In patients with chronic liver disease
Regenerative and dysplastic nodules: Cirrhosis is characterized by the progressive fibrosis of the liver parenchyma and a spectrum of hepatocellular nodules that mark the progression from regenerative nodules (RNs) to low- and high-grade dysplastic nodules (DNs) to, eventually, HCC[19,20]. There is a considerable overlap among these nodules during hepatocarcinogenesis on histopathology and imaging, although the characteristic imaging findings of hepatocarcinogenesis have been relatively well established[15,20].
RNs usually exhibit iso SI or low SI on T2-weighted images and variable SIs on T1-weighted images, while DNs characteristically exhibit high SI on T1-weighted images and iso SI or low SI on T2-weighted images. This is because DNs may contain more copper or iron compared with the background liver. Because these nodules may contain varying amounts of lipids, copper, or iron, they exhibit variable SIs on T1- and T2-weighted images depending on their content. However, RNs and DNs mostly do not exhibit high SI on T2-weighted images or restricted diffusion[21]. Therefore, during the differential diagnosis of RNs or DNs from HCCs, the presence of mild to moderately high SI on T2-weighted images or restricted diffusion strongly indicates the presence of HCC.
Following the injection of gadoxetic acid, because RNs are predominantly supplied by the portal vein, most of them enhance to the same degree as the adjacent liver parenchyma, resulting in iso SI in the hepatic arterial and later phases. Occasionally, they demonstrate slightly lower enhancement, which is observed as mildly low SI in the portal and transitional phases. Meanwhile, because DNs exhibit a decreased number of portal tracts with a relatively lesser increase in the number of unpaired arteries during hepatocarcinogenesis, they mostly demonstrate iso SI or low SI in the hepatic arterial and later phases. However, some DNs may have an increased arterial supply because of neoangiogenesis and enhance more than the liver in the hepatic arterial phase; this may lead to a misdiagnosis of hypervascular HCC[20].
On HBP images obtained using gadoxetic acid, RNs typically show iso SI or high SI because of preserved OATP expression[20] (Figure 3). DNs usually show iso SI or low SI on HBP images because of decreased OATP expression (Figure 4). Therefore, because OATP expression decreases during hepatocarcinogenesis, iso SI to high SI on HBP images is generally suggestive of benign lesions (RNs or low-grade DNs), while low SI on HBP images is a strong predictor of premalignancy or malignancy (high-grade DNs or HCCs).
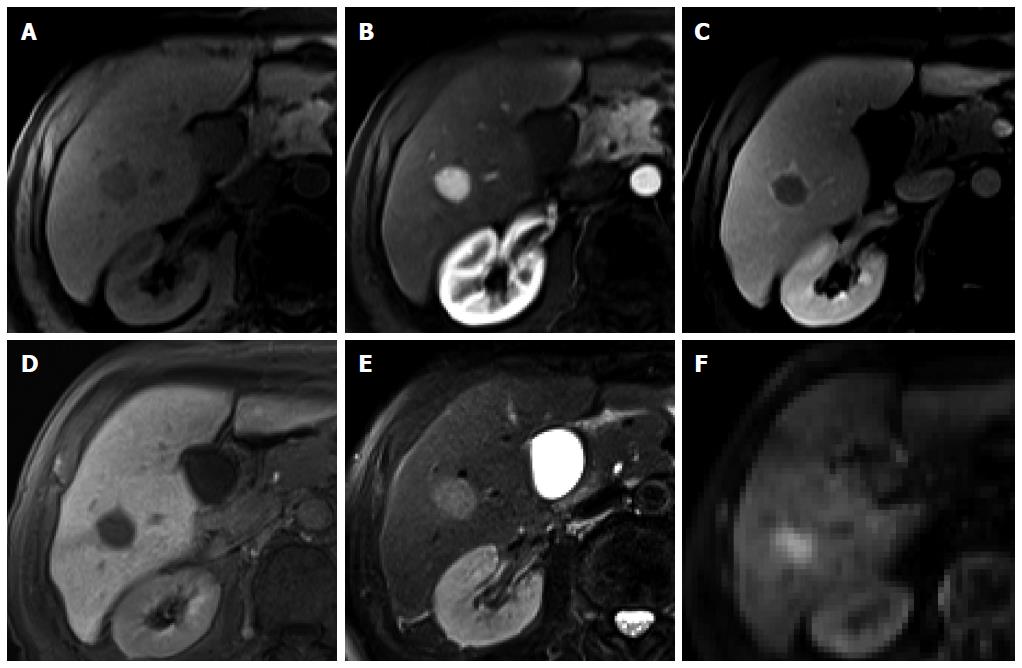
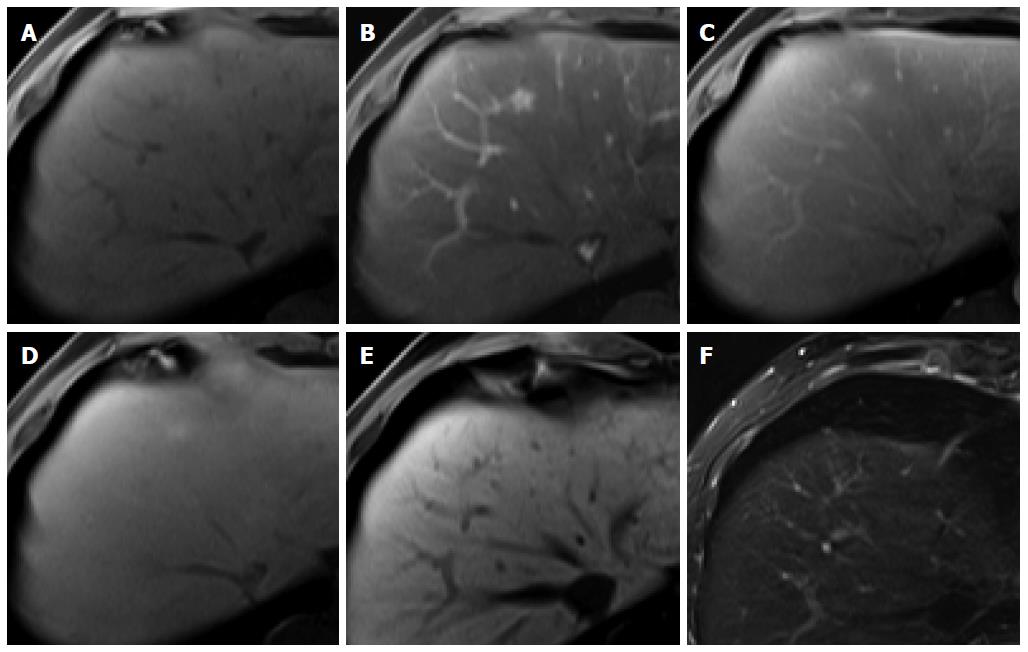
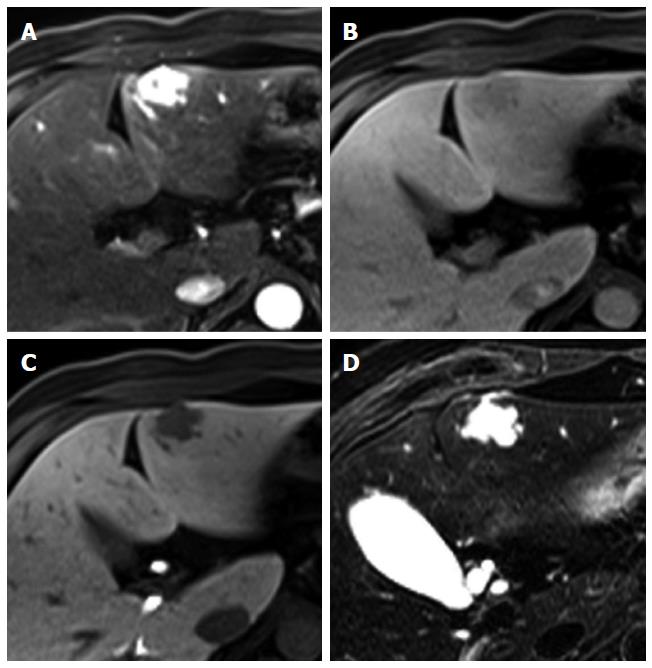
Figure 3 Regenerative nodule in a 61-year-old man with alcoholic liver cirrhosis.
A-D: Precontrast T1-weighted image, hepatic arterial phase, portal venous phase, and transitional phase show no visible lesion in the scanned area; E: Hepatobiliary phase shows a hyperintense nodule (arrow) in hepatic S4; F: T2-weighted image shows isointensity of the tumor.
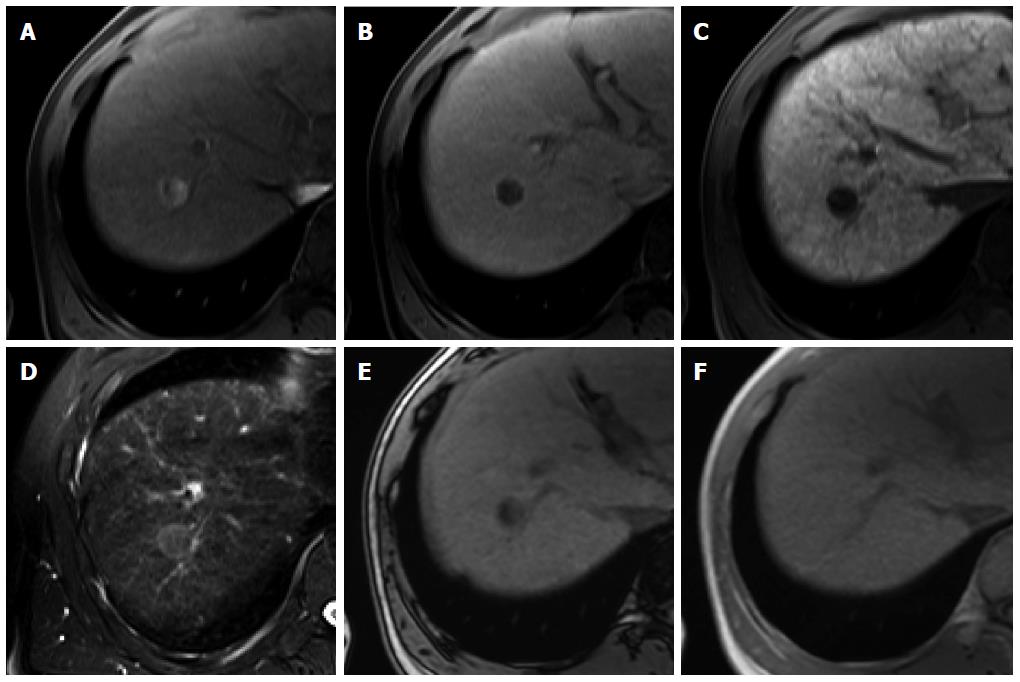
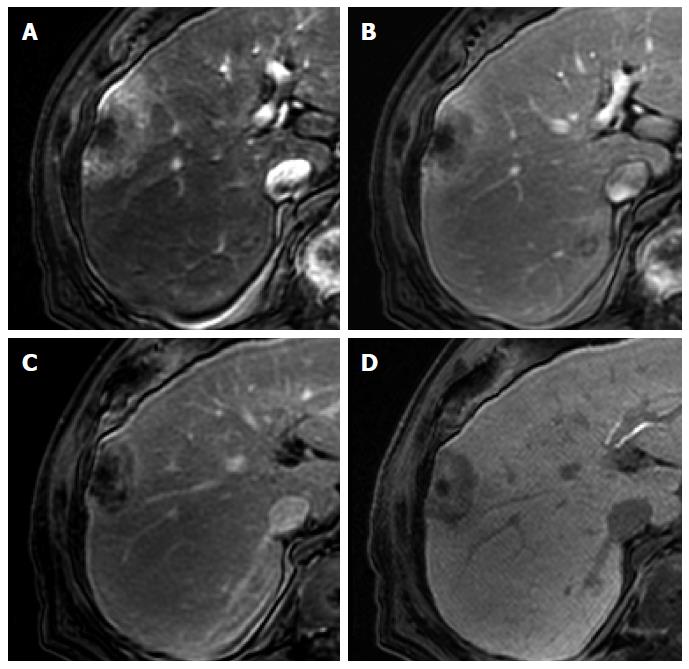
Figure 4 Dysplastic nodule in the same patient as Figure 1.
A: precontrast T1-weighted image shows a hyperintense nodule in segment 8, suggesting high contents of iron or copper; B: Hepatic arterial phase of gadoxetic acid-enhanced MRI shows isointensity of the tumor; C, D: transitional and hepatobiliary phases show hypointensity of the tumor relative to the liver parenchyma; E, F: T2-weighted image and diffusion weighted image (b = 800) show isointensity of the tumor.
Focal nodular hyperplasia-like nodules: Focal nodular hyperplasia (FNH)-like nodules are histopathologically and immunohistochemically identical to classic FNH observed in noncirrhotic livers, although they occur in patients with chronic liver disease or cirrhotic livers[22]. Therefore, the imaging findings of FNH-like nodules are also identical to the characteristic radiological findings of classic FNH on dynamic CT and MRI. Usually, FNH-like nodules are small hypervascular lesions[23] (Figure 5). However, if hypervascular FNH-like nodules are detected in cirrhotic livers, it is often difficult to differentiate them from HCC, particularly in atypical cases[24]. Because of identical imaging features, differences between atypical FNH-like nodules and typical HCCs will be discussed later in the FNH section. On the other hand, with regard to differentiation of typical FNH-like nodules from atypical HCCs with HBP high SI, lack of delayed washout is a key imaging finding for diagnosis of NRH (Kim JW, unpublished data, 2014)[25].
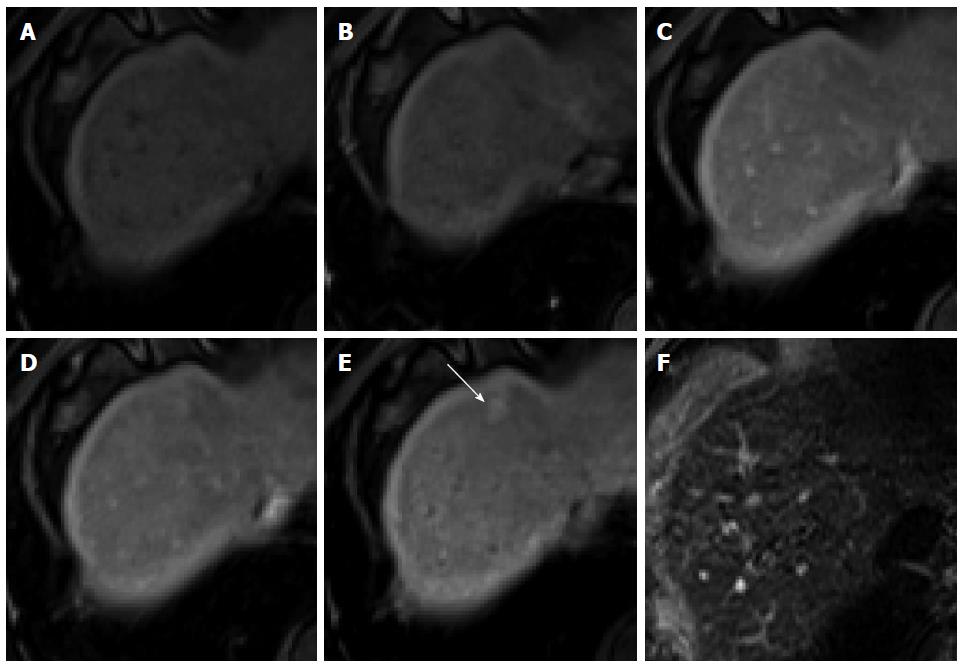
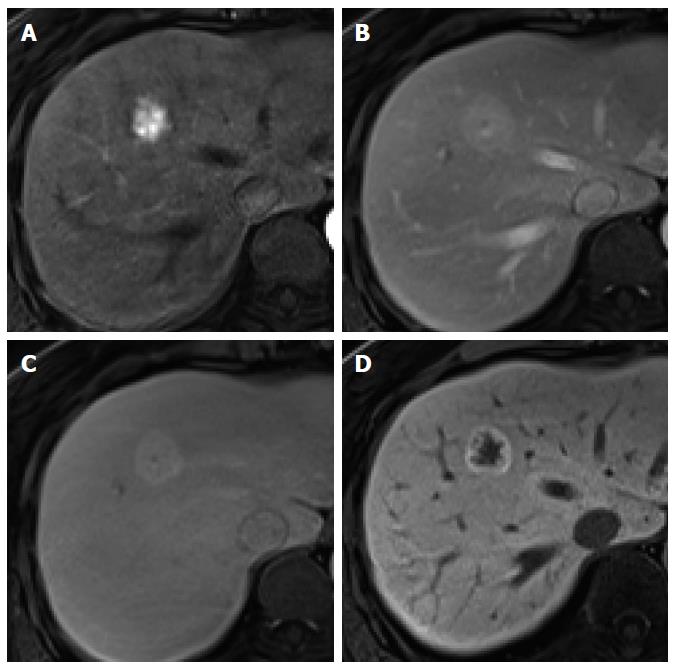
Figure 5 Focal nodular hyperplasia-like nodule in a 45-year-old man with hepatitis B infection.
A: Precontrast T1-weighted image shows isointensity of the tumor; B: Hepatic arterial phase using gadoxetic acid shows lobulating-contoured, marked enhanced nodule in segment 4; C, D: Portal venous and transitional phases show slight hyperenhancement of the tumor relative to the liver parenchyma; E: Hepatobiliary phase shows isointensity or subtle peripheral ring-like enhancement of the tumor; F: T2-weighted image shows isointensity of the tumor.
Nodular regenerative hyperplasia: Nodular regenerative hyperplasia (NRH) is a rare liver condition characterized by the widespread benign transformation of the hepatic parenchyma into small RNs. NRH may lead to the development of noncirrhotic portal hypertension[26] and is often associated with organ transplantation, myeloproliferative disease, or autoimmune processes. NRH exhibits iso SI to high SI on T1-weighted images (93.9%) and iso SI on T2-weighted images (82%), which are slightly different from the T1 and T2 SIs for FNH[27]. In one study using hepatocyte-specific MR agents with gadobenate dimeglumine[27], all NRHs showed arterial enhancement and iso SI to high SI on portal venous, equilibrium, and HBP images. Although the dynamic enhancement pattern of NRH resembles that of FNH, FNHs and FNH-like nodules show strong arterial enhancement and NRHs show mild arterial enhancement[27,28]. According to unpublished data of Kozaka et al[28], NRH appears as peripheral ring-like enhancement of the lesion on HBP images using gadoxetic acid, and they described this appearance as a doughnut-like nodule in HBP images. Therefore, arterial enhancement degree and SIs on T1- and T2-weighted images can provide differentiation of NRHs from FNHs and FNH-like nodules. Furthermore, the absence of washout and either iso to high SI or doughnut-like nodules on HBP images will distinguish this benign lesion from HCC.
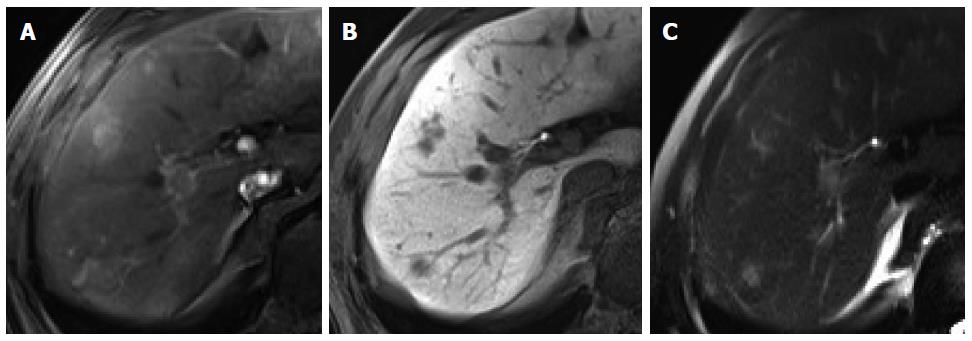
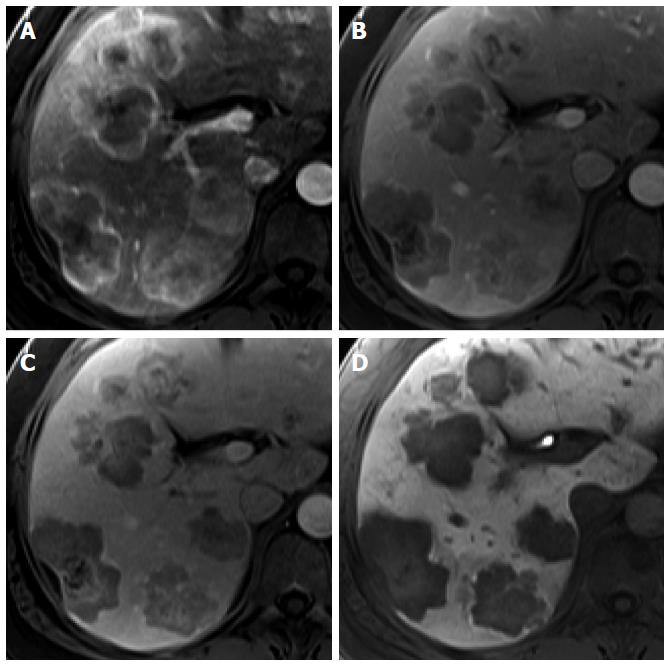
Intrahepatic cholangiocarcinoma: Intrahepatic mass-forming cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy after HCC. The typical enhancement pattern of ICC (77%) is peripheral rim-like arterial enhancement with progressive and concentric fill-in enhancement[29-31] (Figure 6). However, small ICC lesions (less than 3 cm in diameter, up to 6% of ICCs) can show an atypical enhancement pattern characterized by homogeneous arterial enhancement with washout, thus mimicking HCC[30] (Figure 6). Moreover, hepatitis C virus-induced liver cirrhosis has been recognized as an important risk factor for the development of ICC[32]. It may cause difficulty in the differential diagnosis of small hypervascular ICC from HCC in patients with liver cirrhosis, particularly that secondary to hepatitis C infection. Meanwhile, on gadoxetic acid-enhanced HBP images, most ICCs (96%) show low SI. In previous studies, 32%-85% of ICCs showed a central hyperintense area with a peripheral hypointense rim, known as target appearance. Furthermore, a central high SI was described as an EOB cloud, attributed to contrast uptake by the central fibrotic stroma[30,31,33] (Figure 7). In a comparison between ICC and HCC using gadoxetic acid-enhanced MRI[33], the target appearance on HBP images was more common with ICC than with HCC (85.7% vs 17.1%) and was the best predictor for distinguishing ICC from HCC. Furthermore, HBP images were found to show an increased lesion conspicuity (lobulated shape of ICC vs globular shape of HCC) and better delineation of daughter nodules and intrahepatic metastasis[30], which may aid in ICC diagnosis.
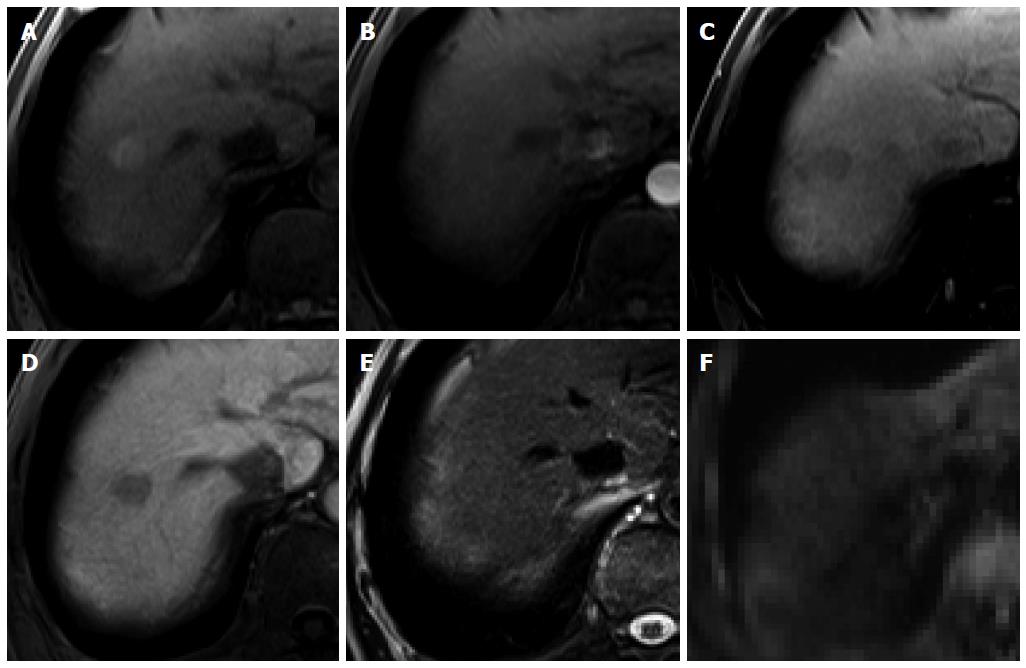
Figure 6 Intrahepatic cholangiocarcinoma in an 80-year-old man.
A: Hepatic arterial phase using gadoxetic acid shows peripheral arterial enhancing mass with capsular retraction at the hepatic segment 8 subcapsular location; B: Portal venous and transitional phases show target appearance with peripheral enhancement and central nonenhancement; C, D: Hepatobiliary phase also shows low signal intensity of the most outer portion, high signal intensity of mid portion, and marked low signal intensity of the center of the tumor.
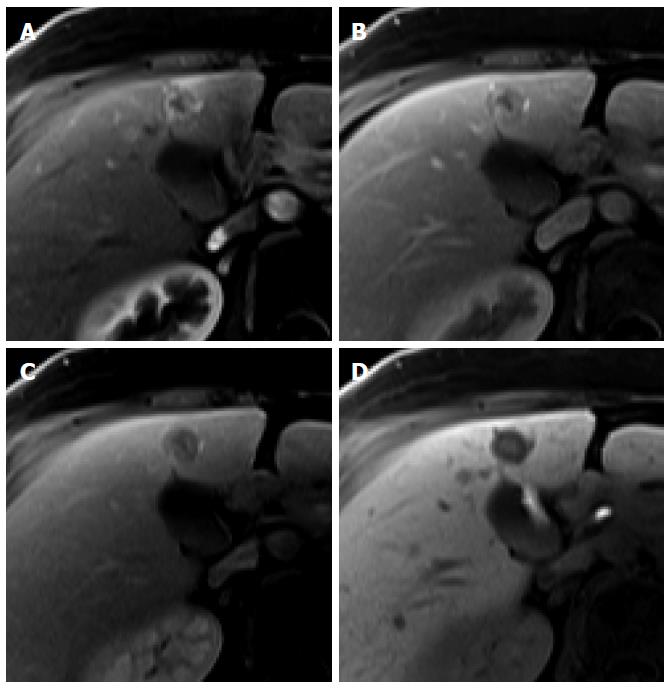
Figure 7 Intrahepatic cholangiocarcinoma in an 80-year-old man.
A: Hepatic arterial phase using gadoxetic acid shows heterogeneously arterial enhancing nodule in hepatic segment 4 dome; B, C: Portal venous and transitional phases show delayed washout; D: Hepatobiliary phase shows target appearance with peripheral low signal intensity and central high signal intensity.
In patients without chronic liver disease or with a normal liver
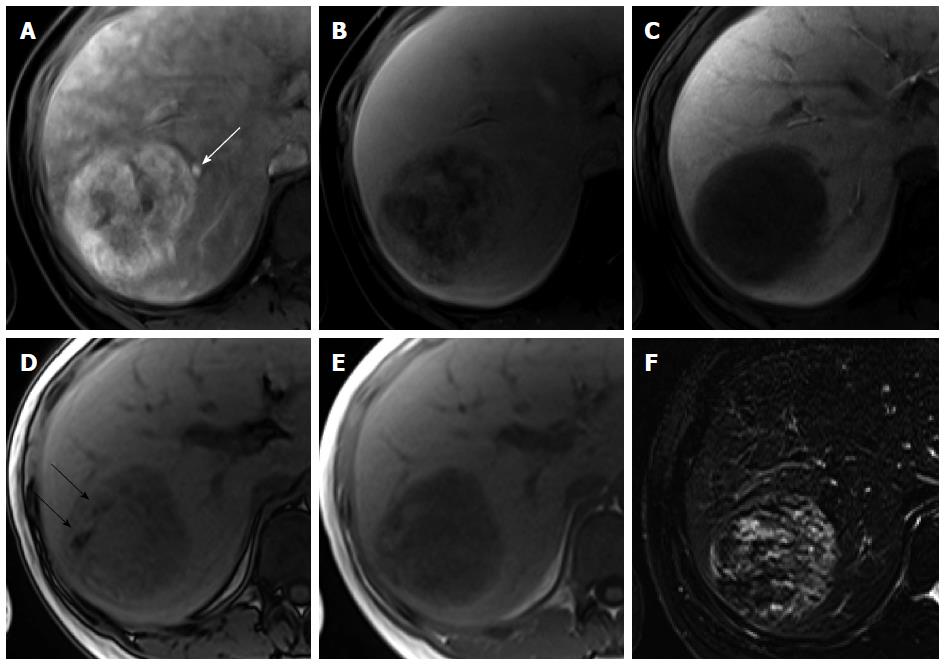
Focal nodular hyperplasia: FNH is the second most common benign hepatic tumor, found more commonly in healthy young and middle-aged women. Histologically, FNH is characterized by a central fibrous scar with surrounding nodules of hyperplastic hepatocytes and small bile ducts. Because of the benign nature of FNH, which usually necessitates conservative management, noninvasive diagnosis is important. Characteristic morphological features and dynamic enhancement patterns have been well demonstrated for FNH[34]. Morphologically, FNH shows a lobulated or microlobulated border without a true tumor capsule and has a central fibrous scar. Similar to the typical imaging findings observed on dynamic CT and MRI using conventional ECF agents, FNH shows intense and homogeneous arterial enhancement that subsequently fades without delayed washout and iso SI or high SI in the portal venous and transitional phases of gadoxetic acid-enhanced MRI[34,35]. Because of continuous contrast uptake by functioning hepatocytes within the tumor, the majority (91%-96%) of FNHs show iso SI or high SI on HBP images[34-37]. Iso SI or high SI on HBP images is a characteristic imaging finding of FNH, allowing accurate diagnosis[35,37-39]. Although a small number of FNHs (23%) show mixed or low SI on HBP images, peripheral ring-like enhancement of the lesion is frequently observed and is crucial for the identification of FNH[36] (Figure 8). On the other hand, the presence of a typical central scar is a reliable radiological sign for FNH diagnosis. However, a macroscopic central scar occurs in 50%-61% of FNHs and is often absent in FNHs measuring less than 3 cm[37,40]. Compared with that observed using ECF contrast agents, a central scar observed using gadoxetic acid does not typically demonstrate delayed enhancement, resulting in markedly low SI on HBP images[41]. Accordingly, FNH with a large central scar rarely shows low SI on HBP images. Because approximately 10% of HCC and less than 10% of FNH lesions show high and low SI, respectively, on HBP images[13,34,35,37], a definitive diagnosis of HCC and FNH is sometimes difficult. However, with regard to hypervascular FNH with low SI on HBP images, female sex, presence in the normal liver, characteristic morphologic features such as lobulated or microlobulated borders and a central scar, lack of delayed washout, and ring-like enhancement with central iso SI to low SI on HBP images are helpful for the diagnosis of FNH.
Figure 8 Focal nodular hyperplasia in a 55-year-old woman.
A: Hepatic arterial phase image of gadoxetic acid-enhanced etic resonance imaging shows lobulating contoured, marked enhanced tumor; B, C: Portal venous and transitional phases show slightly hyperenhancement of the tumor relative to the liver parenchyma, and central hypointense area is suspected as central scar; D: Hepatobiliary phase shows peripheral ring-like enhancement of the tumor with larger area of markedly hypointense central scar as compared with the other phase images.
Hepatocellular adenoma: Hepatocellular adenoma (HCA) is the third most common benign hepatic tumor that particularly affects young and middle-aged women. It was recently subclassified into four groups according to the genotype and phenotype: inflammatory (50%), hepatocyte nuclear factor (NHF)-1α-mutated (35%-40%), β-catenin-mutated (10%-15%), and unclassified (< 10%)[42]. The MRI findings vary on the basis of histological findings and associated complications, and MRI has proven to be an accurate method for subtyping HCAs[43-45]. For several years, the differentiation of HCA from FNH has been a major concern, because these hypervascular tumors are frequently observed in women of a similar age. HCA requires surgical resection because of the risk of hemorrhage and malignant transformation[35,38,39]. On the other hand, on gadoxetic acid-enhanced MRI, 90% of HCAs show mild-to-moderate arterial enhancement, 72% show low SI in the transitional phase, and 93% show low SI on HBP images[35]. In particular, HNF-1α-mutated and β-catenin-mutated HCAs show arterial enhancement with washout and low SI on HBP images, mimicking HCC, while some inflammatory HCAs show arterial enhancement with persistent enhancement and high SI on HBP images, mimicking FNH[35,46]. Therefore, the majority of HCAs shows the typical enhancement pattern shown by HCC. However, the most important fact is that HCA typically occurs in noncirrhotic livers in women of child-bearing age, while HCC primarily occurs in cirrhotic livers. Notwithstanding, differentiation between HCA and HCC in noncirrhotic livers is an issue. However, a larger fat component is more typical for HNF-1α-mutated HCA[44]. A rim-like band with high SI in the periphery of the lesion on T2-weighted images (atoll sign) is observed for 13% of HCA[39,43]. Furthermore, a capsule or a pseudocapsule, which appears as a delayed enhancing rim, is observed less commonly in HCA than in HCC (Figure 9) (25%-31% vs 70%)[16,47,48]. Although there is no study on the advantages of using gadoxetic acid for the differential diagnosis of HCA and HCC, these ancillary findings will be helpful in their differentiation in noncirrhotic livers.
Figure 9 Hepatocellular adenoma in a 45-year-old woman.
A: Hepatic arterial phase using gadoxetic acid shows moderate arterial enhancement; B: Transitional phase shows delayed washout without capsule or pseudocapsule; C: Hepatobiliary phase shows heterogeneous low signal intensity of the tumor; D: T2-weighted image shows a peripheral hyperintense band with moderate high signal intensity of residual tumor.
Hemangioma: Hemangioma is the most common benign hepatic tumor[49]. On dynamic CT and MRI using conventional ECF agents, a typical hemangioma shows early peripheral nodular enhancement with centripetal and prolonged enhancement (Figure 10). High-flow hemangiomas, which account for 16% of all hemangiomas and 42% of hemangiomas measuring less than 1 cm in diameter, show immediate homogeneous arterial enhancement with persistent enhancement in the portal and equilibrium phase[49]. With regard to gadoxetic acid-enhanced MRI, hemangioma shows typically low SI on HBP images because of the absence of hepatocytes in the lesion[50]. Furthermore, it shows low SI in the transitional phase (Figure 11). Because contrast uptake by hepatocytes begins as early as the portal venous phase and parenchymal enhancement gradually increases in the transitional phase and HBP, hemangioma shows a relatively decreased SI compared with the parenchyma, exhibiting washout in the transitional phase[50]. Although this has been described as “pseudowashout”[51], hemangioma shows SI equivalent to that of the portal vein in all phases, with a gradual decrease in SI from the portal phase to HBP (no rapid washout)[50]. Moreover, hemangioma shows typically bright and high SI on T2-weighted images and high SI on diffusion-weighted images with high apparent diffusion coefficient value[51,52]. Therefore, high-flow hemangioma on gadoxetic acid-enhanced MRI, which shows arterial hyperenhancement with pseudowashout, can be confused for HCC. However, bright and high SI on T2-weighted images, high apparent diffusion coefficient value, and SI equivalent to that of the portal vein in all phases may be helpful for the differentiation of hemangioma from HCC.
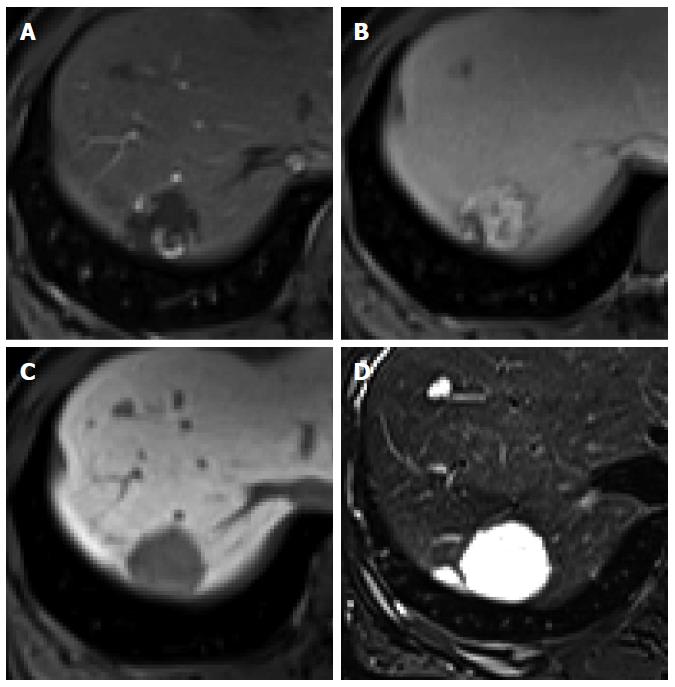
Figure 10 Hepatic hemangioma in a 45-year-old woman.
A: Hepatic arterial phase using gadoxetic acid shows peripheral nodular enhancement of the tumor in segment 7; B: Transitional phase shows centripetal and prolonged enhancement; C: Hepatobiliary phase shows hypointense defect relative to hepatic parenchyma; D: T2-weighted image shows bright and high signal intensity.
Figure 11 Hepatic hemangioma in a 54-year-old man.
A: Hepatic arterial phase in gadoxetic acid-enhanced MRI shows homogeneous marked enhancement of the tumor in segment 3; B: Transitional phase shows slightly low signal intensity of the tumor relative to the liver parenchyma; C: Hepatobiliary phase shows more markedly low signal intensity of the tumor; D: T2-weighted image shows bright and high signal intensity of the tumor.
Angiomyolipoma: Angiomyolipoma (AML) is a benign, nonencapsulated mesenchymal tumor comprising varying proportions of three tissue elements: blood vessels, smooth muscle, and mature adipose tissue. The presence of fat tissue is the most important radiological feature of AML, although it is not specific for AML. The fat component of AML varies from less than 10% to more than 90% of the tumor volume, resulting in a varied imaging appearance and leading to an erroneous diagnosis in most cases. In addition, the epithelioid type of AML, which contains no or a minimal amount of macroscopic fat, demonstrates arterial enhancement and delayed washout, mimicking HCC[53]. Therefore, AML has been commonly misdiagnosed as HCC. On dynamic CT and MRI using conventional ECF agents, the fatty areas of AML are well vascularized and show early enhancement, whereas steatotic foci in HCC are relatively avascular and show less contrast enhancement[54]. With regard to the vascular components of AML, tortuous central tumoral vessels and early draining veins were found to be pathognomonic features of AML[55]. On the other hand, only one study differentiated between AML and HCC using gadoxetic acid-enhanced MRI[56]. Kim et al[56] reported that 100% of AMLs and 85% of HCCs showed arterial enhancement and delayed washout on MRI using gadoxetic acid. Compared with HCC, AML was found to show homogeneous low SI on HBP images more frequently (83% vs 41%) (Figure 12), while the degree of enhancement for this lesion on HBP images was found to be much lower than that for the spleen (92% vs 30%). They explained that AML is devoid of hepatocytes, leading to a more homogeneously lower SI on HBP images, while HCC may contain some dysplastic hepatocytes, leading to a more heterogeneously higher SI on HBP images. Therefore, HBP of gadoxetic acid-enhanced MRI would be the most beneficial sequence for discriminating AML from HCC. In addition, because AML is common in women, younger patients, and patients with a normal liver, the occurrence of lesions in young women with a normal liver would be a helpful clue for its differential diagnosis.
Figure 12 Angiomyolipoma in a 33-year-old woman.
A: Hepatic arterial phase using gadoxetic acid shows heterogeneous arterial enhancement of the tumor. Early venous drainage to the right hepatic vein is seen (white arrow); B: Transitional phase shows heterogeneously low signal intensity; C: Hepatobiliary phase shows homogeneously low signal intensity; D, E: Opposed phase (D) and in-phase (E) T1-weighted gradient echo images reveal hypointense mass with area of signal drop on opposed-phase image (black arrows), indicating fat-containing lesion; F: T2-weighted image shows heterogeneous signal intensity of the tumor.
Focal eosinophilic infiltration: Focal eosinophilic infiltration (FEI) is a focal hepatic lesion caused by eosinophil-induced tissue damage. It is associated with various eosinophilia-related conditions such as parasitic infections, allergic reactions, hypereosinophilic syndrome, and internal malignancies. On imaging, FEI lesions appear as small, ill-defined, nonspherical lesions, with low attenuation in the portal phase during dynamic CT[57]. Nevertheless, radiological differentiation of FEI from hepatic metastasis is difficult because of its multiplicity and higher incidence in patients with an underlying malignancy[58,59]. Furthermore, arterial hypervascularity is infrequently observed in FEI[60]. On gadoxetic acid-enhanced MRI in particular, we observed that FEI showed arterial enhancement [70%; rim (37.1%) and homogeneous (22.9%) enhancement] and low SI in the portal venous phase, transitional phase, and HBP (80%, 88.6%, and 100%, respectively), resulting in 45.7% of lesions showing the typical enhancement pattern of HCC[61] (Figure 13). However, FEI was found to show characteristically mixed or intermingled low SI, irregular margins, and nonspherical shapes on HBP images, which would be helpful for characterizing this lesion[62]. Size discrepancy on HBP images relative to the size on T1- or T2-weighted images is another characteristic finding of FEI, although there are studies on the differentiation of FEI from metastasis using gadoxetic acid[58,59,62]. In addition, iso SI on T1-weighted images is a useful MRI finding for the diagnosis of FEI[59].
Figure 13 Focal eosinophilic infiltration in a 52-year-old man.
A: Hepatic arterial phase of gadoxetic acid-enhanced MRI shows two irregular homogeneously enhancing nodular lesions in segments 7 and 8; B: Hepatobiliary phase shows low signal intensities with ill-defined margin and nonspherical shape; C: Heavily T2-weighted image shows smaller size of the lesions in S8, compared to (B).
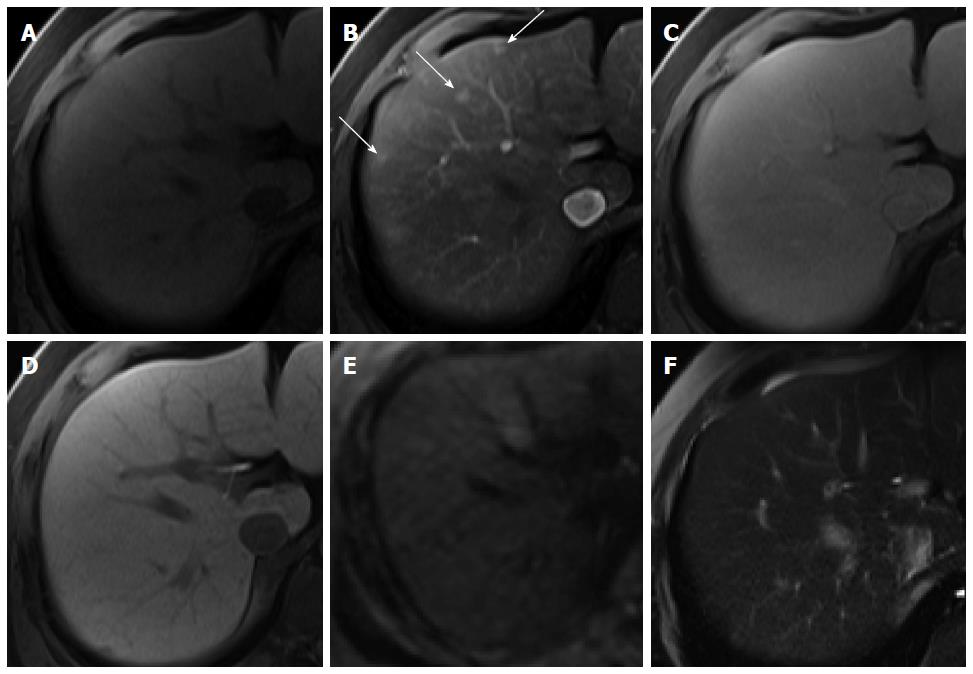
Hypervascular pseudolesions: Hypervascular pseudolesions, also known as arterioportal shunts, are typically wedge-shaped lesions with arterial enhancement in a subcapsular location. Typical lesions are easy to recognize and diagnose[63]. Meanwhile, subcentimeter-sized, small hypervascular pseudolesions tend to be nodular in shape[64] and are one of the primary lesions mimicking HCCs, resulting in difficulties in differential diagnosis. In HBP of gadoxetic acid-enhanced MRI, most hypervascular pseudolesions (94.3%) show iso SI compared with the surrounding liver tissue (Figure 14), which can be attributed to the intact hepatocyte function of these lesions[65], whereas up to 13% of nodular hypervascular pseudolesions show low SI on HBP images[64], also commonly demonstrated by HCCs. However, SI on HBP images is significantly lower for HCCs than for pseudolesions. Therefore, even though hypervascular pseudolesions rarely show low SI on HBP images, this finding may be helpful for accurate differentiation from HCC.
Figure 14 Hypervascular pseudolesion in a 49-year-old man with normal liver.
A: Precontrast T1-weighted image shows no focal hepatic lesion; B: Hepatic arterial phase using gadoxetic acid shows several arterial enhancing nodular lesions in the liver (arrows); C-F: Transitional, hepatobiliary, T2-weighted, and diffusion weighted images show no signal change.
Metastasis: Metastases are the most common malignant hepatic tumor. Metastases usually manifest as multiple discrete nodules or masses but occasionally manifest as a solitary nodule or mass[66]. Hepatic metastases from adenocarcinoma, such as colorectal cancer, are usually hypovascular and have arterial rim-like enhancement. Characteristically, neuroendocrine tumor, renal cell carcinoma, melanoma, and breast cancer are well known for hypervascular metastasis, showing arterial enhancement with delayed washout, like HCC. Although there have been few studies on enhancement pattern using gadoxetic acid, they have been commonly identified as defects on HBP images, owing to no functioning hepatocytes within the tumor[67]. However, in colorectal cancer liver metastasis, homogeneous defects on HBP were not common (27.8%) and heterogeneous defects on HBP were most common (63.3%)[68] (Figure 15). In breast cancer liver metastasis, this commonly manifested as a target sign (62%) with central high SI and peripheral low SI rim on the HBP, like ICC, because of contrast pooling at the central fibrotic area[67] (Figure 16). Therefore, hepatic metastases may appear as homogeneous defects, heterogeneous defects, or with target appearance on HBP images and the most common finding on HBP may vary from tumor to tumor. Meanwhile, the presence of multiple focal lesions in the non-cirrhotic liver of a patient with known malignancy, a homogeneous or heterogeneous defect, or target appearance on HBP image favor the diagnosis of metastasis.
Figure 15 Hepatic metastasis from colon cancer in a 74-year-old man.
A: Hepatic arterial phase using gadoxetic acid shows multiple arterial rim-like enhancing tumors with lobulating margin; B, C: Portal venous and transitional phases show heterogeneously low signal intensity of the tumors; D: Hepatobiliary phase shows peripheral hypointense rim with subtle high signal intensity of the center.
Figure 16 Hepatic metastasis from breast cancer in a 61-year-old woman.
A: Hepatic arterial phase using gadoxetic acid shows arterial rim-like enhancement; B, C: Portal venous and transitional phases show delayed washout of periphery of the tumor with central nonenhancement; D: Hepatobiliary phase shows target appearance with subtle high signal intensity of the center and peripheral low signal intensity rim.