirrhotic patients.
Clinical outcomes: Patients with PUD associated with cirrhosis have higher rates of bleeding complications, of delayed ulcer healing, and of ulcer recurrence as compared to the general population[22,56]. For example, Siringo et al[22] showed that gastric ulcers healed more slowly and recurred more frequently than gastric ulcers in controls without cirrhosis (P = 0.04). The bleeding may be exacerbated by thrombocytopenia or an elevated international normalized ratio (INR) associated with cirrhosis and portal hypertension. Siringo et al[22] further reported that 79% of peptic ulcer recurrences in patients with cirrhosis were asymptomatic. This emphasizes the clinical importance of endoscopic follow-up of gastric ulcers in this population. In a recent, nationwide, database analysis from Taiwan of patients hospitalized for PUB from 1997-2006, patients with cirrhosis had a much higher rate of rebleeding from PUD than patients without cirrhosis (HR = 3.19; 95%CI: 2.62-3.88)[57].
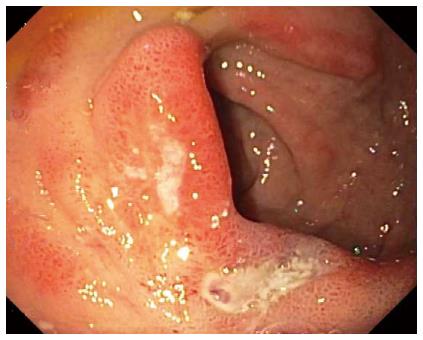
In a recent, retrospective, Finnish study of 518 patients with a first episode of nonvariceal UGI bleeding (NVUGIB), the rebleeding rate at one year among 102 alcoholics was significantly higher at 16.7% than the rate among the 416 nonalcoholic patients of 9.1% (P = 0.027)[58]. About half of the patients bled from PUD in both groups. Figure 2 illustrates the endoscopic findings of an acute 14 mm × 5 mm peptic duodenal ulcer with low risk stigmata of recent hemorrhage (SRH), consisting of a nonbleeding, flat, pigmented spot, in a patient with Child-Pugh class A alcoholic cirrhosis experiencing upper GI bleeding. Despite the absence of high risk SRH, the patient required transfusion of 4 units of packed erythrocytes for the bleeding episode.
Patients with advanced liver disease have increased mortality from PUB. In the aforementioned study from Taiwan, the 9711 patients with cirrhosis had significantly higher mortality-adjusted rebleeding rates than the 38844 matched controls without cirrhosis at 1 year (14.4% vs 11.3%, P < 0.001), 5 years (26.1% vs 22.5%, P < 0.001), and 10 years (28.4% vs 27.1%, P < 0.001)[57]. A population-based study of 21359 patients hospitalized in Denmark from 2004-2011 included 1127 (5.3%) with chronic liver disease, of whom 3.1% had cirrhosis and 2.2% did not have cirrhosis[59]. Cumulative 90-d mortality after discharge was significantly higher in patients with chronic liver disease: 25% mortality in patients with cirrhotic and 21% mortality in patients with chronic liver disease without cirrhosis vs 18% mortality in patients without chronic liver disease. After adjusting for confounding variables, including comorbidities, patients with cirrhosis had more than twice the mortality of patients without liver disease (OR = 2.38, 95%CI: 2.02-2.80), and patients with liver disease without cirrhosis had 46% higher mortality than patients without liver disease. In a cross-sectional, nationwide, study from the United States of 96887 inpatients admitted for PUB in 2009, the 3574 patients (3.7%) with cirrhosis had significantly higher mortality from PUB than patients without cirrhosis (5.5% vs 2.0%, P < 0.01), with an even higher mortality of 6.6% in decompensated cirrhotics[60]. The investigators reported no differences in endoscopic utilization between patients with vs without cirrhosis, but patients with cirrhosis incurred higher hospitalization costs, despite less frequently undergoing surgery for PUB.
Although patients with PUB and cirrhosis have worse clinical outcomes than patients with only PUB, they still have better clinical outcomes than patients with acute variceal bleeding and cirrhosis. A large, multicenter, prospective cohort study of 465 Italian patients with cirrhosis and acute UGI hemorrhage reported that 25% had non-variceal bleeding, most commonly from PUD[25]. Compared to patients with variceal bleeding, patients with PUB had significantly lower risks of uncontrolled initial bleeding (7% vs 15%), rebleeding (9.6% vs 19%), and mortality (15% vs 21%). Risk factors for mortality included alcoholism, serum hyperbilirubinemia, hypoalbuminemia, and presence of hepatic encephalopathy or hepatocellular carcinoma. Predictors of short-term (≤ 5 d) unfavorable outcomes of uncontrolled bleeding, rebleeding, or mortality, for patients with either variceal or nonvariceal bleeding, included active bleeding at EGD, low initial hematocrit, elevated aminotransferase levels, advanced Child-Pugh class, and portal vein thrombosis.
Table 2 summarizes data on UGI bleeding, unrelated to portal hypertension, with a focus on PUB in patients with cirrhosis[22,52,61-63]. The data on PUB in Table 2 are somewhat limited by including other etiologies of nonvariceal UGI bleeding aside from PUD, but all listed studies included a majority of cirrhotic patients bleeding from PUD. Table 3 summarizes the key reported clinical findings regarding PUD in patients with aALD or cirrhosis[22-25,48-50,53,56,57,59,60,64,65].
| Ref. | No. of parents | ALD (%) | Mean age | Prevalence of PUB (%) | Mortality (%) | Predictors of mortality | Rebleeding rate (%) | Length of follow-up |
| González-González et al[52] | 160 | “Most” | 56.5 ± 14.4 | 50.6 | 13.8 | High BUN, Low albumin, Active ulcer bleeding, Need for endoscopic therapy, Cryptogenic cirrhosis | 1.9 | NA |
| Seo et al[61] | 107 | 43 | 55.8 ± 11 | 60 | 14.5 | Failure to control bleeding, High creatinine, High AST, High Child-Pugh score, PVT | 9.3 | 6 wk |
| Morsy et al[62] | 93 | NA | 55.3 ± 11.2 | 30 | 14 | Bacterial infection, Shock, Early rebleeding, Low albumin, Low hemoglobin, Endoscopic therapy | 4.3 | Short-term (in-hospital) |
| Siringo et al[22] | 85 | 40 | 62 ± 12 | 41 | 231 | Low hemoglobin, | 0 | 6 wk |
| Encephalopathy | ||||||||
| Alcoholism, High bilirubin, PVT, High Child-Pugh score, | ||||||||
| High AST/ALT levels, HCC | ||||||||
| Rudler et al[63] | 29 | 24 (83) | 55.0 ± 9.0 | 29 (100)2 | 1 (3) | NA | 2 (7) | 30 d |
| Key finding |
| Increased prevalence of PUD in patients with cirrhosis. Most common etiology of non-variceal hemorrhage in cirrhotics |
| Advanced cirrhosis (Child-Pugh stage C) more strongly associated with PUD than early cirrhosis (Child-Pugh stage A) |
| Most patients with PUD associated with cirrhosis are asymptomatic |
| Patients with cirrhosis are more likely to bleed from PUD than other patients with PUD |
| Higher frequency of complications from bleeding PUD in patients with cirrhosis |
| Higher rate of re-bleeding from PUD in cirrhotics |
| Alcohol impairs ulcer healing and decreases patient compliance with anti-ulcer therapy |
| Higher mortality from PUB in cirrhotics compared to non-cirrhotics |
| Cirrhotic patients with PUD do not have a higher rate of H. pylori infection than non-cirrhotics with PUD |
Pathogenesis: The pathophysiology of the association between PUD and cirrhosis is incompletely understood. Alcohol toxicity, portal hypertension, Helicobacter pylori (H. pylori) infection, and nonsteroidal anti-inflammatory drugs (NSAIDs) have been investigated as etiologic factors.
Patients with aALD have a much higher prevalence of PUD than the general population, and likely have an even higher prevalence of PUD than patients with non-alcoholic cirrhosis[49,65]. This finding is somewhat controversial. In a prospective study of 216 male cirrhotic patients, evaluated for liver transplantation at the University of Pittsburgh, 12.2% of patients with alcoholic cirrhosis had duodenal ulcers, a significantly higher rate of duodenal ulcers than that in patients with non-alcoholic cirrhosis (e.g., cryptogenic, autoimmune, or viral cirrhosis)[49]. Additionally, an alcoholic etiology for cirrhosis is an important predictor of poor outcome 6 wk after acute UGI bleeding, regardless of the bleeding etiology[25]. Furthermore, a recent, prospective, French study reported that 27 of 29 cirrhotics with severe PUB were alcoholics, and that 93% of them had endoscopic evidence of portal hypertension[63]. The clinical outcome from PUB in this study was, surprisingly, not significantly different between patients with cirrhosis vs those without cirrhosis, when assessed for rebleeding rate (7% vs 6.9%), need for arterial embolization (10.3% vs 8.6%), need for salvage surgery (0% vs 1.7%), and mortality (3% vs 1.1%). However, another study reported similar rates of PUD in alcoholic vs nonalcoholic cirrhotics[24].
Alcohol impairs ulcer healing and alcoholism decreases patient compliance with recommended therapies[64]. It is toxic to gastric mucosa, and stimulates gastric acid secretion[43]. However, these factors do not completely explain the increased prevalence of PUD with aALD[66,67].
Data on the role of portal hypertension in PUD pathogenesis in patients with advanced liver disease are somewhat limited and relatively contradictory. Defensive mucosal factors, including gastric mucosal perfusion and mucosal secretion, are impaired in the presence of portal hypertension[68]. Furthermore, reduction of portal hypertension with propranolol reduces alcohol-induced gastric mucosal injury in rats with portal hypertension, and improves the endoscopic appearance of portal hypertensive gastropathy in patients with cirrhosis, suggesting that portal hypertension may promote PUD by impairing mucosal defenses[68,69].
In a recent, retrospective, single-center study from South Korea of 455 patients with cirrhosis or chronic hepatitis, cirrhosis was more strongly associated with PUD than chronic hepatitis (OR = 4.13, P < 0.03), but an elevated hepatic vein pressure gradient (HVPG) was not a risk factor for PUD in either group of patients[70]. However, another study of 245 cirrhotic patients vs 245 healthy controls, matched for age and sex, revealed that an HVPG ≥ 12 was a significant predictive factor for gastric ulcers in cirrhotic patients (24.4%), as compared to either cirrhotic patients with an HVPG < 12 mmHg (4.5%) or controls without cirrhosis (4.0%)[71]. This study, however, excluded patients with duodenal ulcers. Other studies reported an increased prevalence of endoscopic stigmata of portal hypertension, such as esophageal varices, in cirrhotic patients with PUB, as compared to cirrhotic patients without PUB[63,72]. For example, 27 of 29 patients with cirrhosis and severe PUB had endoscopic features of portal hypertension, vs none of 179 patients without cirrhosis and severe PUB had these endoscopic features (P < 0.0001)[63].
In the general population H. pylori is a major risk factor for PUD, especially for duodenal ulcers (DUs)[73]. The role of H. pylori in the pathogenesis of PUD in patients with advanced liver disease is somewhat controversial, and H. pylori likely plays a limited role in such patients. The prevalence of H. pylori infection in patients with cirrhosis reportedly ranges widely from 35%-89%, attributed to differences of H. pylori prevalence in different countries and in different populations within individual countries, and to the use of different diagnostic tests[50,74-79]. Populations in heavily industrialized countries have a much lower prevalence of H. pylori infection[80], while wealthy patients in heavily industrialized countries have the lowest prevalence[81]. Also, studies using serologic testing generally report a higher prevalence of H. pylori infection in patients with cirrhosis compared to studies using pathologic examination of gastric biopsies, partly due to the lower positive predictive value of serologic testing[76]. A recent meta-analysis of 21 studies involving 6135 patients, reported a significant association between cirrhosis and H. pylori infection in patients with PUD (OR = 2.05, 95%CI: 1.33-3.18, P < 0.0001)[82]. However, subgroup analysis revealed significantly higher prevalence of H. pylori infection in patients from Europe (OR = 2.98, 95%CI: 2.02-4.39, P < 0.0001) or from America (OR = 4.75, 95%CI: 1.42-15.95, P = 0.025), but not in patients from Asia (OR = 0.90, 95%CI: 0.48-1.66, not significant).
In a prospective South Korean study in which H. pylori infection was diagnosed by rapid urease testing and histologic analysis of gastric biopsies, the prevalence of H. pylori infection was significantly lower among 288 patients with cirrhosis (35%), than among either 322 patients with non-ulcer dyspepsia (62.4%), or 339 patients with PUD without cirrhosis (73.7%) (P = 0.001)[50]. Moreover, the prevalence of PUD in patients with Child-Pugh class C cirrhosis was significantly higher than in patients with Child-Pugh class A or B cirrhosis (31% vs 21%, P < 0.05), despite patients with Child-Pugh class C cirrhosis having a lower prevalence of H. pylori infection.
Also, patients with alcoholic cirrhosis had a significantly lower prevalence of H. pylori infection than patients with cirrhosis from viral hepatitis (22% vs 42%, P < 0.001), or cryptogenic cirrhosis (22% vs 40%, P < 0.001)[50]. Similarly, in a prospective study of 66 cirrhotic patients, including 44 patients with alcoholic cirrhosis, PUD was significantly associated with recent alcohol intake (< 1 wk before EGD) or portal hypertensive gastropathy, but was not significantly associated with H. pylori infection[65]. Patients with alcoholic cirrhosis had a lower prevalence of H. pylori infection (OR = 0.77, 95%CI: 0.04-16.59, P < 0.0001), than patients with other etiologies of liver disease, including primary biliary cirrhosis (OR = 1.75, 95%CI: 1.15-2.64, P = 0.147), or viral cirrhosis (OR = 2.66, 95%CI: 1.24-5.71, P < 0.0001)[82].
Cirrhotic patients with PUD generally show little or no benefit from H. pylori eradication in preventing PUD recurrence[49,83,84]. In a prospective study of 104 patients with cirrhosis and duodenal ulcer (DU), 54 (52%) had H. pylori infection, and 44 of these patients received a three drug regimen, consisting of a 1-wk course of amoxicillin, clarithromycin, and a proton pump inhibitor (PPI)[48]. Thirty-six (82%) patients had eradication of H. pylori infection after completing the course of therapy. DUs healed in 49 of 54 patients after 8 wk of PPI therapy, and healed in the remaining 5 patients after 16 wk of therapy. DUs recurred within 1 year in 45% of patients remaining H. pylori positive despite receiving antimicrobial therapy, vs 48% of patients without H. pylori infection at enrollment. Interestingly, DU recurred within 1 year in 21 (58%) of patients with successful H. pylori eradication. The risk of bleeding from PUD was similar for all three groups. In a study of 28 patients with cirrhosis and PUD, including 18 with H. pylori infection and 10 without H. pylori infection, followed for up to 2 years after undergoing H. pylori eradication or chronic PPI therapy, H. pylori eradication did not prevent PUD relapse[83]. PUD relapsed in 8 of 18 H. pylori positive and in 9 of 10 H. pylori negative patients (P = 0.04). An absence of H. pylori infection and more severe liver disease were independently associated with shorter time to PUD relapse. In a retrospective study from Asia, 103 patients with PUD and H. pylori, who were eradicated late (> 1 year) after PUD diagnosis had a higher risk of recurrent PUD as compared to 154 patients with H. pylori eradicated early (< 1 year) (HR = 1.58, 95%CI: 1.09-2.28, P = 0.015). However, early or late eradication of H. pylori did not reduce the ulcer recurrence rate in patients with alcoholic cirrhosis[83,84].
NSAIDs constitute an important cause of UGI mucosal injury, including PUD, gastritis, and related complications of GI hemorrhage or GI perforation in the general population[85,86]. NSAIDs are believed to play a role in UGI bleeding from PUD in patients with advanced liver disease, although the data are relatively limited and somewhat contradictory. A recent, retrospective, nationwide, American study evaluated use of NSAIDs in 4876 cirrhotic patients hospitalized for either variceal (n = 3307) or nonvariceal (n = 1569) UGI bleeding[87]. Use of non-selective NSAIDs were associated with a nearly two-fold increased risk of both variceal and nonvariceal UGI bleeding among cirrhotic patients (OR = 1.87, 95%CI: 1.66-2.11). Also, celecoxib use was associated with a non-significant trend of a moderately increased risk of UGI bleeding (OR = 1.44, 95%CI: 0.89-2.31). Concomitant administration of PPIs or histamine-2 receptor antagonists tended to decrease UGI toxicity of non-selective NSAIDs or celecoxib.
In a prospective study, NSAID administration less than one week before hospitalization, was reported by 15 (42.8%) of 35 patients with cirrhosis and PUB, by 102 (58.2%) of 125 non-cirrhotic patients with PUB, and by 6 (8.5%) of 70 patients with cirrhosis and without PUB (P = 0.0001)[88]. This study suggests NSAIDs may play a role in the pathogenesis of PUB in cirrhotic patients.
However, in a recent, prospective study from France of 203 patients admitted to the ICU, NSAIDs or aspirin were used significantly less by cirrhotic patients with PUB [5 (17%) of 29] than by non-cirrhotic patients with PUB [94 (54%) of 174, P < 0.0001][63]. The majority of PUB in cirrhotic patients were idiopathic (i.e., unrelated to H. pylori infection or NSAID use). Patients with advanced liver disease may avoid NSAIDs because physicians commonly warn these patients about the potential hepatotoxicity of NSAIDs[89].
DL is a dilated (caliber-persistent), aberrant, submucosal, end artery that erodes through the thin overlying layer of GI mucosa in the absence of an ulcer to cause bleeding. It is responsible for approximately 1.5% of acute UGI bleeding[31]. About 70% of all DLs occur in the stomach, and about 75% of all gastric DLs occur within 6-10 cm of the gastroesophageal junction[29,90]. Although relatively uncommon, DL constitutes an important cause of acute UGI bleeding, given its propensity to cause massive, life-threatening, and recurrent bleeding[29]. Such features may be particularly clinically significant in patients with coexistent liver disease.
Data on UGI bleeding from DL in patients with underlying liver disease are limited. In a retrospective, single-center, American study of endoscopic records of 4569 consecutive patients with UGI bleeding admitted between 1991-1996, DL was the etiology of bleeding in only 6 (0.13%) patients[91]. However, 5 of these 6 patients had advanced liver disease, defined as cirrhosis or portal hypertension, including 4 patients with alcoholic liver disease. The association between UGI bleeding from DL and advanced liver disease was highly statistically significant (OR = 19.04; 95%CI: 2.1-900.8; P < 0.002), suggesting that DL may be an underappreciated cause of UGI bleeding in patients with advanced liver disease.
Other studies report a moderately strong association of UGI bleeding from DL in patients with advanced liver disease, particularly aALD. In a retrospective study of 480 patients with UGI bleeding, 28 (5.8%) had DLs[92]. Among these 28 patients with DL, 4 (14.3%) had documented alcoholic cirrhosis, and 8 others (29%) were abusing alcohol. Alcohol consumption may promote bleeding from DL because alcohol may weaken the arteriolar wall leading to vessel rupture that precipitates the bleeding[93]. Key clinical findings in DL, with an emphasis on DL associated with cirrhosis, are summarized in Table 4[29,31,90-98].
| Key findings | Rationale | Ref. |
| Typically presents with acute severe bleeding | Micropulsatile bleeding produced by rent in an arteriole which is under high pressure | Nojkov et al[31], Luis et al[94] |
| Bleeding typically painless | Primary vascular event (bursting of a persistent-caliber vessel) without associated inflammation or ulceration | Cappell[29] |
| Appears at endoscopy as an elevated pigmented protuberance with minimal surrounding erosion and no ulceration | Formed by a caliber-persistent artery that erupts through superficial overlying cells on mucosal surface | Nojkov et al[31], Lee et al[93] |
| Lesion most commonly located in stomach, typically within 6 cm below the gastroesophageal junction along the lesser curve | This gastric region is not perfused by a submucosal plexus, but instead is perfused directly from tributaries of the right and left gastric arteries | Cappell et al[29], Fockens et al[90], Lee et al[95] |
| Often (up to 30% of cases) missed at initial esophagogastroduodenoscopy (EGD) | Missed at EGD because lesion is small and inconspicuous | Nojkov et al[31], Chung et al[96] |
| Incidence of 1.5% among general population of patients with upper GI bleeding | Fockens et al[90], Chaer et al[97] | |
| High (25%) mortality if untreated at EGD, which is reduced to about 10% with endoscopic therapy | High risk of rebleeding if not treated endoscopically. Rebleeding is frequently massive | Romãozinho et al[98] |
| Dieulafoy’s lesion may be associated with cirrhosis | Akhras et al[91], Baettig et al[92] | |
| Bleeding from a Dieulafoy’s lesion is associated with alcoholism | Alcohol may precipitate DL rupture manifesting as GI bleeding by weakening the dilated (caliber-persistent) arteriolar wall in Dieulafoy’s lesion | Baettig et al[92], Lee et al[95] |
In MWS patients typically present with hematemesis from longitudinally arrayed mucosal lacerations straddling the gastroesophageal junction and extending into the distal esophagus or proximal stomach that are usually induced by antecedent forceful retching or nonbloody vomiting[99]. The mucosal lacerations cause bleeding from submucosal arteries. This lesion is reliably diagnosed by EGD. The hematemesis is typically mild. MWS accounts for approximately 5% of UGI bleeding[100]. In 40% to 80% of cases, MWS is associated with heavy alcohol consumption, either from binge drinking or chronic alcoholism, that induces the vomiting or retching[100,101]. A hiatal hernia is another important risk factor for MWS[102].
The prevalence of MWS causing UGI bleeding in patients with cirrhosis is reportedly 3%-10% which is similar to its prevalence of 5% in the general population[39,51,100,103], but MWS is likely more prevalent in patients with aALD, given its strong association with alcoholism. For example, among 339 patients with portal hypertension, confirmed by measurements of HVPG, undergoing emergency EGD for UGI bleeding, 55 patients (16.2%) had MWS[26]. Forty (73%) of these 55 patients had alcoholism as the etiology of their liver disease, which was a significantly higher rate than the rate of alcoholism in patients with other etiologies of UGI bleeding [157 (55.3%) of 284]. Moreover, the frequency of bleeding from MWS was significantly higher in patients with more advanced cirrhosis (Child-Pugh class B or C).
In a study of 42 patients with bleeding from MWS, 14 (33%) had underlying liver disease, including six (14%) with aALD[27]. Patients with liver disease had significantly more severe MWS bleeding than patients without liver disease as indicated by units of packed erythrocytes transfused (P < 0.005), but MWS bleeding severity was not correlated with the presence of portal hypertension or Child-Pugh score. Only three patients (21%) required endoscopic therapy; in these three patients epinephrine injection and/or Bicap electrocoagulation successfully stopped the bleeding or rebleeding[27]. There were no fatalities from MWS bleeding in patients with liver disease. Contrariwise, in a small trial of 30 patients with UGI bleeding from MWS, the bleeding was significantly more severe and more difficult to control at EGD in the 8 patients with portal hypertension than in the 22 patients without portal hypertension[28]. Additionally, patients with portal hypertension were more likely to rebleed from MWS [3 (38%) of 8 patients rebled] as compared to patients without portal hypertension [0 (0%) of 22 patients rebled, P = 0.02, Fisher’s exact test, P value calculated by us from original descriptive statistics]. All 8 patients with portal hypertension and MWS bleeding had underlying aALD[28]. A Korean study of 159 patients with acute UGI bleeding from MWS also reported a higher frequency of recurrent bleeding from MWS in patients with cirrhosis[82]. Cirrhosis was present in 33 (21%) of these patients. Seven (41%) of the 17 patients who rebleed from MWS had cirrhosis (P = 0.02). The vast majority of patients who rebled were alcoholics [14 (82%) of 17 patients].
Another study of 224 patients, recruited over 10 years, with acute UGI bleeding from MWS revealed that 68 (31%) of the patients had abused alcohol and had aALD[42]. The bleeding stopped spontaneously in 90% of the 224 patients, with only 20 patients requiring endoscopic or surgical therapy. However, 8 of the patients died, including six patients who died from the acute UGI bleeding from massive hemorrhage, pulmonary aspiration, or hemorrhagic shock, and two patients who died from hepatic complications indirectly related to the acute UGI bleeding.
The prevalence of acute UGI bleeding from MWS in patients with liver disease may be lower in Asian than Western countries. In a recent, prospective, single-center, study from China, 16 (3%) of 519 patients with acute nonvariceal UGI bleeding had MWS. Only one of these patients had cirrhosis[104]. Key clinical features of acute UGI bleeding from MWS in patients with underlying liver disease are summarized in Table 5[27,28,38,82,100,101,105-107].
| Key findings | Ref. |
| Findings generally associated with Mallory-Weiss syndrome | |
| Characterized by longitudinally oriented mucosal lacerations in the distal esophagus or very proximal stomach | Knauer[101] |
| Accounts for about 5% of acute upper GI bleeding | Michel et al[100] |
| Mortality of bleeding from Mallory-Weiss syndrome is only about 3%-5%. Risk factors for mortality include age > 65 yr and significant comorbidities | Ljubičić et al[105] |
| Findings associated with Mallory-Weiss syndrome associated with alcoholism | |
| MWS strongly associated with alcoholism | Knauer[101] |
| MWS very frequently (40%-80%) associated with alcoholism or recent binge drinking | Watts et al[106] |
| Overall prevalence of bleeding from MWS in cirrhosis is up to 10% | del Olmo et al[38], Feng et al[82] |
| Alcoholics with portal hypertension have higher prevalence of bleeding from MWS (up to 16%) | Paquet et al[26], Schuman et al[27] |
| MWS may be the first bleeding episode in > 1/3 (37%) of these patients | |
| Patients with cirrhosis have more severe bleeding from MWS and more likely to rebleed from MWS (compared to non-cirrhotics) | Schuman et al[27], Jensen et al[28], Kim et al[107] |
| Re-bleeding risk particularly high in alcoholics | |
| Contradictory data on effect of portal hypertension on severity of bleeding from MWS | Schuman et al[27], Jensen et al[28] |
| Bleeding from MWS can precipitate liver failure with its attendant mortality in about 3% of patients with alcoholic cirrhosis | del Olmo et al[38] |
Pre-endoscopic therapy: Most patients with severe liver disease should be admitted to an intensive care unit (ICU) for acute UGI bleeding, particularly when manifesting clinical findings suggestive of severe UGI bleeding, including unstable vital signs, active hematemesis, or hematochezia, or when patients have severe comorbidities. Clinical stratification of variceal vs nonvariceal etiology for UGI bleeding is difficult before EGD in these patients because some patients with stigmata of underlying chronic liver disease bleed from PUD, DL, or MWS, as aforementioned. However, physical findings of portal hypertension, such as ascites or caput medusa, favor the diagnosis of variceal bleeding[108]. Endotracheal intubation, to protect the airway, should be considered in patients with severe hematemesis, suspected variceal bleeding, or significant hepatic encephalopathy[109,110]. A Foley catheter is inserted in patients with shock, unstable vital signs, oliguria, or massive bleeding. Nasogastric lavage is often helpful to assess whether the bleeding is from the UGI tract, to assess the tempo of the bleeding, and to clear the gastric field for EGD. This is most helpful in the presence of severe, active, bleeding often associated with variceal or other UGI bleeding in cirrhotic patients.
As for any acute UGI bleeder, volume resuscitation is critical in patients with cirrhosis to prevent systemic hypotension and consequent end-organ damage to heart, brain, or kidneys from hypoperfusion. At least two, large-caliber, peripheral intravenous lines, or a reliable central venous line should be inserted. Volume resuscitation is initially accomplished by crystalline solution, using normal saline or Ringer’s lactate, but transfusion of packed erythrocytes is often required, after typing and crossing of blood. The number of units transfused is guided by the tempo of the UGI bleeding, the vital signs, and serial hematocrit determinations. It is important, however, to avoid over-transfusion in patients with underlying advanced liver disease, as this can precipitate variceal bleeding by increasing the portal hypertension and refilling of the varices[13,111]. Packed erythrocytes should be transfused to maintain the hemoglobin level only at about 8 g/dL. However, patients who have cardiovascular disease and severe comorbidities, or are extremely elderly should be transfused to a somewhat higher level[112].
Coagulopathies are appropriately treated. Thrombocytopenia is common in patients with advanced liver disease because of splenic sequestration from splenomegaly and direct bone marrow toxicity of alcohol[113]. Furthermore, platelet counts decline directly from platelet loss during acute UGI bleeding. Platelets should be transfused to maintain > 50000 platelets/mm3 in actively bleeding patients[114]. Coagulopathy from liver disease should by monitored by the INR, and should be corrected in actively bleeding patients, usually by administration of fresh frozen plasma (FFP). One unit of FFP should also be transfused after transfusion of every four units of packed erythrocytes to replenish depleted clotting factors[115].
Electrolyte abnormalities are appropriately treated. Patients transfused large volumes of blood products should be carefully monitored and treated for hypocalcemia because citrate present in blood products binds to ionized calcium[116]. These patients have an increased risk of hypophosphatemia and hypokalemia, especially if dextrose solutions are used during resuscitation, because insulin release consequent to dextrose administration accelerates the transfer of phosphate and potassium into cells[117]. Prophylactic antibiotics should be administered at presentation, because antibiotics improve survival in cirrhotic patients with UGI bleeding, especially with bleeding from esophageal varices[118], by reducing the risk of spontaneous bacterial peritonitis or other severe infections[119].
Alcoholics should receive thiamine. They should be monitored for symptoms and signs of alcohol withdrawal. Alcohol withdrawal may be difficult to appreciate in alcoholics suffering from variceal bleeding because tachycardia, confusion, and low-grade pyrexia may be attributed to the variceal bleeding alone. Special considerations in the therapy of acute UGI bleeding in patients with aALD are summarized in Table 6[25,109,110,117-129].
| Recommended clinical practice | Rationale | Ref. |
| Consider early intubation for severe upper GI bleeding in a patient with alcoholism or alcoholic cirrhosis | These patients are at higher risk of aspiration because variceal bleeding related to alcoholism or cirrhosis is frequently massive, arises from the esophagus which is much closer to the trachea than other types of gastroduodenal bleeding; and the patient may be obtunded from hepatic encephalopathy from cirrhosis | Herrera[109], Rudolph et al[110] |
| Avoid sedatives and narcotics in patients with advanced liver disease | May precipitate hepatic encephalopathy from cirrhosis | Bamji et al[120], Prabhakar et al[121] |
| Monitor for hepatic encephalopathy | Patients with advanced cirrhosis at risk for hepatic encephalopathy | Rahimi et al[122] |
| Monitor for delirium tremens | Acute alcoholic withdrawal in hospital can induce delirium tremens | Ferguson et al[123], Holloway et al[124] |
| Avoid over-transfusion (maintain hemoglobin level at about 8 gm/dL) | Over-transfusion may exacerbate variceal bleeding by increasing portal hypertension | Herrera[109] |
| Patients often have thrombocytopenia which may contribute to the bleeding | Thrombocytopenia due to splenic sequestration from splenomegaly from portal hypertension and from direct alcohol toxicity to bone marrow | Pradella et al[125] |
| Patients often have a prolonged INR which may contribute to the bleeding | INR prolonged due to inadequate synthesis of liver-dependent clotting factors, such as factor V, due to advanced liver disease | Lata et al[126] |
| Administer thiamine | Prevent Wernicke’s syndrome from thiamine deficiency which is common in alcoholics | Hack et al[127] |
| Monitor for electrolyte abnormalities which may be more prominent in alcoholics | Knochel[117] | |
| Consider early (urgent) esophagogastroduodenoscopy | Important to distinguish esophageal variceal bleeding from other etiologies of upper GI bleeding because esophageal variceal bleeding has different therapies | Buccino et al[37], del Olmo et al[38] |
| Consider empiric octreotide therapy before endoscopy | Alcoholics or patients with cirrhosis frequently have GI bleeding from esophageal varices which can be treated by octreotide therapy | Ludwig et al[128] |
| Perform paracentesis, as necessary, to exclude spontaneous bacterial peritonitis | Patients with cirrhosis and ascites are at high risk to develop spontaneous bacterial peritonitis due to mild immunosuppression with cirrhosis | Goulis et al[119] |
| Administer antibiotics in the presence of acute GI bleeding in a cirrhotic patient | Empiric antibiotic therapy lowers mortality because of decreased sepsis | Bernard et al[118] |
| Monitor BUN and creatinine levels to detect early hepatorenal syndrome. Avoid nephrotoxic medications such as NSAIDs | At high risk for renal deterioration due to decreased renal perfusion associated with cirrhosis and hypovolemia from GI hemorrhage | Ginès et al[129] |
| Exclude acute portal vein thrombosis in patients who suddenly develop severe esophageal varices by abdominal imaging studies (e.g., Doppler ultrasound or CT angiography) | Portal vein thrombosis in a patient with preexistent cirrhosis may exacerbate the portal hypertension and cause acute variceal bleeding | D’Amico et al[25] |
All patients presenting with acute UGI bleeding receive PPI therapy intravenously before endoscopy even though many patients are bleeding from lesions that are not acid-mediated, because the neutralization of intraluminal gastric acid with PPI therapy stabilizes blood clots for all gastric lesions[130,131]. For example, PPIs are standardly administered before EGD even in cirrhotic patients with suspected variceal bleeding. Patients with suspected esophageal variceal bleeding or other bleeding related to portal hypertension, based on patient history, stigmata of chronic liver disease, or abnormal liver function tests, are generally treated with octreotide before EGD to decrease splanchnic blood flow and reduce the risk of bleeding from potential esophageal varices[132].
Diagnostic endoscopy: All patients with acute UGI bleeding generally require EGD, but patients with aALD or nonalcoholic cirrhosis generally require more urgent EGD because of the potential for life-threatening variceal bleeding requiring endoscopic therapy. EGD is highly sensitive and specific for diagnosing the etiology of acute UGI bleeding, with a diagnostic sensitivity of about 95%[133]. UGI bleeding from esophageal varices, portal hypertensive gastropathy, and GAVE are reliably diagnosed at EGD, as are bleeding from PUD or MWS. Occasionally, PUD or a Mallory-Weiss tear may be obscured by active bleeding, or a peptic ulcer may be obscured by an overlying pool of blood or adherent clot. Contrariwise, DL is missed in up to 30% of initial EGDs, especially if the patient is not actively bleeding or oozing at EGD, because of its small size and inconspicuousness and patients sometimes require repeat EGD for the diagnosis[134].
General principles of endoscopic therapy: Focal GI lesions, including PUD, DL, or MWS, are treated by focal endoscopic therapies, including injection, ablation, and mechanical therapy (Table 7)[31,135]. Injection therapy most commonly involves local injection of epinephrine or sclerosing agents (sclerotherapy). Epinephrine injection induces vasospasm and tamponade/mechanical pressure from interstitial injection which promotes stasis, thrombosis, and hemostasis[136]. It is generally diluted in saline to a concentration of 1:10000 and injected in four quadrants circumferentially around a point lesion, such as a peptic ulcer. Relative contraindications to epinephrine therapy include severe tachycardia, life-threatening cardiac arrhythmias such as atrial flutter, unstable vital signs from untreated, profound, hypovolemia, and recent myocardial infarction or unstable angina[31]. However, cardiovascular side effects, including angina, tachycardia, cardiac arrhythmias and systemic hypertension, are relatively uncommon[137]. Epinephrine injection monotherapy arrests about 80% of active bleeding[135]. For example, in a prospective study, 66 (85%) of 78 patients undergoing endoscopic epinephrine injection for a nonbleeding visible vessel or actively bleeding ulcer had no recurrent bleeding[138]. Sclerotherapy promotes vascular inflammation and thrombosis from local tissue irritation. Cyanoacrylate, thrombin, or fibrin glue are infrequently used as injection therapies[139]. Cyanoacrylate hardens as a glue to plug a bleeding artery[140].
| Injection therapies |
| Dilute epinephrine |
| Sclerotherapy |
| Ablation therapies |
| Contact methods |
| Thermocoagulation: heater probe |
| Electrocoagulation: BICAP (bipolar electrocoagulation probe), Gold Probe |
| Noncontact methods |
| APC (argon plasma coagulation) |
| Mechanical therapy |
| Banding |
| Hemoclips |
| Endoscopic suturing |
Ablation modalities include thermocoagulation, electrocoagulation, and argon plasma coagulation (APC) (Table 7). These modalities arrest bleeding by focally applying intense energy, via heat, electricity or a plasma cloud, to destroy and devitalize tissue. In APC the probe is hovered over the lesion without lesion contact[141]. Contrariwise, thermocoagulation and electrocoagulation require direct contact (apposition) of the probe to the lesion, and application of pressure during coagulation (coaptive coagulation) to compress and seal any bleeding vessel[31,135,142]. Nd:YAG (neodymium-doped yttrium aluminum garnet) laser photocoagulation has become obsolete because it can cause deep injury and has an unacceptably high risk of gastrointestinal perforation of approximately 3%[143].
Mechanical therapies, including band ligation, hemoclips, or over the scope endoclips (bear claw), arrest bleeding by mechanically occluding a bleeding vessel[144]. Mechanical therapy requires greater endoscopic skill and experience than injection or ablative therapies because the band or clip must be placed precisely around the bleeding lesion to successfully strangulate it[135]. Mechanical therapy appears to be the therapy of choice in the presence of a moderate-to-severe coagulopathy because it mechanically occludes a bleeding vessel without depending upon thrombosis to stop the bleeding[135]. Thus mechanical therapy may be favored in cirrhotic patients with severe thrombocytopenia or a highly elevated international normalized ratio (INR). Table 7 lists common endoscopic therapies for nonvariceal upper GI bleeding.
Endoscopic therapy for PUD: In a patient with recent bleeding from PUD, therapeutic EGD is generally performed according to endoscopic SRH (Table 8; Forrest classification)[145]. Patients with major SRH at EGD, including active bleeding, oozing, or a non-bleeding visible vessel, should undergo endoscopic therapy[135]. A nonbleeding visible vessel is defined endoscopically as an elevation (projection) from the ulcer base that is pigmented, whether it be red, purple, blue, or gray[146]. The rationale for endoscopic therapy is to prevent rebleeding or ongoing bleeding from PUD, because patients with rebleeding have a very high mortality of up to 30%[147]. For example, patients with a nonbleeding visible vessel have a 40%-60% risk of rebleeding with medical therapy alone that is reduced to about 15% with dual endoscopic therapy[148]. Patients with minor endoscopic SRH (Table 8) should not undergo endoscopic therapy because of a low risk of rebleeding and mortality even without endoscopic therapy. For example, patients with an ulcer that is homogeneous and clean-based or that has a flat pigmented spot have an about 3%-5% or 7%-10% risk, respectively, of rebleeding, even without undergoing endoscopic therapy[147].
| Endoscopic SRH | Endoscopic appearance | Endoscopic therapy | Endoscopic therapy and rationale for therapy |
| Major SRH | |||
| Active bleeding | Active bleeding observed at EGD | Yes | Reduction from 90% to 15% risk of ongoing bleeding with performance of endoscopic therapy |
| Nonbleeding visible vessel | Pigmented elevation (projection) from ulcer base, whether red, blue or gray in color | Yes | Reduction from about 50% to 15% risk of rebleeding with performance of endoscopic therapy |
| Intermediate SRH | |||
| Adherent clot | Focal clot that is resistant to removal by mild-to-moderate irrigation | Recommended by most endoscopists | |
| Active oozing of blood | Active oozing observed at EGD | Generally recommended | May reduce risk of rebleeding from 28% to 15% with endoscopic therapy |
| Minor SRH | |||
| Flat pigmented spot | Pigmented spot, whether red, blue or gray, which lies flat on the ulcer base | No | Low risk of rebleeding of about 13% with medical therapy alone |
| No SRH | |||
| Homogeneous, clean-based ulcer | Simple ulcer with no bleeding, no adherent clot, no visible vessel and no pigmented spot | No | Extremely low risk of rebleeding of about 4% that does not warrant the risks of endoscopic therapy |
Endoscopic therapy for a nonbleeding, adherent clot is somewhat controversial. Many endoscopists, however, advocate aggressive removal of adherent clots. First, the endoscopic diagnosis is often ambiguous without clot removal. A presumed benign ulcer underneath an adherent clot may be exposed as a malignant ulcer or DL after clot removal[135]. Second, clot removal may reveal unexpected underlying major SRH, such as a nonbleeding visible vessel, that mandates endoscopic therapy. Third, even though mechanical clot removal occasionally precipitates active bleeding, endoscopists generally prefer to treat active bleeding immediately at the initial EGD rather than passively wait for potential rebleeding that would necessitate repeat EGD. At EGD, an adherent clot is initially vigorously irrigated using a water pump. If irrigation fails to remove the clot, the clot is decapitated using a snare without electrocoagulation after four-quadrant injection around the clot with epinephrine[149]. Endoscopic therapy is applied to the exposed underlying ulcer, especially if active bleeding or oozing occurs after clot removal or a visible vessel is exposed[150]. Multiple studies support forceful removal of adherent clots. For example, in a randomized, controlled trial of 32 patients with UGI bleeding and nonbleeding adherent clots identified at EGD, patients undergoing endoscopic epinephrine injection and mechanical clot removal followed by thermocoagulation had a 5% rebleeding rate, whereas those receiving medical therapy with a PPI had a 34% rebleeding rate[151]. An adherent clot should generally be considered for forceful removal if the lesion is at a location amenable to endoscopic therapy and if the endoscopist is comfortable with the techniques of forceful endoscopic clot removal and therapy.
Epinephrine injection is the most common initial endoscopic therapy for PUD with high or intermediate SRH because this therapy is relatively easy and quick to apply, even in the face of severely active bleeding, and can help clear the endoscopic field by reducing the tempo of active bleeding to enable application of more efficacious, pinpoint, therapy. Epinephrine therapy, by itself, is not highly efficacious at achieving permanent hemostasis, with a rebleeding rate of about 20%[152]. Endoscopists, therefore, generally perform a second endoscopic therapy after epinephrine injection to further reduce the bleeding rate to 10%-15%[152,153]. Second therapies include ablation with thermocoagulation or electrocoagulation, and mechanical therapy with hemoclips or banding. The choice of second technique depends upon equipment availability and the training and experience of the endoscopist.
Currently employed endoscopic therapies generally have a small, acceptable risk of gastrointestinal perforation, local tissue necrosis, or bleeding exacerbation[154]. For example, the risk of perforation for all current endoscopic therapies is 1% or less[155].
A comprehensive review of the literature revealed no large studies focused on endoscopic therapy for PUD in patients with cirrhosis or aALD. However, endoscopic therapy in patients with major SRH is apparently more important in this patient population than in the general population because of the higher risks of rebleeding or dying from PUB in this population. Currently the same endoscopic therapies should be performed in this population as in the general population. It is important to treat coagulopathy from thrombocytopenia or an elevated INR for endoscopic therapy to be most effective.
Endoscopic therapy for DL: Endoscopic therapy is uniformly performed for DLs, even if SRH are absent, because of the high risk of rebleeding from DLs without endoscopic therapy and the high mortality of this rebleeding. Reducing this high risk of rebleeding justifies the risks of endoscopic therapy[31]. Endoscopic therapy reduces the risk of rebleeding from DLs from 30% to about 10%[156].
The data on individual endoscopic therapies for DL are limited because DL is relatively uncommon. Many endoscopic therapies are generally effective for DLs in the general population. Current data suggest that mechanical hemostasis may be the most effective. A recent, large, literature review encompassing 106 patients undergoing hemoclips and 86 patients undergoing band ligation as monotherapies for bleeding DLs showed that both techniques were nearly always effective at achieving initial hemostasis, and had a low (< 10%) rate of rebleeding[31].
In the same recent literature review, among 68 patients undergoing epinephrine injection therapy and among 13 patients undergoing sclerotherapy, about 12% failed primary hemostasis, and another 25% rebleed after initial hemostasis[31]. Although this efficacy is somewhat lower than that for mechanical therapy, injection therapy appears to be an excellent initial therapy for massive bleeding from DL due to ease and rapidity of the injection so that the DL can then be well visualized to apply mechanical or ablative therapy[31].
Data on efficacy of endoscopic ablation therapies for DL are somewhat limited, with only about 40 reported cases among the 3 different therapies of thermocoagulation, electrocoagulation, and APC, reported in 7 small clinical studies[31]. However, despite these limitations, the data demonstrate that endoscopic ablation is relatively efficacious, with a combined initial success rate of hemostasis of about 95%-97.5% and a rebleeding rate of 12.5%[31].
In summary, the data show that endoscopic therapy is highly effective at achieving initial hemostasis and preventing rebleeding from DLs. Mechanical therapy may be the most effective endoscopic therapy. However, dual endoscopic therapy, with initial epinephrine injection followed by mechanical therapy, may be even more effective than any monotherapy. The high risk of rebleeding from DL justifies the risk of undertaking endoscopic therapy to prevent further bleeding[31].
Limited data exist on the effectiveness of endoscopic therapy for DL in cirrhotic patients. Table 9 summarizes data extrapolated from multiple studies on the efficacy of various endoscopic techniques[91,92,157-160]. Overall, various endoscopic hemostatic techniques, including injection, ablation, and mechanical therapy, can be effectively applied to bleeding DLs in cirrhotic patients. However, patients with cirrhosis likely have increased mortality related to hepatic decompensation. For example, four (18%) of the 22 reported patients in Table 9 died within 5 mo of the UGI bleeding, from hepatic decompensation, despite achieving successful endoscopic hemostasis.
| Endoscopic procedure(Number of patients) | Hemostatictechnique | Lesion location | Study type | Follow-up | Patient outcome | Ref. |
| EGD (12)1 | Epinephrine + Polidocanol | Stomach/duodenum | Retrospective | 5 mo | No rebleeding, 3 of cirrhotic pts died from hepatic decompensation within 5 mo | Baettig et al[92] |
| EGD (6) | Not reported | Stomach/duodenum | Retrospective | NA (6 yr period reviewed) | No rebleeding in 5 of 6 pts (83%), 1 pt (17%) underwent surgery for rebleeding | Akhras et al[91] |
| EGD (5) | Histoacryl (3) | Stomach | Retrospective | 18 mo | No rebleeding | Cheng et al[159] |
| Epinephrine + heater probe (2) | ||||||
| EGD (4) | Hemoclip | Stomach/duodenum | Prospective | 54 mo | 9% of whole cohort of 34 pts rebled. No data whether any of the cirrhotics rebled | Yamaguchi et al[160] |
| EGD (2) | Rubber band ligation | Stomach | Retrospective | 30 d | No rebleeding, 1 of 2 pts died from hepatic decompensation within 30 d | Mumtaz et al[157] |
| EGD (1) | Argon plasma coagulation | Stomach | Retrospective | 29 mo | No rebleeding | Iacopini et al[158] |
In one study of 28 patients with DL, including 4 with documented cirrhosis and 8 alcoholics, endoscopic therapy with epinephrine and polidocanol injection successfully stopped the bleeding in 96% of cases[92]. The bleeding was effectively controlled in 4 patients with alcoholic cirrhosis, without rebleeding or death during the initial hospitalization, but 3 of the 4 cirrhotic patients died during a mean follow-up period of 5 mo, including 2 dying from hepatic coma and 1 dying from variceal bleeding. In a retrospective study of 23 patients with DL-induced acute UGI bleeding, including 2 (8.7%) patients with advanced liver disease, there was no difference in short-term (30 d) clinical outcomes between patients treated with endoscopic rubber band ligation vs endoscopic thermal/injection therapy[157]. Six patients (26%) died within 30 d after bleeding from DL, including 1 of the 2 patients with advanced liver disease, who expired from liver decompensation. In an Italian study with long-term mean follow-up of 29 mo, APC was a safe and effective treatment for 23 patients with GI bleeding from DL including 1 patient (4.3%) with liver cirrhosis[158]. None of the patients died and only 1 patient experienced rebleeding, which was successfully retreated with APC.
Endoscopic therapy for MWS: Endoscopic therapy for MWS depends upon the presence of SRH and other factors. Endoscopic therapy should be applied for MW tears that are actively bleeding at endoscopy. Endoscopic therapy may be considered when other SRH are present, including a protruding visible vessel, pigmented protuberance, or fresh adherent clot, especially in the presence of risk factors for rebleeding of initially severe bleeding or a coagulopathy. However, most patients with MWS do not require endoscopic therapy because the tears usually heal spontaneously with only medical therapy[161]. In particular, endoscopic therapy is usually unnecessary when the bleeding is minor as indicated by absence of hematochezia, stable vital signs at presentation, and lack of blood transfusions; when a coagulopathy is not present; and when SRH are absent.
Data on endoscopic therapies for MWS in the general population are somewhat limited by the retrospective nature of most studies and the relatively small number of patients in individual studies. Currently recommended endoscopic therapies include injection therapy with epinephrine, ablation therapy with electrocoagulation, and mechanical therapy with hemoclips or band ligation. Epinephrine can be used as a monotherapy or combined with a second therapy. For example, in a randomized, controlled trial of bleeding from high-risk MWS, bleeding recurred in 6.8% of patients receiving endoscopic injection therapy vs 25.8% of patients receiving medical therapy alone (P < 0.05)[162]. Electrocoagulation significantly reduced the risk of rebleeding compared to medical therapy in patients with MWS at high risk of rebleeding[163]. Likewise, endoscopic band ligation and hemoclip placement were highly effective at preventing rebleeding from MWS, as demonstrated in a randomized clinical trial of 41 patients[164].
Table 10 summarizes the data on the efficacy of endoscopic therapies to treat acute MWS bleeding in cirrhotic patients[26-28,104,165]. Although the data are limited and retrospective, all the reported therapeutic endoscopic modalities appeared to be safe and effective to control the bleeding in the vast majority of cirrhotic patients. In the aforementioned study by Paquet et al[26] which included 339 consecutive patients with cirrhosis and portal hypertension who underwent EGD for acute UGI bleeding within 6 h of presentation, 55 patients had bled from MWS. All 55 patients were treated endoscopically with injection of aethoxysklerol into the bleeding lesion, which universally resulted in successful hemostasis, with no rebleeding or other adverse effects[26]. In another aforementioned study, 14 patients with cirrhosis and acute bleeding from MWS underwent EGD within 24 h of presentation, but endoscopic therapy (epinephrine injection or electrocoagulation) was deemed necessary in only 3 (21%) of the patients[27]. No patient experienced rebleeding, other complications, or death related to the MWS or the underlying liver disease. Mechanical endoscopic hemostasis (band ligation or hemoclip placement) was also reported effective in cirrhotic patients with bleeding MWS. In a recent French study of 56 patients comparing these 2 mechanical therapies, none of the 7 patients with cirrhosis, treated with band ligation in 4 and hemoclips placement and epinephrine in 3, rebleed or suffered endoscopic complications. On multivariate analyses, liver disease was not a risk factor for rebleeding.
| Number of patients | HemostasisTechnique | n (%) with active alcohol abuse | Time of EGD | Study type | Follow-up | Outcome | Ref. |
| 55 | Aetoxysklerol injection | 40 (73) | Within 6 h | Retrospective | 2 yr | No rebleeding or death | Paquet et al[26] |
| 141 | Epinephrine injection/elec-trocoagulation | 6 (43) | Within 24 h | Retrospective | 5 yr | No rebleeding or death2 | Schuman et al[27] |
| 7 | Band ligation 4 | Not reported | Within 12 h | Retrospective | 5 d | No rebleeding or death | Lecleire et al[165] |
| Hemoclips and epinephrine 3 | |||||||
| 3 | Heater probe/Bicap electrocoagulation | 3 (100) | “Urgently” after presentation | Retrospective | 17 mo | Failed to control bleeding in 1/3 pts (33%) | Jensen et al[28] |
| 1 | Hemoclip and Endoscopic band ligation | 1 (100) | “Emergent-ly” at presentation | Retrospective | NA | Failed to stop bleeding with both techniques; patient underwent surgery and died of liver failure 3 d after surgery | Yin et al[104] |
However, endoscopic therapy is not universally successful in patients with cirrhosis who bleed from MWS. For example, a small report of 8 patients with cirrhosis and MWS bleeding, reported endoscopic failure to control the initial MW bleeding in 1 (33%) of the 3 patients receiving either heater probe thermocoagulation or Bicap electrocoagulation. Additionally, a recent case-series of bleeding MWS from China included one patient with concomitant alcoholic liver cirrhosis. This patient was the only one in this study who failed endoscopic therapy initially with hemoclips placement and subsequently hemoclips and band ligation and died 3 d after undergoing surgery from liver failure[104].
The incidence of PUD, frequency of bleeding from PUD, and severity of bleeding from PUD are all increased in patients with cirrhosis, including those with aALD. However, the pathophysiology of this phenomenon is incompletely understood. Experiments in laboratory animals, such as rats, may help elucidate the pathophysiology, by separately analyzing the effects of alcohol exposure and portal hypertension (e.g., expose rats to alcohol consumption vs experimentally ligating or banding the portal vein to produce experimental portal hypertension with little acute hepatocellular liver injury).
The clinical presentation of PUD associated with cirrhosis or aALD is incompletely understood because many of the studies on the subject lump PUD in cirrhotics with the other etiologies of UGI bleeding not due to portal hypertension (Table 2). Studies are needed which systematically compare patients with PUD and cirrhosis or aALD to patients with PUD without cirrhosis or aALD. The data on endoscopic therapies for MWS and DL for patients with cirrhosis are limited. However, conclusions can be extrapolated from data on endoscopic therapies in noncirrhotic patients bleeding from these lesions. Analysis of larger patient populations of patients bleeding from MWS or DL would be useful to show that the general principles of endoscopic therapy pertain to cirrhotic patients.
H. pylori appears to play only a minor role in the increased observed rate of PUD in cirrhotics. Perhaps the most convincing evidence of this minor role is that H. pylori eradication does not protect against DU recurrence, as it does in the general population.
In conclusion, this review describes more frequent and more severe GI bleeding from PUD, DL, or MWS in patients with aALD than in the general population. These differences have important consequences in the clinical management and prognosis of these conditions in patients with aALD. It is hoped that this review will stimulate further study of this clinically important but incompletely understood subject.
Excessive alcohol consumption is highly prevalent worldwide and can cause several forms of liver injury ranging from alcoholic fatty liver disease to alcoholic hepatitis to alcoholic cirrhosis. While the majority of acute gastrointestinal bleeding with advanced alcoholic liver disease (aALD), including alcoholic hepatitis or alcoholic cirrhosis, is related to portal hypertension, about 30%-40% of acute gastrointestinal (GI) bleeding in patients with aALD is unrelated to portal hypertension. This work shows that patients with aALD have more frequent and more severe bleeding from the following etiologies of nonvariceal upper GI bleeding: peptic ulcer disease (PUD), Dieulafoy’s lesion (DL), and Mallory-Weiss syndrome (MWS). Clinicians should appreciate the important clinical implications of these phenomena.
It is important for clinical practitioners and clinical researchers to appreciate that patients with advanced alcoholic liver disease, including alcoholic hepatitis and alcoholic cirrhosis, have more frequent and more severe nonvariceal upper GI bleeding from PUD, DL, and MWS. This work systematically reviews and critically analyses the literature on this quickly evolving subject and reports the clinical consequences of these findings.
This review is distinguished by being a systematic review of the subject of nonvariceal bleeding in patients with aALD, by reviewing all three etiologies of non-variceal upper GI bleeding severely affected by aALD, and by a focus on the clinical implications of the reported findings. This work aims to provide practicing clinicians and clinical researches an in-depth and up-to-date comprehensive and critical review of this clinically important, but underappreciated, subject.
The currently reported increased frequency and increased severity of bleeding from PUD, DL, and MWS in patients with aALD have clinically important implications regarding the treatment, natural history, and prognosis of these etiologies of upper GI bleeding in patients with aALD. This work also stimulates clinical researchers to better understand the pathophysiology of these reported clinical phenomena.
aALD refers to alcoholic hepatitis and alcoholic cirrhosis. This term is presently used because both of these forms of alcoholic liver disease affect the pathophysiology, natural history, treatment, and prognosis of non-variceal upper GI bleeding from PUD, DL, and MWS.
The manuscript provides a comprehensive review of the recent literature data on the association between PUD, DL, and MWS and aALD or cirrhosis. It is also well supported by the quoted references. This is a well written and interesting contribution of considerable interest to the readership of the Journal.
Biostatistics statement: The statistical analysis in this manuscript was reviewed by a biostatistician (see Methods section).
P- Reviewer: Ji G, Ocker M, Uyanik M S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | World Health Organization. Global status report on alcohol and health. Geneva: World Health Organization 2011; 286. |
| 2. | Welte J, Barnes G, Wieczorek W, Tidwell MC, Parker J. Alcohol and gambling pathology among U.S. adults: prevalence, demographic patterns and comorbidity. J Stud Alcohol. 2001;62:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 4. | Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2283] [Cited by in RCA: 2093] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 5. | MacSween RN, Burt AD. Histologic spectrum of alcoholic liver disease. Semin Liver Dis. 1986;6:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 163] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Méndez-Sánchez N, Almeda-Valdés P, Uribe M. Alcoholic liver disease. An update. Ann Hepatol. 2005;4:32-42. [PubMed] |
| 7. | Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209-219. [PubMed] |
| 8. | Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 9. | Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800-809. [PubMed] |
| 10. | Odelowo OO, Smoot DT, Kim K. Upper gastrointestinal bleeding in patients with liver cirrhosis. J Natl Med Assoc. 2002;94:712-715. [PubMed] |
| 11. | Schlichting P, Christensen E, Fauerholdt L, Poulsen H, Juhl E, Tygstrup N. Main causes of death in cirrhosis. Scand J Gastroenterol. 1983;18:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Yu CH, Xu CF, Ye H, Li L, Li YM. Early mortality of alcoholic hepatitis: a review of data from placebo-controlled clinical trials. World J Gastroenterol. 2010;16:2435-2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1210] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 14. | Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 553] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol. 2014;12:919-928.e1; quiz e51-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Copelan A, Chehab M, Dixit P, Cappell MS. Safety and efficacy of angiographic occlusion of duodenal varices as an alternative to TIPS: review of 32 cases. Ann Hepatol. 2015;14:369-379. [PubMed] |
| 17. | Matsui S, Kudo M, Ichikawa T, Okada M, Miyabe Y. The clinical characteristics, endoscopic treatment, and prognosis for patients presenting with duodenal varices. Hepatogastroenterology. 2008;55:959-962. [PubMed] |
| 18. | Patwardhan VR, Cardenas A. Review article: the management of portal hypertensive gastropathy and gastric antral vascular ectasia in cirrhosis. Aliment Pharmacol Ther. 2014;40:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 19. | Thuluvath PJ, Yoo HY. Portal hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973-2978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | McGorisk T, Krishnan K, Keefer L, Komanduri S. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video). Gastrointest Endosc. 2013;78:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Siringo S, Burroughs AK, Bolondi L, Muia A, Di Febo G, Miglioli M, Cavalli G, Barbara L. Peptic ulcer and its course in cirrhosis: an endoscopic and clinical prospective study. J Hepatol. 1995;22:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Vergara M, Calvet X, Roqué M. Helicobacter pylori is a risk factor for peptic ulcer disease in cirrhotic patients. A meta-analysis. Eur J Gastroenterol Hepatol. 2002;14:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Kamalaporn P, Sobhonslidsuk A, Jatchavala J, Atisook K, Rattanasiri S, Pramoolsinsap C. Factors predisposing to peptic ulcer disease in asymptomatic cirrhotic patients. Aliment Pharmacol Ther. 2005;21:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 597] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 26. | Paquet KJ, Mercado-Diaz M, Kalk JF. Frequency, significance and therapy of the Mallory-Weiss syndrome in patients with portal hypertension. Hepatology. 1990;11:879-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Schuman BM, Threadgill ST. The influence of liver disease and portal hypertension on bleeding in Mallory-Weiss syndrome. J Clin Gastroenterol. 1994;18:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Jensen DM, Tung LA, You S. Etiology and management of Mallory Weiss bleeding in patients with and without portal hypertension. Gastrointest Endosc. 1988;34:204(A). |
| 29. | Cappell MS. Gastrointestinal vascular malformations or neoplasms: Arterial, venous, arteriovenous and capillary. Textbook of Gastroenterology. 5th ed. Chichester (West Sussex), United Kingdom: Wiley-Blackwell 2009; 2785-2810. |
| 30. | Jeon HK, Kim GH. Endoscopic management of Dieulafoy’s lesion. Clin Endosc. 2015;48:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Nojkov B, Cappell MS. Gastrointestinal bleeding from Dieulafoy’s lesion: Clinical presentation, endoscopic findings, and endoscopic therapy. World J Gastrointest Endosc. 2015;7:295-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Lebrec D, De Fleury P, Rueff B, Nahum H, Benhamou JP. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology. 1980;79:1139-1144. [PubMed] |
| 33. | Kumar A, Mishra SR, Sharma P, Sharma BC, Sarin SK. Clinical, laboratory, and hemodynamic parameters in portal hypertensive gastropathy: a study of 254 cirrhotics. J Clin Gastroenterol. 2010;44:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Tran A, Villeneuve JP, Bilodeau M, Willems B, Marleau D, Fenyves D, Parent R, Pomier-Layrargues G. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94:2909-2911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Maslekar S, Toh EW, Adair R, Bate JP, Botterill I. Systematic review of anorectal varices. Colorectal Dis. 2013;15:e702-e710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 36. | Grace ND. Prevention of initial variceal hemorrhage. Gastroenterol Clin North Am. 1992;21:149-161. [PubMed] |
| 37. | Buccino RV, Bogliolo G, Ferrara M, Pietropaolo V, Pecchioli L, Miscusi G, Montori A. Endoscopic approach to patients with portal hypertension: a complex diagnosis. A retrospective study based on 10 years’ experience. Surg Endosc. 1990;4:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | del Olmo JA, Peña A, Serra MA, Wassel AH, Benages A, Rodrigo JM. Predictors of morbidity and mortality after the first episode of upper gastrointestinal bleeding in liver cirrhosis. J Hepatol. 2000;32:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Lecleire S, Di Fiore F, Merle V, Hervé S, Duhamel C, Rudelli A, Nousbaum JB, Amouretti M, Dupas JL, Gouerou H. Acute upper gastrointestinal bleeding in patients with liver cirrhosis and in noncirrhotic patients: epidemiology and predictive factors of mortality in a prospective multicenter population-based study. J Clin Gastroenterol. 2005;39:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Lyles T, Elliott A, Rockey DC. A risk scoring system to predict in-hospital mortality in patients with cirrhosis presenting with upper gastrointestinal bleeding. J Clin Gastroenterol. 2014;48:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Chamberlain CE. Acute hemorrhagic gastritis. Gastroenterol Clin North Am. 1993;22:843-873. [PubMed] |
| 42. | Sugawa C, Benishek D, Walt AJ. Mallory-Weiss syndrome. A study of 224 patients. Am J Surg. 1983;145:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Peterson WL, Barnett C, Walsh JH. Effect of intragastric infusions of ethanol and wine on serum gastrin concentration and gastric acid secretion. Gastroenterology. 1986;91:1390-1395. [PubMed] |
| 44. | Hsieh WJ, Lin HC, Hwang SJ, Hou MC, Lee FY, Chang FY, Lee SD. The effect of ciprofloxacin in the prevention of bacterial infection in patients with cirrhosis after upper gastrointestinal bleeding. Am J Gastroenterol. 1998;93:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Svoboda P, Ehrmann J, Klvana P, Machytka E, Rydlo M, Hrabovský V. [A different view of acute upper gastrointestinal bleeding in liver cirrhosis patients]. Vnitr Lek. 2010;56:1116-1121. [PubMed] |
| 46. | Afessa B, Kubilis PS. Upper gastrointestinal bleeding in patients with hepatic cirrhosis: clinical course and mortality prediction. Am J Gastroenterol. 2000;95:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Svoboda P, Ehrmann J, Klvana P, Machytka E, Rydlo M, Hrabovský V. [The etiology of upper gastrointestinal bleeding in patients with liver cirrhosis]. Vnitr Lek. 2007;53:1274-1277. [PubMed] |
| 48. | Lo GH, Yu HC, Chan YC, Chen WC, Hsu PI, Lin CK, Lai KH. The effects of eradication of Helicobacter pylori on the recurrence of duodenal ulcers in patients with cirrhosis. Gastrointest Endosc. 2005;62:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Rabinovitz M, Schade RR, Dindzans V, Van Thiel DH, Gavaler JS. Prevalence of duodenal ulcer in cirrhotic males referred for liver transplantation. Does the etiology of cirrhosis make a difference? Dig Dis Sci. 1990;35:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Kim DJ, Kim HY, Kim SJ, Hahn TH, Jang MK, Baik GH, Kim JB, Park SH, Lee MS, Park CK. Helicobacter pylori infection and peptic ulcer disease in patients with liver cirrhosis. Korean J Intern Med. 2008;23:16-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Kalafateli M, Triantos CK, Nikolopoulou V, Burroughs A. Non-variceal gastrointestinal bleeding in patients with liver cirrhosis: a review. Dig Dis Sci. 2012;57:2743-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | González-González JA, García-Compean D, Vázquez-Elizondo G, Garza-Galindo A, Jáquez-Quintana JO, Maldonado-Garza H. Nonvariceal upper gastrointestinal bleeding in patients with liver cirrhosis. Clinical features, outcomes and predictors of in-hospital mortality. A prospective study. Ann Hepatol. 2011;10:287-295. [PubMed] |
| 53. | Luo JC, Leu HB, Hou MC, Huang CC, Lin HC, Lee FY, Chang FY, Chan WL, Lin SJ, Chen JW. Cirrhotic patients at increased risk of peptic ulcer bleeding: a nationwide population-based cohort study. Aliment Pharmacol Ther. 2012;36:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Kirk AP, Dooley JS, Hunt RH. Peptic ulceration in patients with chronic liver disease. Dig Dis Sci. 1980;25:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Mitchell CJ, Jewell DP. The diagnosis of the site of upper gastrointestinal haemorrhage in patients with established portal hypertension. Endoscopy. 1977;9:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Di Mario F, Gottardello L, Germanà B, Dotto P, Grassi SA, Vianello F, Battaglia G, Leandro G, Burra P, Salvagnini M. Peptic ulcer in cirrhotic patients: a short- and long-term study with antisecretory drugs. Ital J Gastroenterol. 1992;24:122-125. [PubMed] |
| 57. | Hsu YC, Lin JT, Chen TT, Wu MS, Wu CY. Long-term risk of recurrent peptic ulcer bleeding in patients with liver cirrhosis: a 10-year nationwide cohort study. Hepatology. 2012;56:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Kärkkäinen JM, Miilunpohja S, Rantanen T, Koskela JM, Jyrkkä J, Hartikainen J, Paajanen H. Alcohol abuse increases rebleeding risk and mortality in patients with non-variceal upper gastrointestinal bleeding. Dig Dis Sci. 2015;60:3707-3715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Holland-Bill L, Christiansen CF, Gammelager H, Mortensen RN, Pedersen L, Sørensen HT. Chronic liver disease and 90-day mortality in 21,359 patients following peptic ulcer bleeding--a Nationwide Cohort Study. Aliment Pharmacol Ther. 2015;41:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Venkatesh PG, Parasa S, Njei B, Sanaka MR, Navaneethan U. Increased mortality with peptic ulcer bleeding in patients with both compensated and decompensated cirrhosis. Gastrointest Endosc. 2014;79:605-614.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Seo YS, Kim YH, Ahn SH, Yu SK, Baik SK, Choi SK, Heo J, Hahn T, Yoo TW, Cho SH. Clinical features and treatment outcomes of upper gastrointestinal bleeding in patients with cirrhosis. J Korean Med Sci. 2008;23:635-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Morsy KH, Ghaliony MA, Mohammed HS. Outcomes and predictors of in-hospital mortality among cirrhotic patients with non-variceal upper gastrointestinal bleeding in upper Egypt. Turk J Gastroenterol. 2014;25:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Rudler M, Rousseau G, Benosman H, Massard J, Deforges L, Lebray P, Poynard T, Thabut D. Peptic ulcer bleeding in patients with or without cirrhosis: different diseases but the same prognosis? Aliment Pharmacol Ther. 2012;36:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Reynolds JC. Famotidine therapy for active duodenal ulcers. A multivariate analysis of factors affecting early healing. Ann Intern Med. 1989;111:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Auroux J, Lamarque D, Roudot-Thoraval F, Deforges L, Chaumette MT, Richardet JP, Delchier JC. Gastroduodenal ulcer and erosions are related to portal hypertensive gastropathy and recent alcohol intake in cirrhotic patients. Dig Dis Sci. 2003;48:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Aldoori WH, Giovannucci EL, Stampfer MJ, Rimm EB, Wing AL, Willett WC. A prospective study of alcohol, smoking, caffeine, and the risk of duodenal ulcer in men. Epidemiology. 1997;8:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Armstrong D, Arnold R, Classen M, Fischer M, Goebell H, Schepp W, Blum AL. RUDER--a prospective, two-year, multicenter study of risk factors for duodenal ulcer relapse during maintenance therapy with ranitidine. RUDER Study Group. Dig Dis Sci. 1994;39:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Kitano S, Dolgor B. Does portal hypertension contribute to the pathogenesis of gastric ulcer associated with liver cirrhosis? J Gastroenterol. 2000;35:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Ninomiya K, Kitano S, Yoshida T, Bandoh T, Baatar D, Tsuboi S. Impaired adaptive cytoprotection to ethanol-induced damage in gastric mucosa of portal hypertensive rats. Dig Dis Sci. 1999;44:1254-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Bang CS, Baik GH, Kim JH, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ. Peptic ulcer disease in liver cirrhosis and chronic hepatitis: impact of portal hypertension. Scand J Gastroenterol. 2014;49:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Chen LS, Lin HC, Hwang SJ, Lee FY, Hou MC, Lee SD. Prevalence of gastric ulcer in cirrhotic patients and its relation to portal hypertension. J Gastroenterol Hepatol. 1996;11:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Samloff IM. Multiple gastric red spots, capillary ectasia, hypergastrinemia and hypopepsinogenemia I in cirrhosis: a new syndrome? Hepatology. 1988;8:699-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 74. | Zullo A, Rinaldi V, Meddi P, Folino S, Lauria V, Diana F, Winn S, Attili AF. Helicobacter pylori infection in dyspeptic cirrhotic patients. Hepatogastroenterology. 1999;46:395-400. [PubMed] |
| 75. | Tsai CJ. Helicobacter pylori infection and peptic ulcer disease in cirrhosis. Dig Dis Sci. 1998;43:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Siringo S, Vaira D, Menegatti M, Piscaglia F, Sofia S, Gaetani M, Miglioli M, Corinaldesi R, Bolondi L. High prevalence of Helicobacter pylori in liver cirrhosis: relationship with clinical and endoscopic features and the risk of peptic ulcer. Dig Dis Sci. 1997;42:2024-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Pellicano R, Leone N, Berrutti M, Cutufia MA, Fiorentino M, Rizzetto M, Ponzetto A. Helicobacter pylori seroprevalence in hepatitis C virus positive patients with cirrhosis. J Hepatol. 2000;33:648-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Chen CT, Wang TF, Chan CC, Lee FY, Chang FY, Lin HC, Hou MC, Lu RH, Chu CJ, Wang SS. Role of chronic Helicobacter pylori infection in hyperdynamic circulation of cirrhotic patients. Hepatogastroenterology. 2002;49:208-212. [PubMed] |
| 79. | Chang CS, Kao CH, Yeh HZ, Lien HC, Chen GH, Wang SJ. Helicobacter pylori infection and gastric emptying in cirrhotic patients with symptoms of dyspepsia. Hepatogastroenterology. 1999;46:3166-3171. [PubMed] |
| 80. | Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 Suppl 2:33-39. [PubMed] |
| 81. | Webb PM, Knight T, Greaves S, Wilson A, Newell DG, Elder J, Forman D. Relation between infection with Helicobacter pylori and living conditions in childhood: evidence for person to person transmission in early life. BMJ. 1994;308:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 227] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Feng H, Zhou X, Zhang G. Association between cirrhosis and Helicobacter pylori infection: a meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:1309-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Tzathas C, Triantafyllou K, Mallas E, Triantafyllou G, Ladas SD. Effect of Helicobacter pylori eradication and antisecretory maintenance therapy on peptic ulcer recurrence in cirrhotic patients: a prospective, cohort 2-year follow-up study. J Clin Gastroenterol. 2008;42:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Chang SS, Hu HY. H. pylori eradication lower ulcers in cirrhosis. [Corrected]. J Dig Dis. 2014;15:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 85. | Cappell MS, Schein JR. Diagnosis and treatment of nonsteroidal anti-inflammatory drug-associated upper gastrointestinal toxicity. Gastroenterol Clin North Am. 2000;29:97-124, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 87. | Lee YC, Chang CH, Lin JW, Chen HC, Lin MS, Lai MS. Non-steroidal anti-inflammatory drugs use and risk of upper gastrointestinal adverse events in cirrhotic patients. Liver Int. 2012;32:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Castro-Fernández M, Sánchez-Muñoz D, Galán-Jurado MV, Larraona JL, Suárez E, Lamas E, Rodríguez-Hornillo MC, Pabón M. [Influence of nonsteroidal antiinflammatory drugs in gastrointestinal bleeding due to gastroduodenal ulcers or erosions in patients with liver cirrhosis]. Gastroenterol Hepatol. 2006;29:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol. 2014;29:1356-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Fockens P, Tytgat GN. Dieulafoy’s disease. Gastrointest Endosc Clin N Am. 1996;6:739-752. [PubMed] |
| 91. | Akhras J, Patel P, Tobi M. Dieulafoy’s lesion-like bleeding: an underrecognized cause of upper gastrointestinal hemorrhage in patients with advanced liver disease. Dig Dis Sci. 2007;52:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Baettig B, Haecki W, Lammer F, Jost R. Dieulafoy’s disease: endoscopic treatment and follow up. Gut. 1993;34:1418-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Lee JG, Leung JW. Stigmata of recent hemorrhage in Dieulafoy’s lesion. Gastrointest Endosc. 2000;51:191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Luis LF, Sreenarasimhaiah J, Jiang Tang S. Localization, efficacy of therapy, and outcomes of Dieulafoy lesions of the GI tract – The UT Southwestern GI Bleed Team experience. Gastrointest Endosc. 2008;67:AB 87. |
| 95. | Lee YT, Walmsley RS, Leong RW, Sung JJ. Dieulafoy’s lesion. Gastrointest Endosc. 2003;58:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Chung YF, Wong WK, Soo KC. Diagnostic failures in endoscopy for acute upper gastrointestinal haemorrhage. Br J Surg. 2000;87:614-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Chaer RA, Helton WS. Dieulafoy’s disease. J Am Coll Surg. 2003;196:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Romãozinho JM, Pontes JM, Lérias C, Ferreira M, Freitas D. Dieulafoy’s lesion: management and long-term outcome. Endoscopy. 2004;36:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Mallory GK, Weiss S. Hemorrhages from laceration of cardia orifice of the stomach due to vomiting. Am J Med Sci. 1929;178:506–510. [RCA] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 167] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Michel L, Serrano A, Malt RA. Mallory-Weiss syndrome. Evolution of diagnostic and therapeutic patterns over two decades. Ann Surg. 1980;192:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 95] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Knauer CM. Mallory-Weiss syndrome. Characterization of 75 Mallory-Weiss lacerations in 528 patients with upper gastrointestinal hemorrhage. Gastroenterology. 1976;71:5-8. [PubMed] |
| 102. | Kortas DY, Haas LS, Simpson WG, Nickl NJ, Gates LK. Mallory-Weiss tear: predisposing factors and predictors of a complicated course. Am J Gastroenterol. 2001;96:2863-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 103. | Di Fiore F, Lecleire S, Merle V, Hervé S, Duhamel C, Dupas JL, Vandewalle A, Bental A, Gouerou H, Le Page M. Changes in characteristics and outcome of acute upper gastrointestinal haemorrhage: a comparison of epidemiology and practices between 1996 and 2000 in a multicentre French study. Eur J Gastroenterol Hepatol. 2005;17:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Yin A, Li Y, Jiang Y, Liu J, Luo H. Mallory-Weiss syndrome: clinical and endoscopic characteristics. Eur J Intern Med. 2012;23:e92-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Ljubičić N, Budimir I, Pavić T, Bišćanin A, Puljiz Z, Bratanić A, Troskot B, Zekanović D. Mortality in high-risk patients with bleeding Mallory-Weiss syndrome is similar to that of peptic ulcer bleeding. Results of a prospective database study. Scand J Gastroenterol. 2014;49:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Watts HD, Admirand WH. Mallory-Weiss syndrome. A reappraisal. JAMA. 1974;230:1674-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Kim JW, Kim HS, Byun JW, Won CS, Jee MG, Park YS, Baik SK, Kwon SO, Lee DK. Predictive factors of recurrent bleeding in Mallory-Weiss syndrome. Korean J Gastroenterol. 2005;46:447-454. [PubMed] |
| 108. | Alharbi A, Almadi M, Barkun A, Martel M; REASON Investigators. Predictors of a variceal source among patients presenting with upper gastrointestinal bleeding. Can J Gastroenterol. 2012;26:187-192. [PubMed] |
| 109. | Herrera JL. Management of acute variceal bleeding. Clin Liver Dis. 2014;18:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Rudolph SJ, Landsverk BK, Freeman ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003;57:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1069] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 112. | Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964-971.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 113. | Das SK, Mukherjee S, Vasudevan DM, Balakrishnan V. Comparison of haematological parameters in patients with non-alcoholic fatty liver disease and alcoholic liver disease. Singapore Med J. 2011;52:175-181. [PubMed] |
| 114. | Razzaghi A, Barkun AN. Platelet transfusion threshold in patients with upper gastrointestinal bleeding: a systematic review. J Clin Gastroenterol. 2012;46:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 115. | Maltz GS, Siegel JE, Carson JL. Hematologic management of gastrointestinal bleeding. Gastroenterol Clin North Am. 2000;29:169-187, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Dzik WH, Kirkley SA. Citrate toxicity during massive blood transfusion. Transfus Med Rev. 1988;2:76-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Knochel JP. Hypophosphatemia in the alcoholic. Arch Intern Med. 1980;140:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 461] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 119. | Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 256] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 120. | Bamji N, Cohen LB. Endoscopic sedation of patients with chronic liver disease. Clin Liver Dis. 2010;14:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Prabhakar S, Bhatia R. Management of agitation and convulsions in hepatic encephalopathy. Indian J Gastroenterol. 2003;22 Suppl 2:S54-S58. [PubMed] |
| 122. | Rahimi RS, Rockey DC. End-stage liver disease complications. Curr Opin Gastroenterol. 2013;29:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Ferguson JA, Suelzer CJ, Eckert GJ, Zhou XH, Dittus RS. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 124. | Holloway HC, Hales RE, Watanabe HK. Recognition and treatment of acute alcohol withdrawal syndromes. Psychiatr Clin North Am. 1984;7:729-743. [PubMed] |
| 125. | Pradella P, Bonetto S, Turchetto S, Uxa L, Comar C, Zorat F, De Angelis V, Pozzato G. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 126. | Lata J, Husová L, Juránková J, Senkyrík M, Díte P, Dastych M, Dastych M, Kroupa R. Factors participating in the development and mortality of variceal bleeding in portal hypertension--possible effects of the kidney damage and malnutrition. Hepatogastroenterology. 2006;53:420-425. [PubMed] |
| 127. | Hack JB, Hoffman RS. Thiamine before glucose to prevent Wernicke encephalopathy: examining the conventional wisdom. JAMA. 1998;279:583-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 128. | Ludwig D, Schädel S, Brüning A, Schiefer B, Stange EF. 48-hour hemodynamic effects of octreotide on postprandial splanchnic hyperemia in patients with liver cirrhosis and portal hypertension: double-blind, placebo-controlled study. Dig Dis Sci. 2000;45:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Ginès P, Fernández J, Durand F, Saliba F. Management of critically-ill cirrhotic patients. J Hepatol. 2012;56 Suppl 1:S13-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 130. | Green FW, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74:38-43. [PubMed] |
| 131. | Lau JY, Leung WK, Wu JC, Chan FK, Wong VW, Chiu PW, Lee VW, Lee KK, Cheung FK, Siu P. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 132. | D’Amico G, Politi F, Morabito A, D’Antoni A, Guerrera D, Giannuoli G, Traina M, Vizzini G, Pasta L, Pagliaro L. Octreotide compared with placebo in a treatment strategy for early rebleeding in cirrhosis. A double blind, randomized pragmatic trial. Hepatology. 1998;28:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 133. | Dooley CP, Larson AW, Stace NH, Renner IG, Valenzuela JE, Eliasoph J, Colletti PM, Halls JM, Weiner JM. Double-contrast barium meal and upper gastrointestinal endoscopy. A comparative study. Ann Intern Med. 1984;101:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 111] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Reilly HF, al-Kawas FH. Dieulafoy’s lesion. Diagnosis and management. Dig Dis Sci. 1991;36:1702-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Cappell MS. Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7:214-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Chung SC, Leung JW, Leung FW. Effect of submucosal epinephrine injection on local gastric blood flow. A study using laser Doppler flowmetry and reflectance spectrophotometry. Dig Dis Sci. 1990;35:1008-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 137. | Cappell MS, Iacovone FM. Safety and efficacy of esophagogastroduodenoscopy after myocardial infarction. Am J Med. 1999;106:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 139. | Ramesh J, Limdi JK, Sharma V, Makin AJ. The use of thrombin injections in the management of bleeding gastric varices: a single-center experience. Gastrointest Endosc. 2008;68:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 140. | Aabakken L. Current endoscopic and pharmacological therapy of peptic ulcer bleeding. Best Pract Res Clin Gastroenterol. 2008;22:243-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 141. | Cohen J. Argon plasma coagulation in the management of gastrointestinal hemorrhage. UpToDate. Accessed July 22, 2015. Available from: http://www.uptodate.com/contents/argon-plasma-coagulation-in-the-management-of-gastrointestinal-hemorrhage?source=machineLearning&search=argon plasma coagulation&selectedTitle=1~54§ionRank=1&anchor=H2#H2. |
| 142. | Llach J, Bordas JM, Salmerón JM, Panés J, García-Pagán JC, Feu F, Navasa M, Mondelo F, Piqué JM, Mas A. A prospective randomized trial of heater probe thermocoagulation versus injection therapy in peptic ulcer hemorrhage. Gastrointest Endosc. 1996;43:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Dray X, Camus M, Coelho J, Ozenne V, Pocard M, Marteau P. Treatment of gastrointestinal angiodysplasia and unmet needs. Dig Liver Dis. 2011;43:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 144. | Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 145. | Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 525] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 146. | Freeman ML. Stigmata of hemorrhage in bleeding ulcers. Gastrointest Endosc Clin N Am. 1997;7:559-574. [PubMed] |
| 147. | Katschinski B, Logan R, Davies J, Faulkner G, Pearson J, Langman M. Prognostic factors in upper gastrointestinal bleeding. Dig Dis Sci. 1994;39:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 148. | Kovacs TO, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol. 2007;10:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Bini EJ, Cohen J. Endoscopic treatment compared with medical therapy for the prevention of recurrent ulcer hemorrhage in patients with adherent clots. Gastrointest Endosc. 2003;58:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 150. | Kahi CJ, Jensen DM, Sung JJ, Bleau BL, Jung HK, Eckert G, Imperiale TF. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: a meta-analysis. Gastroenterology. 2005;129:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 151. | Jensen DM, Kovacs TO, Jutabha R, Machicado GA, Gralnek IM, Savides TJ, Smith J, Jensen ME, Alofaituli G, Gornbein J. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002;123:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 152. | Calvet X, Vergara M, Brullet E, Gisbert JP, Campo R. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004;126:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 153. | Vergara M, Bennett C, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high-risk bleeding ulcers. Cochrane Database Syst Rev. 2014;10:CD005584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 154. | Chung SC, Leung JW, Sung JY, Lo KK, Li AK. Injection or heat probe for bleeding ulcer. Gastroenterology. 1991;100:33-37. [PubMed] |
| 155. | Olmos JA, Marcolongo M, Pogorelsky V, Herrera L, Tobal F, Dávolos JR. Long-term outcome of argon plasma ablation therapy for bleeding in 100 consecutive patients with colonic angiodysplasia. Dis Colon Rectum. 2006;49:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 156. | Joarder AI, Faruque MS, Nur-E-Elahi M, Jahan I, Siddiqui O, Imdad S, Islam MS, Ahmed HS, Haque MA. Dieulafoy’s lesion: an overview. Mymensingh Med J. 2014;23:186-194. [PubMed] |
| 157. | Mumtaz R, Shaukat M, Ramirez FC. Outcomes of endoscopic treatment of gastroduodenal Dieulafoy’s lesion with rubber band ligation and thermal/injection therapy. J Clin Gastroenterol. 2003;36:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 158. | Iacopini F, Petruzziello L, Marchese M, Larghi A, Spada C, Familiari P, Tringali A, Riccioni ME, Gabbrielli A, Costamagna G. Hemostasis of Dieulafoy’s lesions by argon plasma coagulation (with video). Gastrointest Endosc. 2007;66:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 159. | Cheng CL, Liu NJ, Lee CS, Chen PC, Ho YP, Tang JH, Yang C, Sung KF, Lin CH, Chiu CT. Endoscopic management of Dieulafoy lesions in acute nonvariceal upper gastrointestinal bleeding. Dig Dis Sci. 2004;49:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 160. | Yamaguchi Y, Yamato T, Katsumi N, Imao Y, Aoki K, Morita Y, Miura M, Morozumi K, Ishida H, Takahashi S. Short-term and long-term benefits of endoscopic hemoclip application for Dieulafoy’s lesion in the upper GI tract. Gastrointest Endosc. 2003;57:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 161. | Bharucha AE, Gostout CJ, Balm RK. Clinical and endoscopic risk factors in the Mallory-Weiss syndrome. Am J Gastroenterol. 1997;92:805-808. [PubMed] |
| 162. | Llach J, Elizalde JI, Guevara MC, Pellisé M, Castellot A, Ginès A, Soria MT, Bordas JM, Piqué JM. Endoscopic injection therapy in bleeding Mallory-Weiss syndrome: a randomized controlled trial. Gastrointest Endosc. 2001;54:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 163. | Laine L. Multipolar electrocoagulation in the treatment of active upper gastrointestinal tract hemorrhage. A prospective controlled trial. N Engl J Med. 1987;316:1613-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 165] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 164. | Cho YS, Chae HS, Kim HK, Kim JS, Kim BW, Kim SS, Han SW, Choi KY. Endoscopic band ligation and endoscopic hemoclip placement for patients with Mallory-Weiss syndrome and active bleeding. World J Gastroenterol. 2008;14:2080-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 165. | Lecleire S, Antonietti M, Iwanicki-Caron I, Duclos A, Ramirez S, Ben-Soussan E, Hervé S, Ducrotté P. Endoscopic band ligation could decrease recurrent bleeding in Mallory-Weiss syndrome as compared to haemostasis by hemoclips plus epinephrine. Aliment Pharmacol Ther. 2009;30:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |