Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.24
Peer-review started: May 20, 2015
First decision: July 14, 2015
Revised: August 6, 2015
Accepted: October 13, 2015
Article in press: October 13, 2015
Published online: January 7, 2016
Processing time: 229 Days and 15.4 Hours
Chronic alcohol consumption is a major cause of liver disease. The term alcoholic liver disease (ALD) refers to a spectrum of mild to severe disorders including steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma. With limited therapeutic options, stem cell therapy offers significant potential for these patients. In this article, we review the pathophysiologic features of ALD and the therapeutic mechanisms of multipotent mesenchymal stromal cells, also referred to as mesenchymal stem cells (MSCs), based on their potential to differentiate into hepatocytes, their immunomodulatory properties, their potential to promote residual hepatocyte regeneration, and their capacity to inhibit hepatic stellate cells. The perfect match between ALD pathogenesis and MSC therapeutic mechanisms, together with encouraging, available preclinical data, allow us to support the notion that MSC transplantation is a promising therapeutic strategy to manage ALD onset and progression.
Core tip: Chronic alcohol consumption is a major cause of liver disease. Stem cells, in particular multipotent mesenchymal stromal cells (MSCs), have been envisioned as a promising tool for the development of therapeutic strategies to treat alcoholic liver diseases (ALD). The advantages of MSC include the regulation of exacerbated inflammatory process, their differentiation into hepatocytes, the production of trophic factors that prevent the apoptosis of parenchymal cells, and the induction of the proliferation of endogenous progenitors. Here, we revise the pathophysiology of ALD to identify therapeutic targets for MSCs. Also, we discuss the rationale to propose an MSC-based therapy to treat ALD.
- Citation: Ezquer F, Bruna F, Calligaris S, Conget P, Ezquer M. Multipotent mesenchymal stromal cells: A promising strategy to manage alcoholic liver disease. World J Gastroenterol 2016; 22(1): 24-36
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/24.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.24
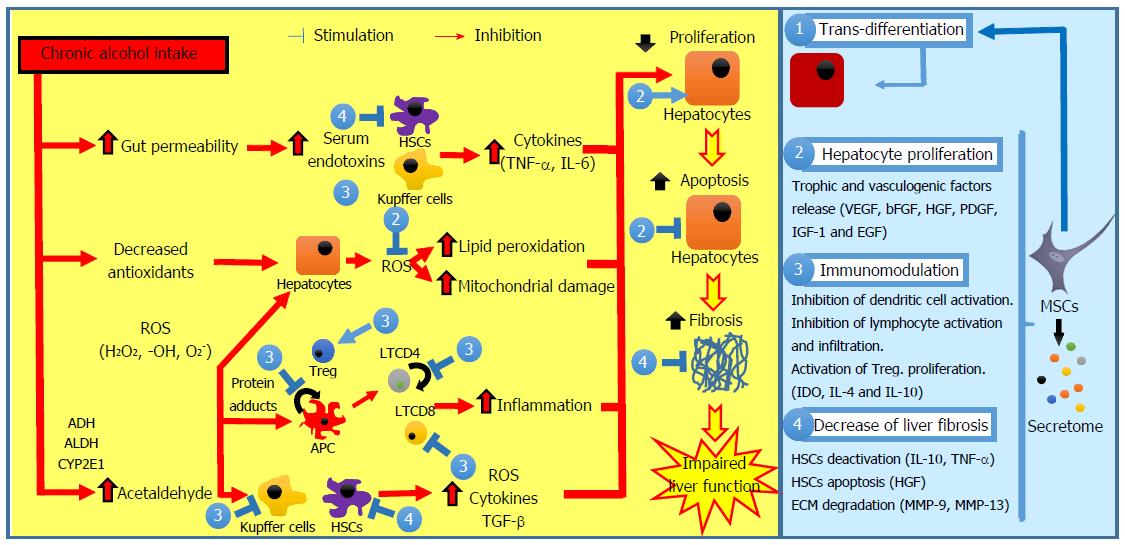
Chronic alcohol consumption is a major cause of liver disease[1-3]. Moreover, alcohol consumption negatively impacts the natural history of other types of chronic liver diseases such as nonalcoholic steatohepatitis, and hepatitis B and C, favoring fibrosis progression[3-5]. Alcoholic liver disease (ALD) comprises a broad spectrum of disorders, encompassing simple steatosis, steatohepatitis, and cirrhosis. The pathomechanism associated with ALD involves complex interactions between the deleterious effects of alcohol and its toxic metabolites on various cell types in the liver, the induction of reactive oxygen species (ROS), and the up-regulation of the proinflammatory cascade[1,3].
Alcoholic steatosis, the earliest manifestation of ALD, is present in more than 90% of heavy drinkers, and is pathologically characterized by microvesicular and macrovesicular fat accumulation within hepatocytes, minimal inflammatory reaction, and no hepatic fibrosis[1]. This stage is asymptomatic and reversible with alcohol abstinence[6]. Alcohol consumption increases the ratio of NADH/NAD+ in hepatocytes, which disrupts mitochondrial β-oxidation of fatty acids, leading to steatosis development[7]. Alcohol consumption also increases fatty acid triglycerides synthesis through the upregulation of the sterol regulatory element binding protein 1c[8] and the downregulation of the peroxisome proliferator-activated receptor-α[9]. ALD progression is characterized by steatosis, a superimposed inflammatory infiltrate of predominantly polymorphonuclear leukocytes and hepatocellular damage. When the inflammation and hepatocellular injury are severe, the condition is termed steatohepatitis and is associated with a high mortality rate[10,11].
The pathogenesis of alcoholic steatohepatitis is complex and multifactorial. In the liver, alcohol is metabolized primarily into acetaldehyde by the enzymes alcohol dehydrogenase in the cytosol, cytochrome P450 in microsomes, and catalase in peroxisomes[12]. Acetaldehyde is highly toxic to hepatocytes because it binds to proteins and DNA, forming adducts that promote glutathione depletion, lipid peroxidation, and mitochondrial damage[13,14]. Additionally, these adducts act as antigens that activate the adaptive immune response, leading to lymphocyte recruitment to the liver[15]. Acetate resulting from acetaldehyde breakdown is rapidly released from the liver into circulation and then metabolized into CO2via the tricarboxylic acid cycle in skeletal muscle, brain, and heart. Although acetate has no direct hepatotoxicity, it is believed that it can regulate the inflammatory response in patients with alcoholic steatohepatitis through the upregulation of proinflammatory cytokines released by macrophages[16].
Alcohol abuse also results in changes in colonic microbiota and increased gut permeability, leading to translocation of bacterial products, such as lipopolysaccharide, into the portal circulation[17]. In Kupffer cells, lipopolysaccharide activates the MyD88-independent signaling pathway through toll-like receptor 4, resulting in the production of oxidative stress and proinflammatory cytokines such as tumor necrosis factor (TNF)-α, contributing to hepatocellular damage[18,19].
Histologic features of alcoholic steatohepatitis include inflammation and necrosis, which are more prominent in the centrilobular region of the hepatic acinus, while hepatocytes are classically ballooned, leading to compression of the sinusoid and portal hypertension[20,21]. Alcoholic cirrhosis is the end stage of ALD and is characterized by distortion of the hepatic architecture, septum formations, rings of scars that surround hepatocyte nodules, the formation of regenerative nodule, and the loss of liver function[22].
Extracellular matrix (ECM), particularly collagen type I, is mainly produced by activated hepatic stellate cells (HSCs), located in the space of Disse between the hepatocytes and sinusoids. HSCs can be activated by neutrophils, damaged hepatocytes, and activated Kupffer cells through various profibrogenic mediators, including transforming growth factor (TGF)-β, TNF-α, and ROS[3,23]. Additionally, ROS downregulate the action of metalloproteinases and upregulate tissue inhibitor of metalloproteinase-1, resulting in greater collagen accumulation[24].
Along with other liver diseases, patients with cirrhosis are at risk for hepatic decompensation (ascites, variceal bleeding, and encephalopathy) and the development of hepatocellular carcinoma[25,26]. Although the most important risk factor for ALD is the absolute amount of alcohol intake, only approximately 35% of heavy drinkers develop advanced ALD, indicating that other factors are involved in host susceptibility to the disease. These factors include sex, obesity, drinking pattern, dietary factors, non-sex-linked genetic factors, and cigarette smoking[27-30].
Despite the profound economic and health impacts of ALD, little progress has been made in the management of patients with this condition, and medical treatment has not changed significantly in the last 45 years[10,31,32]. Although nutritional and supportive management are important, alcohol abstinence is the mainstay therapy for patients with all stages of ALD[33,34]. However, the benefits of alcohol abstinence may not be sufficient for patients with decompensated ALD, such as cirrhosis or severe alcoholic hepatitis[35,36].
Corticosteroids were one of the first pharmacologic therapies investigated for the treatment of alcoholic hepatitis. Despite the widespread awareness and use of this therapy, controversy still exists regarding its true efficacy[37]. Taking into account the participation of TNF-α in ALD pathogenesis, TNF-α antagonists have also been studied for this condition. Although the initial studies were promising, larger clinical trials demonstrated an increased risk of infection and mortality with these agents[38]. In addition, pharmacologic therapy with medications such as disulfiram, baclofen, colchicine, vitamin E, and naltrexone have been considered, although their efficacies are limited[3,39,40].
The most effective therapy for advanced cirrhosis is liver transplant, however, the scarcity of donors, surgical complications, immunologic suppression and rejection, and high medical cost limit its availability and clinical utility[41]. Moreover, liver transplantation is not an option for most patients, and, until now, no other treatment has demonstrated superiority over steroids. Therefore, alternative therapies are needed. To this end, alternative approaches that circumvent the use of the whole organ, such as transplantation of cells of diverse origins, have been proposed in recent years[42].
It is well known that the liver has a high regenerative capacity. Under normal conditions, recovery of liver mass occurs mainly via proliferation of remaining adult hepatocytes. On the other hand, under pathologic conditions in which the proliferation of hepatocytes is inhibited, liver progenitor cells (oval cells) proliferate and differentiate into hepatocytes or biliary epithelial cells[43]. Chronic ethanol exposure and sustained inflammation have been shown to inhibit DNA synthesis in the damaged liver[44,45]. This impaired hepatocyte proliferation is the consequence of oxidative damage by the ROS produced from alcohol metabolism[46]. Moreover, ethanol could inhibit early differentiation of hepatic progenitor cells into functional mature hepatocytes[47].
Cell therapy for the treatment of hepatic fibrosis has been evaluated in different animal models, and some findings have been very encouraging. The transplantation of mature hepatocytes into human patients has provided insights into the way in which human liver disease could be treated by cellular therapies[48]. However, the high number of cells needed for the transplantation, the availability of fresh cells or the quality of cryopreserved ones, and the necessity of immunosuppression to avoid the rejection of transplanted cells are the main limitations of adult hepatocyte transplantation[49,50]. Immunosuppression is a particularly important point, as the hepatic failure itself increases the risk of developing septic complications, which are worsened by the use of immunosuppressive drugs.
Numerous studies have focused on investigating the ability of a variety of stem cells that can be readily isolated using noninvasive procedures to give rise to hepatocytes both in vitro and in vivo[51]. Considering that some of these stem cell populations are present in adults, it would be possible to produce personalized immunologically matched hepatocytes[52]. Moreover, adult stem cells have the ability to reduce the hepatic proinflammatory microenvironment, inhibit the activation or induce apoptosis of HSCs, and promote the regeneration of residual hepatocytes[53,54].
The aim of regenerative medicine is to develop therapeutic strategies for the management of severe injuries or chronic diseases in patients whose endogenous regenerative mechanisms have failed to restore the impaired functions. Over the past years, stem cells have been envisioned as the best tool for this purpose. Stem cell-based intervention is known to act through multiple mechanisms, which is a clear advantage when facing diseases with a complex pathophysiology, such as ALD.
In general terms, adult stem cells are found in all nonembryonic tissues, where they contribute to both maintenance of cellular homeostasis and regeneration of damaged organs. These cells are multipotent and can be isolated from a fetus, newborn, child, or adult, and due to their limited self-renewal potential, they are not teratogenic. Some of them also have plasticity, i.e., they can differentiate into cells from lineages different from their origin[55].
As adult stem cells pose fewer bioethical and technical concerns than embryonic stem cells, the first candidate for a stem cell-based strategy to treat liver regeneration was bone marrow-derived stem cells[53,56-58]. Bone marrow harbors at least two distinct adult stem cell populations: the hematopoietic stem cells that give rise to blood and endothelial cells[59] and the multipotent mesenchymal stromal cells, also referred to as mesenchymal stem cells (MSCs), that provide support to hematopoietic stem cells and drive the process of hematopoiesis[60]. In addition to bone marrow, MSCs have now been isolated from numerous tissues, including liver, lung, umbilical cord, skeletal muscle, dental pulp, spleen, and adipose tissue[61-63]. Thus, it has been postulated that MSCs play a critical role in organ homeostasis by providing supportive factors to the surrounding tissue.
One of the main technical difficulties associated with the therapeutic use of MSCs is the lack of a specific antigen for their identification. Therefore, in 2006, the International Society for Cellular Therapy proposed minimal criteria to define human MSCs (hMSCs): (1) must be plastic-adherent when maintained under standard culture conditions; (2) must express CD105, CD73, and CD90, and lack the expression of CD45, CD34, CD14, CD11b, CD19, and class II human leukocyte antigen surface molecules; and (3) must differentiate into osteoblast, adipocytes, and chondroblasts under in vitro differentiation conditions[64,65].
Despite the scarcity of MSCs (< 0.01% of the mononuclear cells present in the bone marrow), they can be considered as ideal candidates for cell therapy because: (1) they can be obtained from donors without major complications; (2) they can be easily expanded ex vivo; (3) when MSCs are systemically administered, they can selectively migrate to and engraft damaged tissue. The migration of MSCs is facilitated by the release of several molecules from the damaged tissues that interact with different receptors expressed by the MSCs[66,67]; (4) it has been suggested that MSCs cross the germ line barrier and generate cells from the endodermal and ectodermal lineages[55]; (5) MSCs secrete a broad range of bioactive growth factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor, insulin-like growth factor (IGF), hepatocyte growth factor (HGF), and epidermal growth factor (EGF)[68]. Therefore, MSCs could provide trophic support to injured tissue by modifying the microenvironment to induce local precursor proliferation and differentiation, improving damaged tissue irrigation, and preventing parenchymal cell apoptosis[55,68]; and (6) MSCs are hypo-immunogenic[69], which represents the main advantage of MSCs over hematopoietic stem cells for clinical use, as histocompatibility between donor and receptor is not required and the recipients do not need to be conditioned before MSC transplantation[70]. Furthermore, MSCs have been administered to more than 1000 human patients with no evidence of adverse effects or tumor formation[70] (Table 1).
| MSCs in liver inflammation |
| Inhibit the proliferation of CD8 cytotoxic T lymphocytes and increase the relative rate of CD4 Th2 lymphocytes[97,100] |
| Inhibit the maturation of monocytes into dendritic cells[94] |
| Inhibit the secretion of TNF-α, INF-γ, and IL-12 by dendritic cells and increase their secretion of IL-10, reducing the proinflammatory potential[95] |
| Suppress the proliferation, cytolytic activity, and cytokine secretions of the NK cells[96] |
| Express indoleamine 2,3-dioxygenase upon INF-γ stimulation, leading to tryptophan depletion and the inhibition of T-cell proliferation[98] |
| MSCs in liver fibrosis |
| Reduce the proliferation of HSCs and the synthesis of collagen type I through the secretion of TNF-α[125] |
| Induce HSCs apoptosis[124] |
| Express matrix metalloproteinase-9, which degrades the extracellular matrix[128,129] |
| MSCs in liver regeneration |
| Secrete trophic factors (HGF, EGF, and IGF-1) that promote hepatocyte proliferation and function during liver regeneration[68,128,130] |
Multiple mechanisms have been suggested to play a role in amelioration of liver diseases after MSC administration, such as: trans-differentiation of MSCs into hepatocytes, immunomodulation, inhibition of fibrosis development, protective effects on hepatic cells, and restoration of hepatic cell proliferation capacity (Figure 1).
The high degree of plasticity of MSCs has been widely described over the last decade or so[55,71]. Thus, MSCs have the potential cross the germ line barrier and differentiate into non-mesodermal cells (such as hepatocytes and neurons)[72]. It is important to note that MSC-derived hepatocytes need to not only express the genes found in mature liver cells, but express them at a level that is close to what is observed in the normal liver. Therefore, it is crucial to define which characteristics are needed for a differentiated cell to be comparable to a primary hepatocyte. The minimal set of functions of a true hepatocyte includes: (1) metabolic function (detoxification of xenobiotics and endogenous substances); (2) synthetic function (production of albumin, clotting factors, and complement); and (3) storage function (storage of glycogen and fat-soluble vitamins)[73].
Although the protocols for hepatocyte induction have been standardized for cultured MSCs[74,75], an organ-specific microenvironment is the most suitable place for them to differentiate into the required cell types. In this sense, Sato et al[76] were the first to demonstrate the in vivo hepatic differentiation potential of hMSCs. In this study, hMSCs were directly xenografted to the liver of allyl alcohol-treated rats, and they observed that some of the administered hMSCs differentiated into hepatocyte-like cells one month later. Additionally, the in vivo hepatic differentiation potential of MSCs has been demonstrated in rats[77], mice[78], sheep[79], and humans[51].
On the other hand, in vitro differentiated cells were found to express hepatocyte markers (alpha-fetoprotein, albumin, CK18, CK19, CYP1A1, CYP3A4, G6P, and human growth hormone-releasing factor)[80], store glycogen[81], clear ammonia and produce urea[82], and to secrete albumin and uptake low-density lipoprotein[83,84]. However, it is much more challenging to determine whether a cell is a true hepatocyte in vivo. Immunostaining for albumin, CK18, or hepatocyte nuclear factor are recognized indicators of hepatocyte trans-differentiation but not cellular functionality. It is important to note that differentiated MSCs still express mesenchymal markers such as CD90, α-smooth muscle actin, vimentin, and fibronectin, suggesting that complete trans-differentiation is not achieved[85].
Hepatic trans-differentiation potential is essential for MSCs-based therapies in the context of ALD, in which the injured hepatocyte cannot regenerate. However, the initial optimism has been tempered by the recognition of many groups that fusion of MSCs with endogenous hepatocytes is the main mechanism by which new hepatocytes are produced in vivo[86,87]. Hence, irrespective of whether the mechanism is MSC trans-differentiation or fusion, these events do not occur at a sufficiently high frequency to account for the observed functional improvement after MSC administration. Therefore, additional mechanisms may be involved in the regenerative process[88-90].
Liver injury caused by persistent inflammation is accompanied with T cell, B cell and monocyte infiltration of the liver[91,92]. In this respect, MSC immunomodulatory and immunosuppressive properties could potentially be involved in the positive effects that MSC transplantation has in chronic and acute liver diseases.
MSCs regulate the activity of cells from both adaptive and innate immunity[93]. In vitro, they inhibit the differentiation of monocytic precursors into activated dendritic cells[94,95]. Thus, MSCs indirectly limit the cytotoxic expansion and activity of NK T lymphocytes[96]. Both in vitro and in vivo, MSCs downregulate the expression of proinflammatory molecules [interleukin (IL)-1β, IL-12, TNF-α, and interferon-γ] and secrete anti-inflammatory factors (IL-4 and IL-10), shifting the immune response pattern toward a protective Th2 type, and establishing a tolerogenic microenvironment where activated T cells are unable to proliferate and die by apoptosis[97].
Another candidate for the suppressive effects of MSCs is indoleamine 2,3-dioxygenase, which is expressed by MSCs upon interferon-γ stimulation, leading to tryptophan depletion, and thus inhibition of T-cell proliferation[98]. This effect on T lymphocytes indirectly suppresses the function of B lymphocytes because their activation is mainly T-cell dependent. Moreover, MSCs can also modulate B-cell functions by inhibiting their proliferation, differentiation into antibody-secreting cells, and chemotaxis[99].
MSCs also promote the appearance of regulatory T cells, inducing antigen-specific tolerance[100]. Interestingly, it has been shown that the immunologic properties of undifferentiated MSCs are retained when they differentiate into parenchymal cells[101]. Therefore, both undifferentiated and differentiated MSCs will contribute to the maintenance of a microenvironment that allows tissue regeneration.
It is known that MSCs have the ability to secrete, in vitro and in vivo, a wide range of trophic factors, including VEGF, basic fibroblast growth factor, HGF, platelet-derived growth factor, TGF-β, IGF-1, and EGF[68]. The biologic effects of these factors can be both direct, by unleashing intracellular signalization pathways, and indirect, by inducing other cells from the microenvironment to secret additional bioactive factors. Therefore, it has been proposed that MSCs have a catalytic role in tissue regeneration, as once in the damaged tissue, they are able to modify the microenvironment by secreting factors that would: (1) prevent parenchymal cells from dying; (2) induce the proliferation and differentiation of endogenous progenitors; (3) promote neovascularization; and (4) avoid/revert fibrosis development[88,90].
Diverse studies have shown that < 1% of systemically administered MSCs are still present in any organ, including the lung, heart, kidneys, liver, spleen, and gut, one week after administration[102-104]. However, clinically, the beneficial effects associated with MSC administration can be observed for much longer than one week.
MSC-conditioned medium (MSC-CM) administration can recapitulate the beneficial effects of MSCs regarding tissue repair; for instance, data from van Poll et al[105] has provided the first clear evidence that MSC-CM procures trophic support for injured liver by inhibiting hepatocellular death and by stimulating liver regeneration. Although no specific mechanisms of action have been identified, soluble factors, including VEGF, HGF, IGF-1, EGF, IGF-binding protein, and IL-6, have been implicated in those regenerative effects.
Microvesicles (MVs) have recently been considered as important mediators of cell-to-cell communications, as they carry a complex load of proteins, lipids, mRNA, and microRNA, which might affect several cellular processes and pathways[106]. MVs account for approximately 10% of conditioned medium components in terms of protein amount; therefore, MSC-CM therapeutic activity could thus be partially attributed to MVs[107,108].
In addition to the induction of liver regeneration, antifibrotic properties of the MSC secretome have also been described. In this sense, Li et al[109] demonstrated that transplantation of MVs derived from human umbilical cord MSCs can alleviate liver fibrosis induced by carbon tetrachloride administration. These results have also been recapitulated by the administration of ex vivo expanded MSCs[109-112]. However, other studies have reported that MSCs can potentially be fibrogenic and contribute to increased fibrosis[113-115] or have no effect whatsoever[116,117].
These experimental results suggest two apparently contradictory scenarios; a great number of variables contribute to the inconsistences between the different observations. One such inconsistency is the difference in the properties of MSCs prepared in different laboratories, due to differences in the protocols used for MSC isolation and ex vivo expansion. There are also important differences between hMSCs and rodent MSCs, and even between different mouse strains[55]. Finally, another important factor is the dependence of the MSC differentiation process on most of the culture conditions or in vivo microenvironments, especially those developed in damaged tissue. In most of the cases, the signals that drive this differentiation process have not been characterized, so they cannot be replicated in vitro.
Numerous studies have tried to demonstrate the therapeutic potential of MSCs in the treatment of acute and chronic liver diseases, however, to date, a gap in the study of MSC administration for the treatment of ALD remains (Table 2). This gap is due, in part, to the lack of experimental animal models that recapitulate the full progression of ALD in human patients. Nonhuman primates are possibly the most similar model for human diseases[118,119]. For example, exposure of baboons to ad libitum alcohol intake leads to the progression of all stages of liver damage associated with ALD in humans. However, the relevance of nonhuman primates as a model of ALD is outweighed by the prodigious cost of maintaining them, which limits their utility to the field as a whole. Therefore, it is not surprising that the majority of alcohol research performed in animal models involves rodents[118,119]. The major disadvantage of rodent models with regard to experimental ALD is that the obtained liver pathology is limited predominantly to steatosis, with some necroinflammatory changes. The more-severe steatohepatitis and advanced liver damage observed in human patients (fibrosis and cirrhosis) is generally not observed in rodents[118,119].
| Model animal species | Liver injury induction/kind of liver injury | MSCs administration route | Number andsource of transplanted MSCs | Therapeutic effect | Proposed mechanisms | Ref. |
| Rat | Allyl alcohol (ip administration)/chronic damage | Intrahepatic | 1 × 106 MSCs from human BM | Hepatocyte regeneration | Hepatocyte differentiation without evidence of cell fusion | [76] |
| Mouse | Low-level of radiation/minimal, hepatic damage | Tail vein | 2 × 104 MSCs from mouse BM | Hepatocyte regeneration | Hepatocyte differentiation | [78] |
| Mouse | Chronic exposure to high fat diet/NASH | Tail vein | 0.5 × 106 MSCs from mouse BM | Prevention of NASH onset | Paracrine promotion of hepatic proliferation | [110] |
| Preclusion of the inflammatory process | Increase in the fatty-acid oxidation enzymes expression | |||||
| Mouse | Chronic exposure to atherogenic diet/NASH | Splenic capsule | 0.1 × 106 MSCs from mouse adipose tissue | Restoration of albumin expression in hepatic parenchymal cells | Modulation of inflammation | [111] |
| Amelioration of fibrosis | Increase in MMP expression | |||||
| Suppression of persistent hepatic inflammation | ||||||
| Mouse | CCl4 (ip administration)/liver fibrosis | Spleen | 0.5 × 106 MSCs from human amniotic membrane | Reduction of liver fibrosis | Inactivation of HSCs | [126] |
| Improvement of hepatic function | Reduction of hepatocyte apoptosis | |||||
| Promotion of liver regeneration | ||||||
| Differentiation of hepatocyte-like cells | ||||||
| Mouse | CCl4 (ip administration)/liver fibrosis | Tail vein | 0.5 × 106 MSCs from human BM | Reduction of liver fibrosis | Induction of MMP-9 expression | [121] |
| Reduction in TGF-β expression | ||||||
| Rat | D-galactosamine (ip administration)/fulminant hepatic failure | Penile vein | Conditioned medium from human BM MSCs | Reduction in the mortality rate | Modulation of the immune response | [105,122] |
| Reduction in panlobular leukocyte infiltrates | Trophic factor release (i.e., VEGF, HGF, and IGF-BP) | |||||
| Reduction in hepatocellular death | ||||||
| Mouse | CCl4 (ip administration)/liver fibrosis | Intrahepatic | Exosomes derived from human umbilical cord MSCs | Recovery of serum aspartate aminotransferase activity | Not determined | [109] |
| Decrease in collagen type I and III, TGF-β1 level |
Several in vivo studies have been performed to evaluate the therapeutic potential of MSCs in the context of liver fibrosis[54,56]. In most of the studies, liver fibrosis was induced by intraperitoneal or subcutaneous injection of carbon tetrachloride, however, this model cannot provide a perfect simulation of human etiology[120,121].
Application of MSCs in the in vivo models of liver fibrosis/cirrhosis ameliorates the development of the disease[54,56,111,112]. Similar results were obtained when MSC-CM or MVs were applied[105,108,109,122], suggesting that long-term survival of MSCs might not be necessary for their beneficial effects. In these studies, the reduction of fibrosis was correlated with the decrease in the synthesis of collagen I and matrix metalloproteinase inhibitors, with a concomitant decrease in activated HSCs. Multiple mechanisms have been suggested to participate, such as immunomodulation[123], selective apoptosis of[124,125] or reversion to a quiescent state of HSCs, and production of protective factors[126,127].
Studies of in vitro co-cultures of MSCs with activated stellate cells have shown that, even in small numbers, MSCs can paracrinally inhibit the fibrogenic activity of activated stellate cells. This inhibition can be a consequence of the secretion of IL-10 and TNF-α by MSCs. Moreover, MSCs are able to induce apoptosis in reactive stellate cells, a process mediated in part by the secretion of HGF[125]. These results support the hypothesis that the therapeutic effects of MSCs on fibrosis inhibition are the result of the secretion of paracrine factors that modulate the proliferation, viability, and function of resident stellate cells. The production of matrix metalloproteinases can also be effective at reverting hepatic fibrosis. MSCs are capable of secreting and inducing the expression of matrix metalloproteinase-9 and -13 in other cells, the latter being the main rodent and human interstitial collagenase[128,129].
In ALD, as well as in more prominent cirrhotic liver, hepatocytes are reported to have reduced proliferative capacity, which may reflect either the inhibitory effect of adjacent collagen I or that they have reached replicative senescence after many rounds of injury and repair[44,45]. MSC infusion may increase the intrinsic ability of hepatocytes to proliferate by the release of proliferative trophic factors and cytokines, or by facilitating the breakdown of scar tissue, thereby removing a block to proliferation[130].
In our laboratory, we found that intravenous administration of bone marrow-derived MSCs into animals suffering from diet-induced metabolic syndrome and obesity restores liver function and avoids progression from steatosis to nonalcoholic-steatohepatitis[110]. Such MSC-mediated hepatoprotection was unrelated to metabolic syndrome reversion. Nevertheless, this has been associated with the potential of MSCs to enhance liver regeneration and/or manage the second hit required for the transition from steatosis to nonalcoholic-steatohepatitis, as an increased hepatic proliferation rate was found as well as an increased expression of fatty-acid oxidation enzymes. Thus, MSC administration could prevent the progression of ALD by reducing the impairment of fatty-acid oxidation.
Finally, the question of the ideal route of MSC injection remains one of the main unsolved issues regarding efficient administration of MSCs. Even if the tail vein seems to be the most often used administration route in animals, the portal vein and intrahepatic injections also seem to be efficient[129,131]. The optimal dose of cells or conditioned medium also needs to be evaluated because there are significant variations among studies in terms of the number of cells injected per animal.
MSCs have been successfully used in humans to treat different pathologies such as osteogenesis imperfecta[132], idiopathic aplastic anemia[133], graft-versus-host disease[134], and acute myelogenous leukemia[135]. Other applications have been to specifically avoid lung fibrosis injury after bleomycin challenge[136], and for the protection of cardiac function after a myocardial infarction[137]. In every case, clear therapeutic effects with no complications have been reported.
In the same direction, the translation of preclinical research on MSCs to clinical use for cirrhotic patients has generated great interest due to the growing population of patients with advanced liver diseases and the critical shortage of available liver donors.
To date, some clinical trials using hMSCs to treat patients with liver fibrosis have been published[112,138-145]. Unfortunately, in general, the studies were heterogeneous in their design and did not distinguish between the various etiologies of cirrhosis. ALD patients and viral hepatitis patients were mixed together in small case series.
Recently, Jang et al[140] evaluated the effect of autologous bone marrow-derived MSC transplantation on hepatic fibrosis in patients with alcoholic cirrhosis. After MSC administration, liver histologic improvements were observed in 6/11 patients, and recovery of liver function in 10 patients associated with a decreased expression of TGF-β1, collagen type I, and α-smooth muscle actin, without significant complications or side effects during the study period[140]. These results support the use of these cells as a therapy for patients with alcoholic cirrhosis. However, further prospective, controlled studies are needed before MSC administration can be accepted as new strategy for antifibrosis therapy.
Knowledge regarding MSC biology and their application in liver fibrosis treatment has significantly increased over the past years. Nevertheless, the clinical use of MSCs for liver regeneration, in particular ALD, is still in its beginnings, and fundamental questions remain to be addressed.
Although clinical trials have provided hope that MSCs could be a valuable resource for cell-based therapies for liver fibrosis, these results must be interpreted with some caution given the limited number of patients enrolled in each trial and the lack of appropriate controls. For example, patients with acute alcoholic hepatitis normally receive a high dose of prednisone therapy. However, the effect of high-dose steroids on the transplantation of MSCs is not well studied. There is some evidence that MSCs are glucocorticoid sensitive and are induced to differentiate into adipocytes with steroid exposure[146].
Clinical trials have shown that MSC-based therapy is relatively safe, and no serious detrimental effects have been reported in humans to date. However, some concerns have risen over the use of replicating cells, which may escape control as time elapses[147]. Some potential complications could also arise from intravascular administration of MSCs leading to vascular occlusion. Preclinical studies have not excluded the differentiation of injected MSCs into ectopic structures[148], myocardial calcification[149], and enhanced accumulation of fibroblasts and myofibroblasts in the lungs[150], as these events have been reported following MSC treatment.
Stem cell-based therapy represents a newly emerging therapeutic approach to treat ALD. MSCs are an attractive tool because they have been shown to trigger the regeneration of damaged liver tissue, with no evidence of significant adverse effects in either preclinical or clinical studies.
Due to the relation between pathologic events that occur in ALD development and the cellular and molecular mechanisms associated with MSC therapeutic effects, we believe that MSC transplantation could be a promising therapeutic strategy to manage ALD progression.
The authors thank Dr. Amelina Albornoz for the English editing of the manuscript.
P- Reviewer: Heydtmann M S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [PubMed] |
| 2. | Moon KM, Kim G, Baik SK, Choi E, Kim MY, Kim HA, Cho MY, Shin SY, Kim JM, Park HJ. Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin Mol Hepatol. 2013;19:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28 Suppl 1:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 4. | Clouston AD, Jonsson JR, Powell EE. Steatosis as a cofactor in other liver diseases: hepatitis C virus, alcohol, hemochromatosis, and others. Clin Liver Dis. 2007;11:173-189, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Gitto S, Micco L, Conti F, Andreone P, Bernardi M. Alcohol and viral hepatitis: a mini-review. Dig Liver Dis. 2009;41:67-70. [PubMed] |
| 6. | Pateria P, de Boer B, MacQuillan G. Liver abnormalities in drug and substance abusers. Best Pract Res Clin Gastroenterol. 2013;27:577-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Baraona E, Lieber CS. Alcohol and lipids. Recent Dev Alcohol. 1998;14:97-134. [PubMed] |
| 8. | You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem. 2002;277:29342-29347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 415] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Kim W, Kim DJ. Severe alcoholic hepatitis-current concepts, diagnosis and treatment options. World J Hepatol. 2014;6:688-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 11. | Spengler EK, Dunkelberg J, Schey R. Alcoholic hepatitis: current management. Dig Dis Sci. 2014;59:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Rusyn I, Bataller R. Alcohol and toxicity. J Hepatol. 2013;59:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Farfán Labonne BE, Gutiérrez M, Gómez-Quiroz LE, Konigsberg Fainstein M, Bucio L, Souza V, Flores O, Ortíz V, Hernández E, Kershenobich D. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol. 2009;25:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 547] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 16. | Kendrick SF, O’Boyle G, Mann J, Zeybel M, Palmer J, Jones DE, Day CP. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049-3056. [PubMed] |
| 19. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 20. | Bataller R, Rombouts K, Altamirano J, Marra F. Fibrosis in alcoholic and nonalcoholic steatohepatitis. Best Pract Res Clin Gastroenterol. 2011;25:231-244. [PubMed] |
| 21. | Neuman MG, French SW, French BA, Seitz HK, Cohen LB, Mueller S, Osna NA, Kharbanda KK, Seth D, Bautista A. Alcoholic and non-alcoholic steatohepatitis. Exp Mol Pathol. 2014;97:492-510. [PubMed] |
| 22. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4117] [Article Influence: 205.9] [Reference Citation Analysis (3)] |
| 23. | Wang JH, Batey RG, George J. Role of ethanol in the regulation of hepatic stellate cell function. World J Gastroenterol. 2006;12:6926-6932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Arthur MJ, Iredale JP, Mann DA. Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:940-943. [PubMed] |
| 25. | Bolondi L, Gramantieri L. From liver cirrhosis to HCC. Intern Emerg Med. 2011;6 Suppl 1:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Lee SS, Shin HS, Kim HJ, Lee SJ, Lee HS, Hyun KH, Kim YH, Kwon BW, Han JH, Choi H. Analysis of prognostic factors and 5-year survival rate in patients with hepatocellular carcinoma: a single-center experience. Korean J Hepatol. 2012;18:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Anstee QM, Daly AK, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin Liver Dis. 2011;31:128-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Crocè L, Sasso F, Pozzato G, Cristianini G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845-850. [PubMed] |
| 30. | Stroffolini T, Cotticelli G, Medda E, Niosi M, Del Vecchio-Blanco C, Addolorato G, Petrelli E, Salerno MT, Picardi A, Bernardi M. Interaction of alcohol intake and cofactors on the risk of cirrhosis. Liver Int. 2010;30:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311-321. [PubMed] |
| 32. | Jaurigue MM, Cappell MS. Therapy for alcoholic liver disease. World J Gastroenterol. 2014;20:2143-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Borowsky SA, Strome S, Lott E. Continued heavy drinking and survival in alcoholic cirrhotics. Gastroenterology. 1981;80:1405-1409. [PubMed] |
| 34. | Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45-53. [PubMed] |
| 35. | Menachery J, Duseja A. Treatment of decompensated alcoholic liver disease. Int J Hepatol. 2011;2011:219238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Morgan MY. The prognosis and outcome of alcoholic liver disease. Alcohol Alcohol Suppl. 1994;2:335-343. [PubMed] |
| 37. | Singal AK, Walia I, Singal A, Soloway RD. Corticosteroids and pentoxifylline for the treatment of alcoholic hepatitis: Current status. World J Hepatol. 2011;3:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40-46. [PubMed] |
| 40. | O’Shea RS, McCullough AJ. Treatment of alcoholic hepatitis. Clin Liver Dis. 2005;9:103-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: Current status and future development. World J Hepatol. 2011;3:215-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Strom SC, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, Miki T. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15 Suppl 1:S105-S110. [PubMed] |
| 43. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 44. | Duguay L, Coutu D, Hetu C, Joly JG. Inhibition of liver regeneration by chronic alcohol administration. Gut. 1982;23:8-13. [PubMed] |
| 45. | Wands JR, Carter EA, Bucher NL, Isselbacher KJ. Effect of acute and chronic ethanol intoxication on hepatic regeneration. Adv Exp Med Biol. 1980;132:663-670. [PubMed] |
| 46. | Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 427] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 47. | Gao W, Zhou P, Ma X, Tschudy-Seney B, Chen J, Magner NL, Revzin A, Nolta JA, Zern MA, Duan Y. Ethanol negatively regulates hepatic differentiation of hESC by inhibition of the MAPK/ERK signaling pathway in vitro. PLoS One. 2014;9:e112698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Schneider A, Attaran M, Meier PN, Strassburg C, Manns MP, Ott M, Barthold M, Arseniev L, Becker T, Panning B. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation. 2006;82:1115-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Serralta A, Donato MT, Orbis F, Castell JV, Mir J, Gómez-Lechón MJ. Functionality of cultured human hepatocytes from elective samples, cadaveric grafts and hepatectomies. Toxicol In Vitro. 2003;17:769-774. [PubMed] |
| 50. | Serralta A, Donato MT, Martinez A, Pareja E, Orbis F, Castell JV, Mir J, Gómez-Lechón MJ. Influence of preservation solution on the isolation and culture of human hepatocytes from liver grafts. Cell Transplant. 2005;14:837-843. [PubMed] |
| 51. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 52. | Hannan NR, Segeritz CP, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 2013;8:430-437. [PubMed] |
| 53. | Almeida-Porada G, Zanjani ED, Porada CD. Bone marrow stem cells and liver regeneration. Exp Hematol. 2010;38:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol. 2015;21:742-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 55. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 56. | Fiore EJ, Mazzolini G, Aquino JB. Mesenchymal Stem/Stromal Cells in Liver Fibrosis: Recent Findings, Old/New Caveats and Future Perspectives. Stem Cell Rev. 2015;11:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Levine P, McDaniel K, Francis H, Kennedy L, Alpini G, Meng F. Molecular mechanisms of stem cell therapy in alcoholic liver disease. Dig Liver Dis. 2014;46:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | He N, Zhang L, Cui J, Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014;2014:128436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340. [PubMed] |
| 61. | De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101-109. [PubMed] |
| 62. | in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845-852. [PubMed] |
| 63. | Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 950] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 64. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12673] [Article Influence: 704.1] [Reference Citation Analysis (2)] |
| 65. | Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001;226:507-520. [PubMed] |
| 66. | Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 432] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 67. | Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 68. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2224] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 69. | Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2697] [Article Influence: 168.6] [Reference Citation Analysis (0)] |
| 71. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] |
| 72. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 73. | Hengstler JG, Brulport M, Schormann W, Bauer A, Hermes M, Nussler AK, Fandrich F, Ruhnke M, Ungefroren H, Griffin L. Generation of human hepatocytes by stem cell technology: definition of the hepatocyte. Expert Opin Drug Metab Toxicol. 2005;1:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Taléns-Visconti R, Bonora A, Jover R, Mirabet V, Carbonell F, Castell JV, Gómez-Lechón MJ. Human mesenchymal stem cells from adipose tissue: Differentiation into hepatic lineage. Toxicol In Vitro. 2007;21:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 76. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 77. | Shu SN, Wei L, Wang JH, Zhan YT, Chen HS, Wang Y. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol. 2004;10:2818-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 696] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 79. | Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, Pixley J, Zanjani ED, Porada CD, Almeida-Porada G. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46:1935-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Liang XJ, Chen XJ, Yang DH, Huang SM, Sun GD, Chen YP. Differentiation of human umbilical cord mesenchymal stem cells into hepatocyte-like cells by hTERT gene transfection in vitro. Cell Biol Int. 2012;36:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Bornstein R, Macias MI, de la Torre P, Grande J, Flores AI. Human decidua-derived mesenchymal stromal cells differentiate into hepatic-like cells and form functional three-dimensional structures. Cytotherapy. 2012;14:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Ayatollahi M, Soleimani M, Tabei SZ, Kabir Salmani M. Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J Stem Cells. 2011;3:113-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 84. | Prasajak P, Leeanansaksiri W. Developing a New Two-Step Protocol to Generate Functional Hepatocytes from Wharton’s Jelly-Derived Mesenchymal Stem Cells under Hypoxic Condition. Stem Cells Int. 2013;2013:762196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 86. | Berisio R, Schluenzen F, Harms J, Bashan A, Auerbach T, Baram D, Yonath A. Structural insight into the role of the ribosomal tunnel in cellular regulation. Nat Struct Biol. 2003;10:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 897] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 87. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1114] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 88. | Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med. 2010;14:2190-2199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 89. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1372] [Cited by in RCA: 1224] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 90. | Lee T. Stem cell therapy independent of stemness. World J Stem Cells. 2012;4:120-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30 Suppl 1:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 92. | Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 525] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 93. | Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 634] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 94. | Liu YL, Jiang XX, Su YF, Huo SW, Zhu H, Wu Y, Mao N, Zhang Y. Endothelial cells from human umbilical vein inhibit generation of monocyte-derived dendritic cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:480-484. [PubMed] |
| 95. | Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 96. | Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 819] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 97. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3276] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 98. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 99. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1270] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 100. | Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, Conget P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012;30:1664-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 101. | Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890-896. [PubMed] |
| 102. | Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. [PubMed] |
| 103. | Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1574] [Cited by in RCA: 1448] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 104. | Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 493] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 105. | van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 106. | Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 288] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 107. | Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 108. | Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 109. | Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 680] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 110. | Ezquer M, Ezquer F, Ricca M, Allers C, Conget P. Intravenous administration of multipotent stromal cells prevents the onset of non-alcoholic steatohepatitis in obese mice with metabolic syndrome. J Hepatol. 2011;55:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 111. | Seki A, Sakai Y, Komura T, Nasti A, Yoshida K, Higashimoto M, Honda M, Usui S, Takamura M, Takamura T. Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology. 2013;58:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 112. | Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 113. | Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955-963. [PubMed] |
| 114. | di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 115. | Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH, Gonelle-Gispert C. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4:e6657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 116. | Carvalho AB, Quintanilha LF, Dias JV, Paredes BD, Mannheimer EG, Carvalho FG, Asensi KD, Gutfilen B, Fonseca LM, Resende CM. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26:1307-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 117. | Kim S, Kim HS, Lee E, Kim HO. In vivo hepatic differentiation potential of human cord blood-derived mesenchymal stem cells. Int J Mol Med. 2011;27:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Arteel GE. Animal models of alcoholic liver disease. Dig Dis. 2010;28:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G819-G823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 121. | Tanimoto H, Terai S, Taro T, Murata Y, Fujisawa K, Yamamoto N, Sakaida I. Improvement of liver fibrosis by infusion of cultured cells derived from human bone marrow. Cell Tissue Res. 2013;354:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 123. | Han Z, Jing Y, Zhang S, Liu Y, Shi Y, Wei L. The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell Biosci. 2012;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 124. | Lin N, Hu K, Chen S, Xie S, Tang Z, Lin J, Xu R. Nerve growth factor-mediated paracrine regulation of hepatic stellate cells by multipotent mesenchymal stromal cells. Life Sci. 2009;85:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 125. | Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 126. | Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 2011;6:e16789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 127. | Fiore EJ, Bayo JM, Garcia MG, Malvicini M, Lloyd R, Piccioni F, Rizzo M, Peixoto E, Sola MB, Atorrasagasti C. Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 128. | Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 129. | Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, Luo GH, Chen TM, Lee RP, Lin SZ, Harn HJ. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009;85:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 130. | Chagoya de Sánchez V, Martínez-Pérez L, Hernández-Muñoz R, Velasco-Loyden G. Recovery of the Cell Cycle Inhibition in CCl(4)-Induced Cirrhosis by the Adenosine Derivative IFC-305. Int J Hepatol. 2012;2012:212530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | Wang Y, Lian F, Li J, Fan W, Xu H, Yang X, Liang L, Chen W, Yang J. Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. J Transl Med. 2012;10:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | Le Blanc K, Götherström C, Ringdén O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607-1614. [PubMed] |
| 133. | Fouillard L, Francois S, Bouchet S, Bensidhoum M, Elm’selmi A, Chapel A. Innovative cell therapy in the treatment of serious adverse events related to both chemo-radiotherapy protocol and acute myeloid leukemia syndrome: the infusion of mesenchymal stem cells post-treatment reduces hematopoietic toxicity and promotes hematopoietic reconstitution. Curr Pharm Biotechnol. 2013;14:842-848. [PubMed] |
| 134. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [PubMed] |
| 135. | Lee ST, Jang JH, Cheong JW, Kim JS, Maemg HY, Hahn JS, Ko YW, Min YH. Treatment of high-risk acute myelogenous leukaemia by myeloablative chemoradiotherapy followed by co-infusion of T cell-depleted haematopoietic stem cells and culture-expanded marrow mesenchymal stem cells from a related donor with one fully mismatched human leucocyte antigen haplotype. Br J Haematol. 2002;118:1128-1131. [PubMed] |
| 136. | Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407-8411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1058] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 137. | Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol. 2012;57:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 138. | Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS, Fouad HA, Youssef YA. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 140. | Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, Park SY, Kim BR, Kim JW. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 141. | Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 142. | Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459-466. [PubMed] |
| 143. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 144. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 145. | Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 146. | Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054-1063. [PubMed] |
| 147. | Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr. 2012;6:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 148. | Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18:1754-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 149. | Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 460] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 150. | Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |