Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.275
Peer-review started: May 27, 2015
First decision: August 31, 2015
Revised: October 29, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: January 7, 2016
Processing time: 220 Days and 4 Hours
Glypican-3 (GPC3) is a cell surface oncofetal proteoglycan that is anchored by glycosylphosphatidylinositol. Whereas GPC3 is abundant in fetal liver, its expression is hardly detectable in adult liver. Importantly, GPC3 is overexpressed in hepatocellular carcinoma (HCC), and several immunohistochemical studies reported that overexpression predicts a poorer prognosis for HCC patients. Therefore, GPC3 would serve as a useful molecular marker for HCC diagnosis and also as a target for therapeutic intervention in HCC. Indeed, some immunotherapy protocols targeting GPC3 are under investigations; those include humanized anti-GPC3 cytotoxic antibody, peptide vaccine and immunotoxin therapies. When considering the clinical requirements for GPC3-targeting therapy, companion diagnostics to select the appropriate HCC patients are critical, and both immunohistochemical analysis of tissue sections and measurement of serum GPC3 level have been suggested for this purpose. This review summarizes current knowledge regarding the clinical implication of GPC3 detection and targeting in the management of patients with HCC.
Core tip: Glypican-3 is frequently overexpressed in hepatocellular carcinoma (HCC). Accumulating evidence indicates that high glypican-3 expression is a significant prognostic factor that predicts poor outcome of patients with HCC. Thus, it serves as a promising molecular target for the development of novel therapies for HCC, and preclinical and clinical trials targeting glypican-3 are currently underway. Evaluation of the glypican-3 levels in HCC tissues or in sera of patients with HCC would be of value for predicting the patients’ prognosis and companion diagnostics for future glypican-3-targeting therapies.
- Citation: Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol 2016; 22(1): 275-283
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.275
Hepatocellular carcinoma (HCC) is the most common form of liver cancer and is the fifth most common malignant neoplasm worldwide[1]. Despite progress in surgical and non-surgical therapies, the prognosis of HCC remains poor. Although the multi-kinase inhibitor sorafenib prolonged median survival and the time to progression by nearly 3 mo[2], new biomarkers and molecular targets are urgently needed to develop novel treatment strategies.
Glypican-3 (GPC3) is a member of the heparan sulfate (HS) proteoglycan family. It attaches to cell membranes by a glycosylphosphatidylinositol (GPI) anchor[3,4]. GPC3 is widely expressed in human embryos, and it regulates morphogenesis or growth, possibly through insulin-like growth factor, bone morphogenic protein, fibroblast growth factor (FGF) or hedgehog signaling[5-7]. GPC3 gene mutation results in Simpson-Golabi-Behmel syndrome (SGBS), in which patients display fetal macrosomia and continue to grow and gain weight at an unusual rate with a varying range of dysmorphisms[8,9]. In fact, GPC3-deficient mice exhibit several of the clinical features observed in SGBS, including developmental overgrowth, perinatal death, cystic and dysplastic kidney and abnormal lung development[10]. In the liver, normal expression of GPC3 was identified from gestational weeks 18 to 30, and no GPC3 expression was observed in any normal adult liver tissue[5,11,12]. On the other hand, significantly high levels of GPC3 are expressed in HCC cells compared to normal liver and non-neoplastic liver lesions[11,12]. Therefore, GPC3 is a promising tumor marker and may be a potential molecular target for the development of innovative therapies for HCC[3]. This review focuses on the expression of GPC3 and discusses the possible usefulness of GPC3 as a prognostic marker and an immune-therapeutic target for patients with HCC.
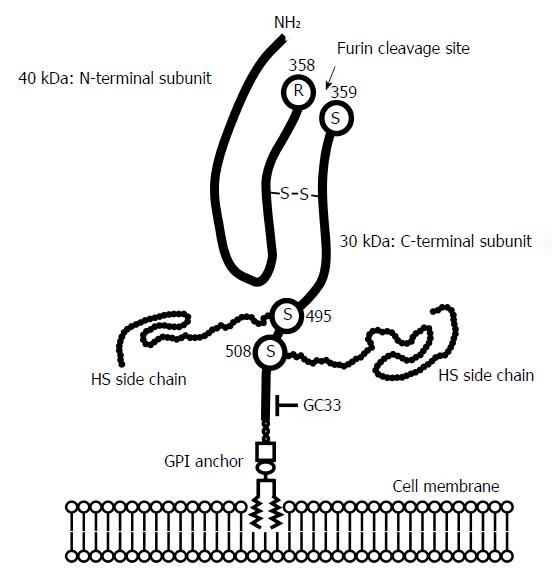
In 1988, Filmus et al[13] isolated a developmentally regulated cDNA clone, called OCI-5, from a rat small intestine cell line. As the OCI-5 gene encoded a protein highly homologous to the glypican family, the human gene was renamed GPC3[14], and it was found to be located on the human X chromosome (Xp26)[15]. The glypican family consists of six members (GPC1 - GPC6), all of which have a cysteine-rich repeat domain at similar positions[4]. GPC3 is abundantly expressed in the placenta and fetal tissues such as liver, lung and kidney; however, its expression is significantly reduced in adult organs[4]. The GPC3 gene encodes 580 amino acids that produce a core protein with a mass of 70 kDa. After cleavage between Arg358 and Cys359 by furin, two subunits linked by disulfide bonds (a 40-kDa N-terminal subunit and a 30-kDa C-terminal subunit) are generated[16]. The mature GPC3 heterodimer is expressed on the cellular surface as a GPI-anchored protein with two HS chains attached to the C-terminal region close to the cell membrane (Figure 1)[3,4]. GPC3 can be released from the cell surface into the extracellular environment. Several forms of secreted GPC3, such as glycated forms with a molecular weight larger than 100 kDa or a 50 kDa fragment lacking HS side chain have been reported[11,17,18]. Therefore, several mechanisms may be involved in the shedding of GPC3. One such mechanism is mediated by notum, a kind of lipase that cleaves GPI-anchored proteins, and it results in the release of the full-length glycated form of GPC3[19]. As shorter forms of soluble GPC3 can be detected in culture supernatant of human HCC cells, an alternative shedding or cleaving enzyme may also be present, area requiring further analysis.
In 1997, Hsu et al[20] identified an mRNA (MXR7) that was highly expressed in HCC tissue, and it was identical to GPC3 mRNA. The mRNA was hardly detectable in adult non-neoplastic liver (3.2%) but was overexpressed in most HCC tissues (74.8%). They also showed a close correlation between the GPC3 mRNA level and elevated serum alpha-fetoprotein (AFP) level[20]. Since then, a number of studies have analyzed GPC3 immunohistochemistry (IHC), and the results indicated specific and enhanced expression of GPC3 in HCC tissues[3,11,12,21]. Many IHC studies used the anti-GPC3 mouse monoclonal antibody 1G12 that recognizes a C-terminal portion of GPC3 near its membrane-bound site[11]. One study used two monoclonal antibodies (A1836A and GPC3-C02) that recognized N-terminal and C-terminal portions of GPC-3, respectively, and showed similar immunostaining patterns[4]. Evidence obtained by those IHC studies revealed acceptable specificity and sensitivity of GPC3 IHC for diagnostic purposes in HCC management. While GPC3 was undetectable in normal adult liver, 70%-100% of HCC cases were positive with enhanced immunoreactivity in less-differentiated tumors[3,11,12,21]. Dysplastic regenerative nodules in cirrhotic liver also showed weak and focal immunoreactivity; however, GPC3 was hardly detectable in hepatocellular adenoma and intrahepatic cholangiocarcinoma[11,12,21]. It should be noted that some extrahepatic cancers with known AFP expression, such as yolk-sac tumor, hepatoid adenocarcinoma and other AFP-producing digestive tract cancer variants, also showed high GPC3 expression[22-29], indicating that GPC3 may be an oncofetal protein like AFP. Indeed, evidence suggested that both the GPC3 and the AFP genes may be regulated by a similar transcription factor[30]. In addition, recent IHC studies have suggested that GPC3 can be expressed in other tumors, including thyroid cancers and ovarian clear cell carcinoma[29,31,32].
Recently, another mouse monoclonal antibody (GC33 and its humanized version) was developed, and it recognized an epitope similar to that of 1G12[33]. Notably, GC33 showed a significant cytotoxic activity mediated by antigen-dependent cell cytotoxicity (ADCC) and complement-dependent cell cytotoxicity (CDC) on GPC3-expressing cells[33,34]. Therefore, the humanized GC33 would have significant implications in the development of GPC3-targeting immunotherapy. To develop GPC3-targeting therapies for HCC, it would be necessary to evaluate the GPC3 expression level in individual patients (i.e., companion diagnostics). In formalin-fixed paraffin-embedded tissue sections, anti-GC33 antibody detected HCC cells with a sensitivity and specificity similar to 1G12[35,36]. Four distinct patterns of GPC3 immuno-localization were observed in HCC cells: peri-canalicular membranous, luminal membranous, circumferential membranous and intracytoplasmic[21,36]. Considering the targeting of GPC3 by humanized GC33, it is reasonable to postulate that the circumferential membranous expression of GPC3 in HCC cells is particularly important. Therefore, an IHC scoring system that placed particular emphasis on circumferential membranous immunoreactivity was proposed in the GPC3 IHC study using anti-GC33 antibody[36].
After the diagnostic utility of GPC3 IHC was established in HCC histopathology, extensive studies searching for the prognostic significance of GPC3 expression were conducted in patients with HCC. The clinicopathological studies with GPC3 IHC revealed that higher expression of GPC3 in HCC cells was corelated to a poorer prognosis for patients after curative partial hepatectomy[36,37], and subsequent studies supported this trend[38-41]. The circumferential membranous immunoreactivity scoring system may be superior in predicting the patients’ prognosis than a scoring system simply reflecting the positive area ratio[36]. Subsequently, meta-analytic studies of the prognostic significance of GPC3 expression were published. They confirmed that a strong GPC3 IHC score was of prognostic value as it was correlated with shorter overall survival (OS) and disease-free survival (DFS) of HCC patients[42,43]. Therefore, these patients may potentially benefit from adjuvant therapy, particularly that targeting GPC3.
In addition, these IHC studies revealed significant intra-tumoral heterogeneity of GPC3 expression levels in HCC tissue, casting doubt on the usefulness of needle biopsy specimens for the evaluation of GPC3 expression in HCC[21,36,44]. The immunoreactivity observed in a small needle biopsy specimen may not represent the overall level of GPC3 expression of the tumor. This may be a critical issue for needle biopsy specimens if one attempts to use GPC3 IHC as a biomarker of HCC.
As mentioned earlier, GPC3 can be released from the cell surface, and soluble GPC3 is detectable as serum GPC3 (sGPC3). Therefore, measuring sGPC3 levels may be a promising alternative for the estimation of GPC3 expression level in HCC tissue. Indeed, there have been several reports that attempted to measure sGPC3 by enzyme-linked immunosorbent assay (ELISA) in patients with HCC or other chronic liver diseases[11,17,45-54]. The details of each study are displayed in Table 1. However, the reported values of sGPC3 differed considerably between the studies even in healthy controls. This is presumably because of the different antibody epitopes used in each ELISA setting and/or possible heterogeneity in the molecular forms of sGPC3. Meta-analysis of the literatures has suggested that sGPC3 is indeed higher in HCC patients than normal subjects. However, its diagnostic utility is questionable, and further studies are required[55]. Moreover, prognostic analyses were not performed in those studies.
| Ref. | Epitope(AA)1 | sGPC3 (ng/mL), median (range) | ||
| n | ||||
| (mean ± SD) | ||||
| Healthy | CH/LC | HCC | ||
| Capurro et al[11] 2003 | C-terminal subunit | ND (53) | ND/0 (0-117) (18/20) | 167.5 (0-2924) (34) |
| (511-580) | (ND/5.85 ± 26.16) | (441 ± 669.8) | ||
| Hippo et al[17] 2004 | N-terminal subunit | - (96) | - (38) | - (69) |
| (25-358) | (0.65 ± 0.32) | (1.09 ± 0.74) | (4.84 ± 8.91) | |
| Beale et al[46] 2008 | ELISA kit (BioMosaics, Burlington, VT) | - | - (41) | - (50) |
| (125.41 ± 281.05) | (161.41 ± 422.33) | |||
| Tangkijvanich et al[47] 2009 | C-terminal subunit | ND (40) | 0 (0-43.6) (100) | 46.3 (0-7826.6) (100) |
| (511-580) | (-) | (-) | ||
| Liu et al[48] 2010 | ELISA kit (BioMosaics) | - | - (32) | - (37) |
| (3 cases are > 300 ng/mL) | (16 cases are > 300 ng/mL) | |||
| Yasuda et al[49] 2010 | ELISA kit (BioMosaics) | - | 1.16 (200) | 0.92 (200) |
| (-) | (-) | |||
| Ozkan et al[50] 2011 | ELISA kit (Wuhan EIAab Science, Wuhan, China) | 0.004 (0.004 - 0.008) (28) | 0.006 (0.004 - 0.24) (55) | 0.005 (0.004-0.09) (75) |
| (-) | (-) | (-) | ||
| Qiao et al[51] 2003 | ELISA kit (USCN Life Science, Wuhan, China) | - (30) | - (18/40) | - (101) |
| (5.93 ± 5.46) | (9.98 ± 9.60/12.09 ± 9.69) | (29.29 ± 17.34) | ||
| Chen et al[52] 2013 | N-terminal subunit | 0 (0 - 563.2) (136) | 0 (0-563.2)/6 (0-365.7) (180/124) | 15.11 (0-2400) (155) |
| (350-364) | (4.14 ± 31.65) | (10.45 ± 46.02/19.44 ± 50.88) | (99.94 ± 267.2) | |
| Lee et al[53] 2014 | ELISA kit (Cusabio Biotech, Wuhan, China) | - | 66.4 (40) | 75.8 (21.7-482.5) (120) |
| (-) | (-) | |||
| Abd El Gawad et al[54] 2014 | ELISA kit (USCN Life Science) | 0.99 (0.86-1.67) (10) | 2.74 (1.99 - 5.93) (10) | 7.7 (4.9-11) (40) |
| (-) | (-) | (-) | ||
| Haruyama et al[56] 2015 | N-terminal subunit | 0.12 (0.04 - 0.17) (25) | 0.11 (9) | 0.24 (0.05-2.96) (115) |
| (321-350) | (0.11 ± 0.04) | (0.12 ± 0.60) | (0.41 ± 0.51) | |
Very recently, we reported a novel sandwich ELISA system that recognized the N-terminal subunit of sGPC3 (sGPC3N)[56]. This ELISA system was highly sensitive compared to others that have been reported (Table 1). Using this ELISA system, sGPC3N antigen levels of 25 healthy volunteers and 115 HCC patients who had undergone curative partial hepatectomy were measured, and the relationship between sGPC3N and clinicopathological parameters was analyzed[56]. The mean ± standard deviation (SD) of sGPC3 levels in healthy controls was 110.12 ± 37.70 pg/mL, with a median value of 115.95 pg/mL. In HCC patients, sGPC3N levels were significantly increased compared to healthy controls and showed mean and median values of 405.16 pg/mL and 236.19 pg/mL, respectively. About 60% of HCC cases showed abnormally high sGPC3N levels (i.e., > mean GPC3 + 2 SD of healthy controls) in preoperative sera and the levels declined significantly after curative partial hepatectomy[56]. Notably, we observed that high preoperative sGPC3N levels were significantly associated with shorter OS and DFS after hepatectomy and also with larger GPC3 IHC-positive areas in the resected HCC tissues. More importantly, multivariate analysis revealed that elevated sGPC3N was an independent prognostic marker for poor OS or DFS[56].
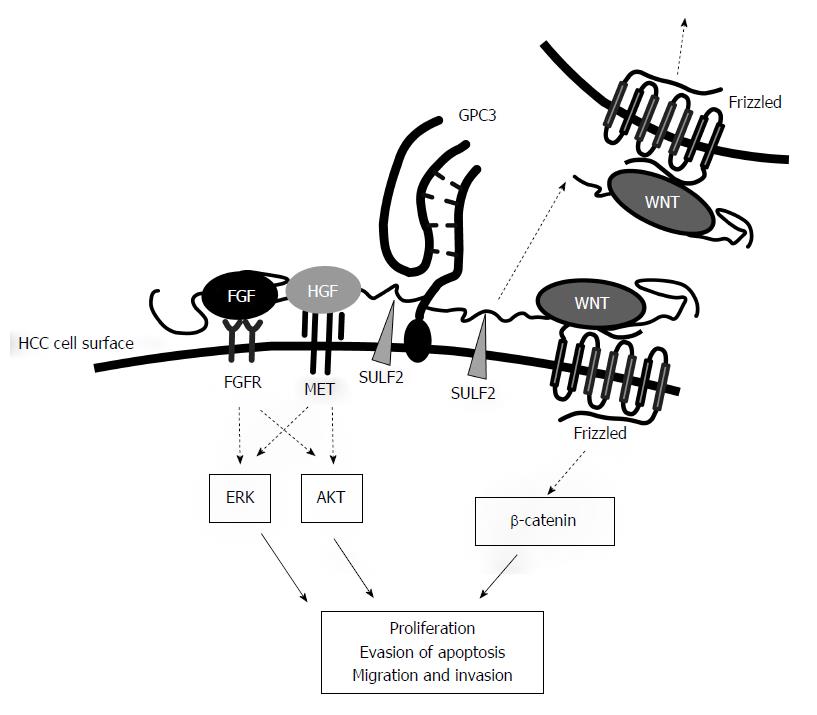
An important remaining question is whether GPC3 has a direct role in the aggressive behavior of HCC cells, or whether the expression is simply an epiphenomenon of malignant progression. Some experimental evidence has suggested that it is a direct relationship, but interpretation is complicated by the roles of cellular GPC3 in HCC cell biology. Cell surface GPC3 forms a complex with Wnt via its HS side chains and stimulates Wnt/β-catenin signaling in HCC cells (Figure 2)[57]. Sulfatase 2 (SULF2), an enzyme that removes 6-O-sulfate groups from HS, is also overexpressed in HCC cells and releases Wnt from the GPC3/Wnt complex, which also upregulates Wnt signaling[58]. Cell surface GPC3 may also act as a storage site for heparin-binding growth factors, such as FGF, hepatocyte growth factor (HGF) and heparin-binding epidermal growth factor, all of which are likely involved in the invasive growth of HCC cells via ERK and/or AKT signaling (Figure 2)[59,60]. A recent study suggested that high expression levels of GPC3 may promote the epithelial-mesenchymal transition (EMT) of HCC cells through ERK activation[61], and EMT is known to be involved in the metastatic phenotype and drug resistance of cancer cells[62]. On the other hand, enhanced shedding of GPC3 with HS side chains may eliminate GPC3-attached Wnt and growth factors from HCC’s cell surface or may show dominant-negative effects by neutralizing these GPC3-binding factors in the pericellular microenvironment[59]. Therefore, GPC3 may have both positive and negative effects on Wnt signaling and pericellular growth factor activities.
The oncoprotein c-Myc may also contribute to the presumed GPC3-induced malignant phenotypes. In the GPC3 gene promoter region, Li et al[63] identified a binding site for c-Myc, and the binding of c-Myc directly activated the transcription of the GPC3 gene. Interestingly, GPC3 also upregulated c-Myc expression, which eventually forms a positive feedback signaling loop between GPC3 and c-Myc in HCC cells[63].
Studies with GPC3-deficient mice indicated that loss of GPC3 function impaired the differentiation of macrophage lineage cells, resulting in the reduction of monocyte/macrophage precursor-derived osteoclasts in the diaphysis of the bone[64]. This evidence may suggest a relationship between macrophage function and GPC3. In many solid tumors, intratumoral infiltration of macrophages and the shift of their polarity to M2 phenotype have important roles in malignant progression[65]. Thus, we used IHC to assess the association of GPC3 expression with the number of tumor-associated macrophages (TAM) and their polarity in HCC tissues. Our results indicated that enhanced circumferential membranous staining of GPC3 (i.e., a high A-Cm score) was associated with increased TAM with an M2-polarized phenotype[66,67]. We also observed a correlation between a high A-Cm score and the expression of monocarboxylate transporter 4 (MCT4) in HCC cells, and patients with MCT4-positive HCC also had poor prognoses[67]. MCT4 is an important proton symporter that regulates intracellular pH, and its expression is regulated by HIF1 signaling. Enhanced expression of MCT4 has been reported in many solid cancers[68]. It is currently unknown whether GPC3 expression and MCT4 expression are functionally related in HCC. Nonetheless, the pericellular microenvironment of HCC cells may have important roles in GPC3 expression.
HCC is an extremely malignant tumor. Many researchers and clinicians are searching for novel treatment strategies for this deadly disease, including molecular targeting therapy, immune therapy, oncolytic virotherapy and microRNA-based therapy[69-73]. The humanized anti-GPC3 monoclonal antibody GC33 shows significant cytotoxic activity against GPC3-expressing human HCC cell lines in vivo through ADCC and/or CDC[33,34]. The first clinical phase I study of humanized GC33 was performed in the United States, and the results were reported in 2013[74]. Twenty patients with advanced HCC were treated with humanized GC33 antibody (2.5-20 mg/kg, iv, weekly), and there were no dose-limiting toxicities[74]. Another phase I trial in Japanese HCC patients also showed that GC33 given up to 20 mg/kg weekly was well tolerated[75]. A randomized phase II trial of humanized GC33 was performed in 185 advanced HCC patients, the results of which were presented at the 2014 ASCO meeting by Yen et al[76] Prior to randomization of the patients, they were separated into 3 groups based on the GPC3 expression levels judged by IHC. The 121 randomized patients were then treated with humanized GC33 (1600 mg every two weeks, iv, after two weekly doses), and 64 patients were treated with a placebo. Median progression-free survival (PFS) in the humanized GC33 and placebo groups were 2.6 and 1.5 mo, respectively (HR: 0.97, P = 0.87)[76]. Therefore, treatment of humanized GC33 did not show a benefit in this trial. However, exposure-efficacy analysis suggested that higher exposure of GC33 with FcγR3A-158V polymorphism or CD16 expression intensity may correlate with prolonged PFS[76]. As GC33 induces cytotoxic effects through ADCC and/or CDC, antibody concentration and efficacy of immune responses in HCC tissue might be critical. Further studies analyzing the immuno-microenvironmental factors in GPC3-expressing HCC are warranted.
GPC3 is also considered an immunotherapeutic target for peptide vaccine therapies[77-79]. Komori et al[78] developed HLA-A2 and -A24-restricted GPC3-derived peptide vaccines (GPC3144-152: FVGEFFTDV and GPC3298-306: EYILSLEEL, respectively). The patients who were induced by the peptides showed increased GPC3-specific cytotoxic T cells (CTLs). Then, by using these two peptides, a phase I trial was performed, in which 39 Japanese patients with advanced HCC were enrolled[80]. No severe common adverse events were observed, and one patient showed partial response and 19 patients showed stable disease. Notably, GPC3-specific CTLs were increased in 30 patients, and the frequency of the GPC3-specific CTLs correlated with OS[80]. On the other hand, development of T cells expressing GPC3-targeting chimeric antigen receptor was reported, which potently eliminated GPC3-positive HCC cell xenografts[81].
The chimeric proteins composed of an antibody fragment fused to a toxin (i.e., immunotoxins) may also have a therapeutic potential in GPC3-targeting therapies. Recently, Gao et al[82] reported successful regression of tumor xenografts of two human liver cancer cell lines, Hep3B and HepG2, by treatment with anti-GPC3 immunotoxin. They used two anti-GPC3 monoclonal antibodies (HN3 and YP7) conjugated to Pseudomonas exotoxin A. HN3 inhibited Wnt signaling induced by GPC3[83] and YP7 recognized an epitope in the C-terminus portion of GPC3. HN3-immunotoxin treatment showed superior anti-tumor effects compared to YP7-immunotoxin[81].
GPC3 is frequently overexpressed in HCC, and its expression level serves as a promising prognostic biomarker. GPC3 may also be a promising molecular target for the development of innovative therapies to improve prognosis of HCC patients. Although a clinical benefit of GPC3-targeting therapy has not yet been confirmed in HCC patients, researchers are actively investigating novel strategies to develop GPC3-targeted therapies for the treatment of HCC.
P- Reviewer: Ding HG, El-Hawary AK, Tziomalos K S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 3. | Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280:2471-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 464] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Iglesias BV, Centeno G, Pascuccelli H, Ward F, Peters MG, Filmus J, Puricelli L, de Kier Joffé EB. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol Histopathol. 2008;23:1333-1340. [PubMed] |
| 6. | Paine-Saunders S, Viviano BL, Zupicich J, Skarnes WC, Saunders S. glypican-3 controls cellular responses to Bmp4 in limb patterning and skeletal development. Dev Biol. 2000;225:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell. 2008;14:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996;12:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 556] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 9. | Pellegrini M, Pilia G, Pantano S, Lucchini F, Uda M, Fumi M, Cao A, Schlessinger D, Forabosco A. Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome. Dev Dyn. 1998;213:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999;146:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 683] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 12. | Yamauchi N, Watanabe A, Hishinuma M, Ohashi K, Midorikawa Y, Morishita Y, Niki T, Shibahara J, Mori M, Makuuchi M. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Filmus J, Church JG, Buick RN. Isolation of a cDNA corresponding to a developmentally regulated transcript in rat intestine. Mol Cell Biol. 1988;8:4243-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Li M, Choo B, Wong ZM, Filmus J, Buick RN. Expression of OCI-5/glypican 3 during intestinal morphogenesis: regulation by cell shape in intestinal epithelial cells. Exp Cell Res. 1997;235:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Huber R, Mazzarella R, Chen CN, Chen E, Ireland M, Lindsay S, Pilia G, Crisponi L. Glypican 3 and glypican 4 are juxtaposed in Xq26.1. Gene. 1998;225:9-16. [PubMed] |
| 16. | De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B, David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Hippo Y, Watanabe K, Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y, Nakano K. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64:2418-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Capurro M, Filmus J. Glypican-3 as a serum marker for hepatocellular carcinoma. Cancer Res. 2005;65:372; author reply 372-373. [PubMed] |
| 19. | Traister A, Shi W, Filmus J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem J. 2008;410:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57:5179-5184. [PubMed] |
| 21. | Wang HL, Anatelli F, Zhai QJ, Adley B, Chuang ST, Yang XJ. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med. 2008;132:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol. 2006;30:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Saikali Z, Sinnett D. Expression of glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 2000;89:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Kai K, Nakamura J, Ide T, Masuda M, Kitahara K, Miyoshi A, Noshiro H, Tokunaga O. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol Int. 2012;62:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Hishinuma M, Ohashi KI, Yamauchi N, Kashima T, Uozaki H, Ota S, Kodama T, Aburatani H, Fukayama M. Hepatocellular oncofetal protein, glypican 3 is a sensitive marker for alpha-fetoprotein-producing gastric carcinoma. Histopathology. 2006;49:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, Gotoda T, Kinjo F, Fujita J, Shimoda T. Histologic and immunohistochemical analyses of α-fetoprotein--producing cancer of the stomach. Am J Surg Pathol. 2012;36:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Takahashi N, Aoyama F, Hiyoshi M, Kataoka H, Sawaguchi A. Establishment and biological characterization of a novel cell line derived from hepatoid adenocarcinoma originated at the ampulla of Vater. Int J Oncol. 2014;44:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Ushiku T, Uozaki H, Shinozaki A, Ota S, Matsuzaka K, Nomura S, Kaminishi M, Aburatani H, Kodama T, Fukayama M. Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomas. Cancer Sci. 2009;100:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Wang SK, Zynger DL, Hes O, Yang XJ. Discovery and diagnostic value of a novel oncofetal protein: glypican 3. Adv Anat Pathol. 2014;21:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Morford LA, Davis C, Jin L, Dobierzewska A, Peterson ML, Spear BT. The oncofetal gene glypican 3 is regulated in the postnatal liver by zinc fingers and homeoboxes 2 and in the regenerating liver by alpha-fetoprotein regulator 2. Hepatology. 2007;46:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Yamanaka K, Ito Y, Okuyama N, Noda K, Matsumoto H, Yoshida H, Miyauchi A, Capurro M, Filmus J, Miyoshi E. Immunohistochemical study of glypican 3 in thyroid cancer. Oncology. 2007;73:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Maeda D, Ota S, Takazawa Y, Aburatani H, Nakagawa S, Yano T, Taketani Y, Kodama T, Fukayama M. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009;22:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M, Furugaki K, Kinoshita Y, Ishiguro T, Hamakubo T. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832-9838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Takai H, Kato A, Ishiguro T, Kinoshita Y, Karasawa Y, Otani Y, Sugimoto M, Suzuki M, Kataoka H. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3. Acta Histochem. 2010;112:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Yorita K, Takahashi N, Takai H, Kato A, Suzuki M, Ishiguro T, Ohtomo T, Nagaike K, Kondo K, Chijiiwa K. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int. 2011;31:120-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 38. | Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, Fang-yin Z, Da-yong Z, Rong-cheng L. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee WC, Chen MF, Tsai CN. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann Surg Oncol. 2012;19 Suppl 3:S455-S463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Wang YL, Zhu ZJ, Teng DH, Yao Z, Gao W, Shen ZY. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol. 2012;18:2408-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Fu SJ, Qi CY, Xiao WK, Li SQ, Peng BG, Liang LJ. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery. 2013;154:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Li J, Gao JZ, Du JL, Wei LX. Prognostic and clinicopathological significance of glypican-3 overexpression in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20:6336-6344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Xiao WK, Qi CY, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of glypican-3 in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Anatelli F, Chuang ST, Yang XJ, Wang HL. Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol. 2008;130:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 46. | Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, Trerotoli P, Giannelli G, Manas D, Reeves H. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, Kongtawelert P. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 2010;25:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan YF, Li N, Ding HG. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410-4415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 49. | Yasuda E, Kumada T, Toyoda H, Kaneoka Y, Maeda A, Okuda S, Yoshimi N, Kozawa O. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma. Hepatol Res. 2010;40:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Ozkan H, Erdal H, Koçak E, Tutkak H, Karaeren Z, Yakut M, Köklü S. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. J Clin Lab Anal. 2011;25:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Qiao SS, Cui ZQ, Gong L, Han H, Chen PC, Guo LM, Yu X, Wei YH, Ha SA, Kim JW. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2003;58:1718-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Chen M, Li G, Yan J, Lu X, Cui J, Ni Z, Cheng W, Qian G, Zhang J, Tu H. Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma. Clin Chim Acta. 2013;423:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Lee HJ, Yeon JE, Suh SJ, Lee SJ, Yoon EL, Kang K, Yoo YJ, Kim JH, Seo YS, Yim HJ. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver. 2014;8:177-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Abd El Gawad IA, Mossallam GI, Radwan NH, Elzawahry HM, Elhifnawy NM. Comparing prothrombin induced by vitamin K absence-II (PIVKA-II) with the oncofetal proteins glypican-3, Alpha feto protein and carcinoembryonic antigen in diagnosing hepatocellular carcinoma among Egyptian patients. J Egypt Natl Canc Inst. 2014;26:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Yang SL, Fang X, Huang ZZ, Liu XJ, Xiong ZF, Liu P, Yao HY, Li CH. Can serum glypican-3 be a biomarker for effective diagnosis of hepatocellular carcinoma? A meta-analysis of the literature. Dis Markers. 2014;2014:127831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Haruyama Y, Yorita K, Yamaguchi T, Kitajima S, Amano J, Ohtomo T, Ohno A, Kondo K, Kataoka H. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int J Cancer. 2015;137:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245-6254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 58. | Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Nakamura I, Han T, Aderca I, Isomoto H. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology. 2010;52:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 59. | Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126:1291-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Gao W, Kim H, Ho M. Human Monoclonal Antibody Targeting the Heparan Sulfate Chains of Glypican-3 Inhibits HGF-Mediated Migration and Motility of Hepatocellular Carcinoma Cells. PLoS One. 2015;10:e0137664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Wu Y, Liu H, Weng H, Zhang X, Li P, Fan CL, Li B, Dong PL, Li L, Dooley S. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int J Oncol. 2015;46:1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 63. | Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, Liu K, Li N, Chen D. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology. 2012;56:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Viviano BL, Silverstein L, Pflederer C, Paine-Saunders S, Mills K, Saunders S. Altered hematopoiesis in glypican-3-deficient mice results in decreased osteoclast differentiation and a delay in endochondral ossification. Dev Biol. 2005;282:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 505] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 66. | Takai H, Kato A, Kato C, Watanabe T, Matsubara K, Suzuki M, Kataoka H. The expression profile of glypican-3 and its relation to macrophage population in human hepatocellular carcinoma. Liver Int. 2009;29:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Ohno A, Yorita K, Haruyama Y, Kondo K, Kato A, Ohtomo T, Kawaguchi M, Marutuska K, Chijiiwa K, Kataoka H. Aberrant expression of monocarboxylate transporter 4 in tumour cells predicts an unfavourable outcome in patients with hepatocellular carcinoma. Liver Int. 2014;34:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 69. | Giannelli G, Rani B, Dituri F, Cao Y, Palasciano G. Moving towards personalised therapy in patients with hepatocellular carcinoma: the role of the microenvironment. Gut. 2014;63:1668-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 70. | Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut. 2015;64:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 71. | Pan Q, Huang Y, Chen L, Gu J, Zhou X. SMAC-armed vaccinia virus induces both apoptosis and necroptosis and synergizes the efficiency of vinblastine in HCC. Hum Cell. 2014;27:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Sidhu K, Kapoor NR, Pandey V, Kumar V. The “Macro” World of microRNAs in Hepatocellular Carcinoma. Front Oncol. 2015;5:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Zhao C, Li Y, Zhang M, Yang Y, Chang L. miR-126 inhibits cell proliferation and induces cell apoptosis of hepatocellular carcinoma cells partially by targeting Sox2. Hum Cell. 2015;28:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Ikeda M, Ohkawa S, Okusaka T, Mitsunaga S, Kobayashi S, Morizane C, Suzuki I, Yamamoto S, Furuse J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci. 2014;105:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Yen CJ, Daniele B, Kudo M, Merle P, Park JW, Ross P. Randomized phase II trial of intravenous RO5137382/GC33 at 1600 mg every other week and placebo in previously treated patients with unresectable advanced hepatocellular carcinoma. J Clin Oncol. 2014;32 Suppl 5:4102a. |
| 77. | Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, Furukawa Y, Ogawa M, Nakamura Y, Nishimura Y. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin Cancer Res. 2004;10:8630-8640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2689-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 79. | Motomura Y, Ikuta Y, Kuronuma T, Komori H, Ito M, Tsuchihara M, Tsunoda Y, Shirakawa H, Baba H, Nishimura Y. HLA-A2 and -A24-restricted glypican-3-derived peptide vaccine induces specific CTLs: preclinical study using mice. Int J Oncol. 2008;32:985-990. [PubMed] |
| 80. | Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-3696. [PubMed] |
| 81. | Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418-6428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 82. | Gao W, Tang Z, Zhang YF, Feng M, Qian M, Dimitrov DS, Ho M. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun. 2015;6:6536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Ho M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci USA. 2013;110:E1083-E1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |