Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.221
Peer-review started: May 12, 2015
First decision: August 25, 2015
Revised: October 18, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 7, 2016
Processing time: 246 Days and 11.7 Hours
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. Significant efforts have been devoted to identify new biomarkers for molecular imaging and targeted therapy of HCC. Copper is a nutritional metal required for the function of numerous enzymatic molecules in the metabolic pathways of human cells. Emerging evidence suggests that copper plays a role in cell proliferation and angiogenesis. Increased accumulation of copper ions was detected in tissue samples of HCC and many other cancers in humans. Altered copper metabolism is a new biomarker for molecular cancer imaging with position emission tomography (PET) using radioactive copper as a tracer. It has been reported that extrahepatic mouse hepatoma or HCC xenografts can be localized with PET using copper-64 chloride as a tracer, suggesting that copper metabolism is a new biomarker for the detection of HCC metastasis in areas of low physiological copper uptake. In addition to copper modulation therapy with copper chelators, short-interference RNA specific for human copper transporter 1 (hCtr1) may be used to suppress growth of HCC by blocking increased copper uptake mediated by hCtr1. Furthermore, altered copper metabolism is a promising target for radionuclide therapy of HCC using therapeutic copper radionuclides. Copper metabolism has potential as a new theranostic biomarker for molecular imaging as well as targeted therapy of HCC.
Core tip: Copper is required for cell proliferation and tumor angiogenesis. This article provided an up-to-date review of copper metabolism as a novel theranostic biomarker in hepatocellular carcinoma. Altered copper metabolism is not only a novel biomarker for molecular imaging of extrahepatic metastasis of hepatocellular carcinoma using radioactive copper, but is also a promising target for copper modulation and radionuclide therapy of hepatocellular carcinoma.
- Citation: Wachsmann J, Peng F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J Gastroenterol 2016; 22(1): 221-231
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.221
Copper is a trace metal that is required for numerous metabolically important enzymes involved in various metabolic pathways of human physiology[1,2]. These include ceruloplasmin, superoxide dismutase, dopamine monooxygenase, lysyl oxidase, cytochrome c oxidase, factor V, and tyrosinase. These enzymes are used for a variety of purposes such as melatonin production, bone production, thrombosis and neurotransmitter synthesis. The amount of daily dietary copper required for an average adult is 1.0 to 1.6 mg according to the third National Health and Nutrition Survey[3]. Zero point nine mg per day of copper is the recommended daily allowance, and less than 10 mg per day is recommended by the National Academy of Sciences[4]. The adult human body contains about 75 mg of copper[5]. The liver and brain contain about one third of the overall quantity present, but copper is distributed throughout the human body and found in many organ systems, including the heart, kidneys, pancreas, spleen, bone and muscle[5].
The majority of daily copper intake is from vegetables and legumes, with other sources such as various meats. On average, vegetable sources of dietary copper require a more complex enzymatic process for absorption, compared to non-vegetable sources such as meat or milk. The variable amount of copper in food sources is dependent on the various amounts of copper in the soil as well as different food processing techniques[1,6]. When copper is ingested via food sources, dietary absorption of copper predominantly occurs in the stomach and small bowel, with only approximately 30%-40% of ingested copper being absorbed by those living in industrialized countries. However, depending on dietary intake of copper, the human body can theoretically absorb as much as 63%-67% in a copper deficient diet, or as little as 12% in those whose copper intake is very high. The high acidic environment in the stomach is believed to cause the release of copper from natural organic complexes. Subsequently, absorption in the small bowel is influenced by a change in the pH as well as pancreatic enzymes[6-8].
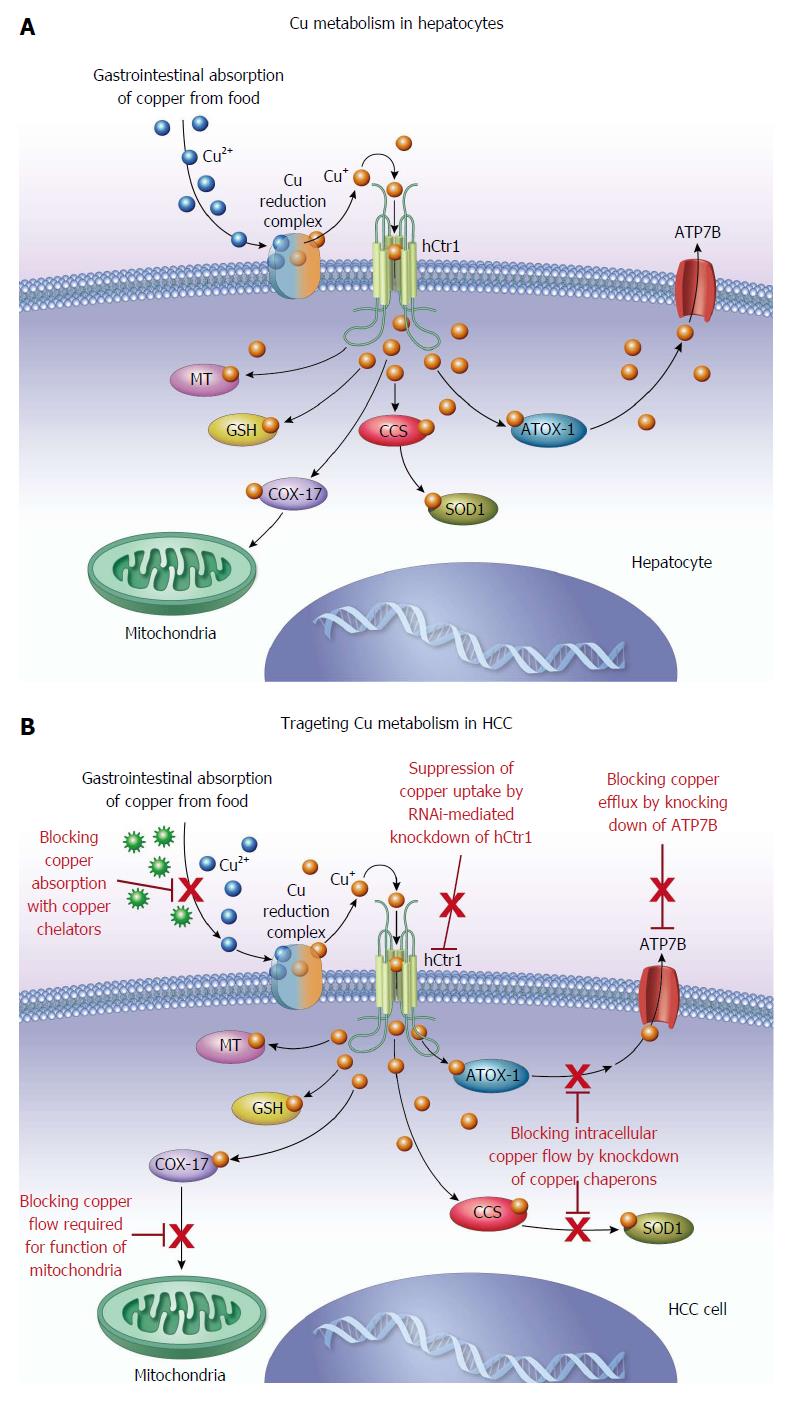
Metallothionein within the absorptive cells of the bowel are able to bind copper via mercaptide bonds and then release it in the plasma cell membrane on the serosal side. After being released from the intestinal mucosa, copper is bound to amino acids and albumin in the portal venous system. A small portion of the copper in the portal venous system is able to pass through to the systemic circulation, while the remainder is transferred into the cytosol of hepatocytes via cell membrane receptors. Within the liver, copper is bound to various proteins, but preferentially metallothionein[5,9].
The liver is a critical organ in the systemic regulation of copper metabolism and the maintenance of copper homeostasis. Wilson’s disease (WD) is an inherited copper metabolism disorder caused by mutation of the ATP7B gene located on chromosome 13, for which numerous specific mutations have been identified[10-12]. Long-Evans Cinnamon rat, an animal model of WD, has a deletion in the copper transporting ATPase gene and develops hereditary hepatitis followed by spontaneous hepatocellular carcinoma (HCC)[13]. When these rats are treated with the copper chelating agent D-pencillamine, as commonly used in humans with WD, prevention of the onset of hepatitis and the inhibition of elevated serum transaminases were observed[14]. Togashi et al[14] therefore concluded that abnormal copper accumulation in the liver of Long-Evans Cinnamon rats was associated with the pathogenesis of hereditary hepatitis and subsequent development of HCC. Both low and high molecular weight copper binding species have been described. The high molecular weight species predominate in gallbladder bile, while low molecular weight species are more prevalent in hepatic bile. The low molecular weight species are thought to assist in the membrane transport of copper across the biliary canaliculus. The high molecular weight portion of copper binding species is principally related to copper homeostasis[9,15]. This is supported by the inability to adequately remove hepatic copper in the absence of the higher molecular weight copper binding species, in the setting of protein synthesis inhibitors[16]. Copper that is tightly bound to bile salts is unable to be absorbed in the gastrointestinal tract, and is lost in feces, which is the predominant route of excretion[5,6,9,17].
The plasma concentration of copper has been shown to increase throughout life, peaking around the age of 60, and then having a minimal decline[18]. This process is thought to be related to a progressive reduction in biliary clearance later in life, rather than an increase in gastrointestinal absorption[6,19]. Differences in the plasma concentration of copper have also been demonstrated due to gender, with females on average having higher concentrations than men. Women between the ages of 20 and 59 were shown to absorb more and have a quicker turnover of radiolabeled copper in a meal, when compared to men. Higher levels of ceruloplasmin are also present in females[18].
HCC is the fifth most common cancer worldwide. It is the third leading cause of cancer-related death worldwide. Overall, about 75%-80% of HCC occurs in patients with hepatitis B or C, with many other known risk factors including aflatoxin B1, obesity, alcohol usage, diabetes, and tobacco[20,21]. It was demonstrated that the copper content in hepatic parenchyma of patients with HCC was significantly higher than in those without HCC, with no significant difference in hepatic iron levels. In fact, the copper liver level was the only significant factor associated with the presence of HCC in the cohort of patients with hepatitis C and chronic liver disease[22]. There were reports of an increased incidence of HCC in patients diagnosed with WD[23,24]. The copper content and level of copper binding proteins in HCC has been shown to be higher than those seen in other liver malignancies such as cholangiocellular carcinoma and metastatic tumors[25,26]. In addition, the serum copper to zinc ratio was significantly higher in patients with HCC than matched controls[27].
There have also been reports of a decreased incidence of HCC in patients with copper metabolism disorders[28]. It has been proposed that WD patients treated with D-penicillamine have an elevated risk of developing HCC[29]. This may be secondary to the associated decrease in copper content in the liver, when on chelation therapy. This discrepancy could reflect either a carcinogenic or a protective role of copper in the pathogenesis of HCC, which remains to be elucidated in further studies.
Currently, the detection of liver masses is predominantly evaluated using anatomic imaging modalities[30], such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). Molecular imaging is gaining momentum and is being used in various disease states[31]. Positron emitting fluorine-18-2-deoxy-gluocose (F-18 FDG) is a radioactive tracer used for the assessment of glucose metabolism in both benign and malignant tissues. After being delivered to the cells via blood flow, F-18 FDG is transported by GLUT transporters and then phosphorylated intracellularly. Typically, the FDG-6-phosphatase is trapped within the cells, unless there is a high level of phosphatase activity, as seen in the liver[32]. Secondary to the high level of phosphatase in the liver, the sensitivity for detecting well differentiated HCC is poor. However, there is usually high F-18 FDG uptake in moderately and poorly differentiated HCC. Positron emission tomography/computed tomography using F-18 FDG (F-18 FDG PET/CT) is also useful for the detection of recurrence and extrahepatic metastasis of HCC[33,34].
The sensitivity of FDG PET/CT in the detection of HCC was found to be approximately 50%, compared to the sensitivity of CT (75%)[35]. However, Wang et al[36] showed improved performance in the detection of HCC when an early dynamic F-18 FDG PET/CT was performed 240 s after tracer injection. Even better detection rates were obtained when early dynamic and conventional delayed whole body information was used in combination. The detection rates improved from 56.7% to 91.9% when using whole body delay versus the combination of early dynamic and whole body scans, respectively. In patients who were scheduled to undergo liver transplantation, F-18 FDG PET/CT was found to be useful for predicting microvascular invasion by HCC. The presence of microvascular invasion by HCC was predicted when the ratio of maximum standardized uptake value (SUV) of HCC to mean SUV of normal liver parenchyma was 1.2 or greater[37].
C-11 acetate, a tracer that evaluates free fatty acid synthesis, may have better sensitivity than that of F-18 FDG[38]. According to a study performed by Ho et al[39], the sensitivity of HCC detection in patients with less than 3 lesions was 87% for C-11 acetate and 47% for F-18 FDG. When this was correlated with histologic findings, it appears that well differentiated tumors were better detected by C-11 acetate, while the poorly differentiated tumors were better detected by F-18 FDG. None of the non-HCC tumors demonstrated abnormal C-11 acetate uptake. The use of dual phase C-11 acetate, using the change in uptake values in early and conventional imaging, correctly differentiated between small, 1-3 cm, well differentiated HCC from focal nodular hyperplasia and hemangiomas[40].
The tracer C-11 choline is used to evaluate the metabolism of phospholipids subsequently used as constituents of the cell membrane. F-18 FDG negative HCC showed elevated uptake of C-11 choline, which was predominantly seen in the moderately differentiated group[41]. F-18 fluorocholine has also been shown to perform better than F-18 FDG for well differentiated HCC, with a combination of both tracers appearing to be the best option[42]. Compared to a single modality, a combination of imaging modalities, including F-18 FDG PET, CT, MRI and ultrasound, currently has higher sensitivity, with minimal effects on specificity[38].
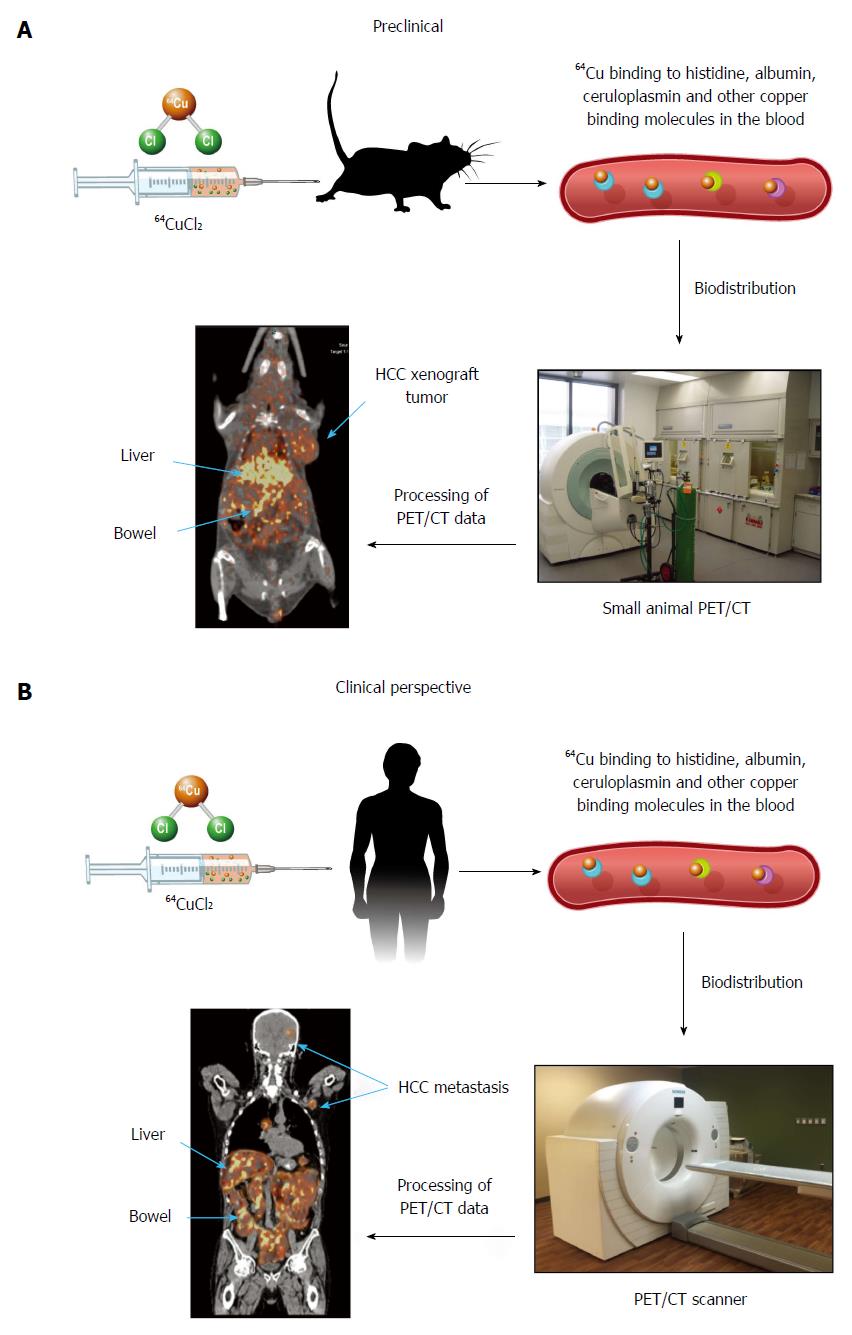
Continuous efforts are being made to develop new tracers for molecular imaging of HCC. Radioactive copper has been used for the assessment of copper metabolism disorders in patients diagnosed with WD using nuclear imaging for at least 45 years[43-46]. When exploring copper metabolism as a biomarker for molecular imaging of HCC, Peng et al[47], for the first time, demonstrated that mouse extrahepatic hepatoma could be visualized by PET using copper-64 chloride (64CuCl2) as a tracer, based on increased copper uptake mediated by mouse copper transporter 1 (mCtr1). There was relatively less 64Cu uptake in the hepatoma compared to the liver, which was thought to be related to several factors: less mCtr1 in the tumor relative to the liver, the possibility that endogenous mCtr1 may be less active on the tumor, other copper transporters in normal hepatocytes not expressed on the tumor, and more rapid efflux of copper in tumor cells than in normal hepatocytes[48]. More recently, human HCC xenografts in athymic mice were also visualized by PET after intravenous injection of 64CuCl2 as a tracer[49]. There was abundant physiologic distribution of 64Cu in the liver, which resulted in limited evaluation of primary HCC. Given the normal intense uptake of FDG by cortical brain tissue and low physiological cerebral uptake of 64Cu[47-53], 64CuCl2-PET is a promising technique for non-invasive assessment of intracranial and other extrahepatic metastasis of HCC located in areas with low physiological copper uptake (Figure 1). The prognosis for patients with intracranial HCC metastasis is poor as they are often resistant to systemic chemotherapy. The use of 64CuCl2-PET/CT for early detection of HCC intracranial metastasis is significant for improving the prognosis of patients with metastatic HCC. On the other hand, 64CuCl2-PET is expected to be useful for excluding extra-hepatic metastases in pre-transplant work up of patients who are considered candidates for liver transplantation. Positron emitting 64Cu radionuclide has a half-life of 12.7 h, making it possible to ship it to an imaging facility distant from the production site of this radiotracer. Preclinical radiation dosimetry of 64CuCl2 using the Atp7b-/- knockout mouse model of WD was comparable to that of F-18 FDG[50], supporting the use of 64CuCl2 as a radiotracer for PET of HCC metastasis, with the exception of the metastatic lesions in the abdomen due to excreted 64Cu in the intestinal tract.
Early detection and treatment are most critical for reducing mortality in patients with HCC[54,55]. The use of conventional transarterial chemoembolization (TACE) for the treatment of unresectable HCC has been found to improve the overall survival of patients compared to available supportive care[56]. The use of cisplatin or doxorubicin in a large review comparing chemoembolization showed a significant benefit compared to embolization alone[57]. A major limitation in the literature regarding TACE is the lack of consistent methods between various investigators[56]. The use of TACE with drug eluting beads (DEB-TACE), primarily using doxorubicin allows for slow drug release and lower levels of systemic chemotherapeutic agents when compared to TACE using lipiodol[58]. Although no survival benefit was shown, Malagari et al[59] were also able to show longer times to progression, less recurrence, and a better local response when using doxorubicin-eluting beads compared to bland embolization. Despite additional studies not showing a difference in radiographic response, survival or adverse events[60], Sieghart et al[56], still recommend that any future trials should include drug eluting bead TACE secondary to lower systemic levels of doxorubicin and then a possible reduction in drug-drug interactions.
The ability to bridge a patient to liver transplant has been achieved using several types of neo-adjuvant therapies including TACE, radiofrequency ablation, trans-arterial radioembolization (TARE), external beam radiotherapy and surgical resection. Bridging has been shown to decrease waiting list dropout, reduce HCC recurrence, and improve post-transplant survival with the goal of obtaining similar post-transplant outcomes to non-HCC patients[61].
Palliation for patients with end-stage or terminal HCC includes various options, with the primary goal of improving patient symptoms rather than definitive treatment[62]. Average survival for patients with end-stage or terminal HCC is 3-4 mo, and includes about 15%-20% of all HCC patients at presentation. The treatment options for end-stage disease are opiates, acetaminophen and corticosteroids[62]. HCC can be difficult to treat despite significant efforts devoted to the development of effective therapies for the treatment of this devastating disease[55]. Continuous efforts are being made to identify new targets for the treatment of HCC. Angiogenesis is an important pathway in tumor growth and copper is an important angiogenic factor for tumor growth[63]. Copper has been shown to be a cofactor in several mediators of angiogenesis including angiogenin, matrix metalloproteinase and fibroblast growth factor[64-66]. Moriguchi et al[67] demonstrated the antiangiogenic effects of the copper chelator, trientine dihydrochloride, on HCC in a rat model. Other groups have also shown that the copper chelator, pencillamine, together with diet modification can lower copper levels and microvascular density in cerebral rabbit models. Brem et al[68] also concluded that using pharmacologic withdrawal and dietary depletion of copper suppressed intracerebral tumor angiogenesis. However, prolonged anti-copper cancer therapy with copper chelators or long-term use of D-pencillamine for anti-inflammatory treatment in rheumatoid arthritis has been shown to cause toxicity such as bone marrow suppression, rash and neurologic symptoms[69,70]. Significant advancement has been made in understanding the molecular biology of copper transporters and chaperons regulating cellular copper homeostasis[71]. Recent advances in understanding the role of copper in the signal transduction pathway of cellular proliferation[53,72-76] support further study of copper metabolism as a target for molecular therapy of HCC. The selection of patients with copper hypermetabolic, metastatic HCC using 64CuCl2-PET/CT may be helpful for improving the efficacy of anti-copper therapy of HCC. Human copper transporter 1 (hCtr1) is a high affinity copper transporter which mediates cellular copper uptake in humans[77]. To overcome the side effects of anti-copper therapy with long-term or high-dose use of copper chelators, RNAi-mediated knockdown of hCtr1[53] may be a promising approach for targeted anti-copper therapy of HCC.
The use of external beam radiation for HCC has been limited as the liver is considered a radiosensitive organ, which may have led to early under-dosing of patients[78]. This limitation can be compounded when HCC occurs in the setting of an already diseased liver as seen with hepatitis C. Radiation-induced liver disease in patients subjected to external beam radiation can cause endothelial damage, platelet activation, fibrin thrombus and venous occlusion. These changes can lead to subsequent hepatic fibrosis. However, there may be a role for radiotherapy in patients with tumors that are in challenging locations, for palliative purposes, a bridge to transplant, or in combination with other treatment options[79]. External beam radiation as well as percutaneous cementoplasty has been used for palliative purposes with successful management of symptoms[80,81].
The targeted delivery of radionuclide therapy has been carried out by intra-arterial delivery of various conjugates radiolabeled with therapeutic radioisotopes including yttrium-90, iodine-131, holmium-166 and rhenium-188[82,83]. Yttrium-90 labeled microspheres are used for interventional radionuclide therapy of HCC[84]. Currently, there are both glass- and resin-based particles available for radioembolization of HCC. The glass-based form has a smaller size with a reduced embolic effect and lower incidence of post-embolization syndrome. One limitation of TACE is possible decompensation of the liver after use in patients with hepatic artery and portal thrombus. The use of Y-90 glass microspheres in patients with HCC and branch or lobar portal vein thrombosis showed favorable tumor response rates and was safe in a trial which included 108 patients[85]. Y-90 does not emit gamma rays and is therefore not optimal for imaging. In contrast, Rhenium-188 is a therapeutic radionuclide with a physical half-life of 16.9 h and emits both beta and gamma rays. The use of intra-arterial Rhenium-188-conjugates for radioembolization of HCC has been shown to inhibit tumor growth[86]. Attempts were also made to develop I-131 radiogene therapy of HCC based on tumor-specific expression of the human sodium/iodide symporter (hNIS) under control of the alpha fetoprotein promoter and enhancer[87-89]. Tumor-specific expression of the hNIS in HCC cells was achieved by transfection of HCC cells with a vector encoding the hNIS gene driven by an alpha fetoprotein promoter/enhancer. Increased uptake of I-131 by the cells expressing hNIS was detected by gamma counting in vitro and by imaging with a gamma camera in vivo. Growth of extrahepatic tumor xenografts derived from cells expressing hNIS was inhibited, secondary to radiation effects of 131I accumulated in the transfected HCC cells expressing hNIS[89]. The development of technologies to allow safe and efficient delivery of the vector encoding the hNIS gene is critical for the clinical application of I-131 radiogene therapy of HCC, based on the findings in preclinical studies.
Multiple copper isotopes are available for cancer imaging and therapy[90-93]. Copper-64 emits both β+ and β- particles and has potential as a theranostic copper radionuclide for both cancer imaging and therapy. Apelgot et al[94] demonstrated that 64Cu had a lethal effect in mammalian cells similar to that of 67Cu radionuclide. Significant efforts have been made to develop 64Cu-radiolabeled conjugates for cancer imaging and therapy[95-99]. Based on its simplicity and increased tumor uptake of 64Cu demonstrated by PET[47-49,52,53,100,101], ionic 64CuCl2 has potential as a therapeutic radiopharmaceutical for the treatment of tumors expressing high levels of hCtr1. Recently, it was reported that growth of malignant melanoma overexpressing hCtr1 was suppressed in mice treated with 64CuCl2[102]. In addition to its potential as a reporter gene for tracking gene delivery with PET, targeted overexpression of hCtr1 may be used for copper radiogene therapy of tumors expressing low levels of endogenous hCtr1[103]. The findings from preclinical studies support further investigation of ionic 64CuCl2 as a radiopharmaceutical for targeted radionuclide therapy of HCC, in addition to copper modulation therapy with copper chelators (Figure 2).
Copper is a transitional metal required for the regulation of cell proliferation and angiogenesis. The exact role of copper in the development of HCC is still poorly understood, as demonstrated by the paradoxical suppression or increase of HCC in patients with copper metabolic disorders such as WD. The findings of increased uptake of radioactive copper by extrahepatic HCC xenografts in mice invite clinical exploration of altered copper metabolism as a new imaging biomarker for metabolic imaging of HCC metastasis with PET using 64CuCl2 as a radioactive tracer. In addition, copper metabolism has potential as a target for copper modulation gene therapy of HCC based on RNAi-mediated knockdown of hCtr1 followed by administration of copper chelators. Furthermore, 64CuCl2 or 67CuCl2 may be used as radiopharmaceuticals for radionuclide therapy of HCC and ablation of extrahepatic HCC metastasis.
P- Reviewer: Cho YS, Piiper A S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Wang CH
| 1. | Johnson MA, Kays SE. Copper: its role in human nutrition. Nutrition Today. 1990;25:6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Danks DM. Copper deficiency in humans. Annu Rev Nutr. 1988;8:235-257. [PubMed] |
| 3. | Food and Nutrition Board of the Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press, Washington DC, 2000. Available from: http://www.nap.org. |
| 4. | Price CT, Langford JR, Liporace FA. Essential Nutrients for Bone Health and a Review of their Availability in the Average North American Diet. Open Orthop J. 2012;6:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Mason KE. A conspectus of research on copper metabolism and requirements of man. J Nutr. 1979;109:1979-2066. [PubMed] |
| 6. | Wapnir RA. Copper absorption and bioavailability. Am J Clin Nutr. 1998;67:1054S-1060S. [PubMed] |
| 7. | Turnlund JR, King JC, Gong B, Keyes WR, Michel MC. A stable isotope study of copper absorption in young men: effect of phytate and alpha-cellulose. Am J Clin Nutr. 1985;42:18-23. [PubMed] |
| 8. | Turnlund JR, Keyes WR, Anderson HL, Acord LL. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr. 1989;49:870-878. [PubMed] |
| 9. | Evans GW. Copper homeostasis in the mammalian system. Physiol Rev. 1973;53:535-570. [PubMed] |
| 10. | Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1311] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 11. | Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 910] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Cox DW, Prat L, Walshe JM, Heathcote J, Gaffney D. Twenty-four novel mutations in Wilson disease patients of predominantly European ancestry. Hum Mutat. 2005;26:280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wu J, Forbes JR, Chen HS, Cox DW. The LEC rat has a deletion in the copper transporting ATPase gene homologous to the Wilson disease gene. Nat Genet. 1994;7:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 264] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Togashi Y, Li Y, Kang JH, Takeichi N, Fujioka Y, Nagashima K, Kobayashi H. D-penicillamine prevents the development of hepatitis in Long-Evans Cinnamon rats with abnormal copper metabolism. Hepatology. 1992;15:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Gollan JL, Davis PS, Deller DJ. Binding of copper by human alimentary secretions. Am J Clin Nutr. 1971;24:1025-1027. [PubMed] |
| 16. | Gregoriadis G, Sourkes TL. Role of protein in removal of copper from the liver. Nature. 1968;218:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Wolters MG, Schreuder HA, van den Heuvel G, van Lonkhuijsen HJ, Hermus RJ, Voragen AG. A continuous in vitro method for estimation of the bioavailability of minerals and trace elements in foods: application to breads varying in phytic acid content. Br J Nutr. 1993;69:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Johnson PE, Milne DB, Lykken GI. Effects of age and sex on copper absorption, biological half-life, and status in humans. Am J Clin Nutr. 1992;56:917-925. [PubMed] |
| 19. | Madarić A, Ginter E, Kadrabová J. Serum copper, zinc and copper/zinc ratio in males: influence of aging. Physiol Res. 1994;43:107-111. [PubMed] |
| 20. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 634] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 21. | Chatterjee R, Mitra A. An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. Int Immunopharmacol. 2015;24:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Ebara M, Fukuda H, Hatano R, Yoshikawa M, Sugiura N, Saisho H, Kondo F, Yukawa M. Metal contents in the liver of patients with chronic liver disease caused by hepatitis C virus. Reference to hepatocellular carcinoma. Oncology. 2003;65:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Iwadate H, Ohira H, Suzuki T, Abe K, Yokokawa J, Takiguchi J, Rai T, Orikasa H, Irisawa A, Obara K. Hepatocellular carcinoma associated with Wilson’s disease. Intern Med. 2004;43:1042-1045. [PubMed] |
| 24. | Xu R, Hajdu CH. Wilson disease and hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2008;4:438-439. [PubMed] |
| 25. | Haratake J, Horie A, Takeda S, Kobori K, Sato H, Tokudome S. Tissue copper content in primary and metastatic liver cancers. Acta Pathol Jpn. 1987;37:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Maeda T, Shimada M, Harimoto N, Tsujita E, Maehara S, Rikimaru T, Tanaka S, Shirabe K, Maehara Y. Role of tissue trace elements in liver cancers and non-cancerous liver parenchyma. Hepatogastroenterology. 2005;52:187-190. [PubMed] |
| 27. | Poo JL, Rosas-Romero R, Montemayor AC, Isoard F, Uribe M. Diagnostic value of the copper/zinc ratio in hepatocellular carcinoma: a case control study. J Gastroenterol. 2003;38:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Wilkinson ML, Portmann B, Williams R. Wilson’s disease and hepatocellular carcinoma: possible protective role of copper. Gut. 1983;24:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Cheng WS, Govindarajan S, Redeker AG. Hepatocellular carcinoma in a case of Wilson’s disease. Liver. 1992;12:42-45. [PubMed] |
| 30. | Honda H, Onitsuka H, Murakami J, Kaneko K, Murayama S, Adachi E, Kanematsu T, Sugimachi K, Masuda K. Characteristic findings of hepatocellular carcinoma: an evaluation with comparative study of US, CT, and MRI. Gastrointest Radiol. 1992;17:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 979] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 32. | Wachsmann JW, Gerbaudo VH. Thorax: normal and benign pathologic patterns in FDG-PET/CT imaging. PET Clin. 2014;9:147-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, Tanaka A, Yamaoka Y, Yamamoto K, Konishi J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36:1811-1817. [PubMed] |
| 34. | Shiomi S, Kawabe J. Clinical applications of positron emission tomography in hepatic tumors. Hepatol Res. 2011;41:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, Zeuzem S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314-3319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Wang SB, Wu HB, Wang QS, Zhou WL, Tian Y, Li HS, Ji YH, Lv L. Combined early dynamic (18)F-FDG PET/CT and conventional whole-body (18)F-FDG PET/CT provide one-stop imaging for detecting hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2015;39:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Ahn SY, Lee JM, Joo I, Lee ES, Lee SJ, Cheon GJ, Han JK, Choi BI. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid-enhanced MR and (18)F-FDG PET/CT. Abdom Imaging. 2015;40:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, Zakher B, Pappas M, Graham E, Sullivan S. Imaging Techniques for the Diagnosis and Staging of Hepatocellular Carcinoma [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014; 14(15)-EHC048-EF. [PubMed] |
| 39. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 40. | Huo L, Dang Y, Lv J, Xing H, Li F. Application of dual phase imaging of 11C-acetate positron emission tomography on differential diagnosis of small hepatic lesions. PLoS One. 2014;9:e96517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Yamamoto Y, Nishiyama Y, Kameyama R, Okano K, Kashiwagi H, Deguchi A, Kaji M, Ohkawa M. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med. 2008;49:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 43. | Bush JA, Mahoney JP, Markowitz H, Gubler CJ, Cartwright GE, Wintrobe MM. Studies on copper metabolism. XIV. Radioactive copper studies in normal subjects and in patients with hepatolenticular degeneration. J Clin Invest. 1955;34:1766-1778. [PubMed] |
| 44. | Osborn SB, Szaz KF, Walshe JM. Studies with radioactive copper (64Cu and 67Cu): abdominal scintiscans in patients with Wilson’s disease. Q J Med. 1969;38:467-474. [PubMed] |
| 45. | Walshe JM, Potter G. The pattern of the whole body distribution of radioactive copper (67Cu, 64Cu) in Wilson’s Disease and various control groups. Q J Med. 1977;46:445-462. [PubMed] |
| 46. | Peng F. Positron emission tomography for measurement of copper fluxes in live organisms. Ann N Y Acad Sci. 2014;1314:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Peng F, Liu J, Wu JS, Lu X, Muzik O. Mouse extrahepatic hepatoma detected on MicroPET using copper (II)-64 chloride uptake mediated by endogenous mouse copper transporter 1. Mol Imaging Biol. 2005;7:325-329. [PubMed] |
| 48. | Peng F, Lu X, Janisse J, Muzik O, Shields AF. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. J Nucl Med. 2006;47:1649-1652. [PubMed] |
| 49. | Zhang H, Cai H, Lu X, Muzik O, Peng F. Positron emission tomography of human hepatocellular carcinoma xenografts in mice using copper (II)-64 chloride as a tracer. Acad Radiol. 2011;18:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Peng F, Lutsenko S, Sun X, Muzik O. Positron emission tomography of copper metabolism in the Atp7b-/- knock-out mouse model of Wilson’s disease. Mol Imaging Biol. 2012;14:70-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Peng F, Lutsenko S, Sun X, Muzik O. Imaging copper metabolism imbalance in Atp7b (-/-) knockout mouse model of Wilson’s disease with PET-CT and orally administered 64CuCl2. Mol Imaging Biol. 2012;14:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Jørgensen JT, Persson M, Madsen J, Kjær A. High tumor uptake of (64)Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl Med Biol. 2013;40:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Cai H, Wu JS, Muzik O, Hsieh JT, Lee RJ, Peng F. Reduced 64Cu uptake and tumor growth inhibition by knockdown of human copper transporter 1 in xenograft mouse model of prostate cancer. J Nucl Med. 2014;55:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 55. | Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S80-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 57. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] |
| 58. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 59. | Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 60. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (1)] |
| 61. | Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19:7515-7530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 62. | Kumar M, Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S130-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis--clinical implications. J Trace Elem Med Biol. 2004;18:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 64. | Soncin F, Guitton JD, Cartwright T, Badet J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem Biophys Res Commun. 1997;236:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 65. | Siméon A, Emonard H, Hornebeck W, Maquart FX. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000;67:2257-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Engleka KA, Maciag T. Inactivation of human fibroblast growth factor-1 (FGF-1) activity by interaction with copper ions involves FGF-1 dimer formation induced by copper-catalyzed oxidation. J Biol Chem. 1992;267:11307-11315. [PubMed] |
| 67. | Moriguchi M, Nakajima T, Kimura H, Watanabe T, Takashima H, Mitsumoto Y, Katagishi T, Okanoue T, Kagawa K. The copper chelator trientine has an antiangiogenic effect against hepatocellular carcinoma, possibly through inhibition of interleukin-8 production. Int J Cancer. 2002;102:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Brem S, Tsanaclis AM, Zagzag D. Anticopper treatment inhibits pseudopodial protrusion and the invasive spread of 9L gliosarcoma cells in the rat brain. Neurosurgery. 1990;26:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1-10. [PubMed] |
| 70. | Singh G, Fries JF, Williams CA, Zatarain E, Spitz P, Bloch DA. Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis. J Rheumatol. 1991;18:188-194. [PubMed] |
| 71. | Hasan NM, Lutsenko S. Regulation of copper transporters in human cells. Curr Top Membr. 2012;69:137-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem. 2009;284:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 73. | Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci USA. 2013;110:19507-19512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 312] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 74. | Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509:492-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 445] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 75. | Grubman A, White AR. Copper as a key regulator of cell signalling pathways. Expert Rev Mol Med. 2014;16:e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 76. | Safi R, Nelson ER, Chitneni SK, Franz KJ, George DJ, Zalutsky MR, McDonnell DP. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014;74:5819-5831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 77. | Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94:7481-7486. [PubMed] |
| 78. | Sharma H. Role of external beam radiation therapy in management of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S122-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Sandroussi C, Dawson LA, Lee M, Guindi M, Fischer S, Ghanekar A, Cattral MS, McGilvray ID, Levy GA, Renner E. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int. 2010;23:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Jiang W, Zeng ZC, Zhang JY, Fan J, Zeng MS, Zhou J. Palliative radiation therapy for pulmonary metastases from hepatocellular carcinoma. Clin Exp Metastasis. 2012;29:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Kodama H, Aikata H, Uka K, Takaki S, Mori N, Waki K, Jeong SC, Kawakami Y, Shirakawa H, Takahashi S. Efficacy of percutaneous cementoplasty for bone metastasis from hepatocellular carcinoma. Oncology. 2007;72:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Britz-Cunningham SH, Adelstein SJ. Molecular targeting with radionuclides: state of the science. J Nucl Med. 2003;44:1945-1961. [PubMed] |
| 83. | Sundram F. Radionuclide therapy of hepatocellular carcinoma. Biomed Imaging Interv J. 2006;2:e40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 85. | Salem R, Lewandowski R, Roberts C, Goin J, Thurston K, Abouljoud M, Courtney A. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004;15:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 86. | Keng GH, Sundram FX, Yu SW, Somanesan S, Premaraj J, Oon CJ, Kwok R, Htoo MM. Preliminary experience in radionuclide therapy of hepatocellular carcinoma using hepatic intra-arterial radio-conjugates. Ann Acad Med Singapore. 2002;31:382-386. [PubMed] |
| 87. | Willhauck MJ, Sharif Samani BR, Klutz K, Cengic N, Wolf I, Mohr L, Geissler M, Senekowitsch-Schmidtke R, Göke B, Morris JC. Alpha-fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther. 2008;15:214-223. [PubMed] |
| 88. | Jin YN, Chung HK, Kang JH, Lee YJ, Kimm KI, Kim YJ, Kim S, Chung JK. Radioiodine gene therapy of hepatocellular carcinoma targeted human alpha fetoprotein. Cancer Biother Radiopharm. 2008;23:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Ma XJ, Huang R, Kuang AR. AFP promoter enhancer increased specific expression of the human sodium iodide symporter (hNIS) for targeted radioiodine therapy of hepatocellular carcinoma. Cancer Invest. 2009;27:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Blower PJ, Lewis JS, Zweit J. Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol. 1996;23:957-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 289] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Sun X, Anderson CJ. Production and applications of copper-64 radiopharmaceuticals. Methods Enzymol. 2004;386:237-261. [PubMed] |
| 92. | Evangelista L, Luigi M, Cascini GL. New issues for copper-64: from precursor to innovative PET tracers in clinical oncology. Curr Radiopharm. 2013;6:117-123. [PubMed] |
| 93. | Niccoli Asabella A, Cascini GL, Altini C, Paparella D, Notaristefano A, Rubini G. The copper radioisotopes: a systematic review with special interest to 64Cu. Biomed Res Int. 2014;2014:786463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Apelgot S, Coppey J, Gaudemer A, Grisvard J, Guille E, Sasaki I, Sissoeff I. Similar lethal effect in mammalian cells for two radioisotopes of copper with different decay schemes, 64Cu and 67Cu. Int J Radiat Biol. 1989;55:365-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Lewis MR, Wang M, Axworthy DB, Theodore LJ, Mallet RW, Fritzberg AR, Welch MJ, Anderson CJ. In vivo evaluation of pretargeted 64Cu for tumor imaging and therapy. J Nucl Med. 2003;44:1284-1292. [PubMed] |
| 96. | Chong HS, Mhaske S, Lin M, Bhuniya S, Song HA, Brechbiel MW, Sun X. Novel synthetic ligands for targeted PET imaging and radiotherapy of copper. Bioorg Med Chem Lett. 2007;17:6107-6110. [PubMed] |
| 97. | Yuan J, You Y, Lu X, Muzik O, Oupicky D, Peng F. Synthesis of Poly[APMA]-DOTA-64Cu conjugates for interventional radionuclide therapy of prostate cancer: assessment of intratumoral retention by micro-positron emission tomography. Mol Imaging. 2007;6:10-17. [PubMed] |
| 98. | Jin ZH, Furukawa T, Claron M, Boturyn D, Coll JL, Fukumura T, Fujibayashi Y, Dumy P, Saga T. Positron emission tomography imaging of tumor angiogenesis and monitoring of antiangiogenic efficacy using the novel tetrameric peptide probe 64Cu-cyclam-RAFT-c(-RGDfK-)4. Angiogenesis. 2012;15:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Yuan J, Zhang H, Kaur H, Oupicky D, Peng F. Synthesis and characterization of theranostic poly(HPMA)-c(RGDyK)-DOTA-64Cu copolymer targeting tumor angiogenesis: tumor localization visualized by positron emission tomography. Mol Imaging. 2013;12:203-212. [PubMed] |
| 100. | Sparks R, Peng F. Positron emission tomography of altered copper metabolism for metabolic imaging and personalized therapy of prostate cancer. J Radiol Radiat Ther. 2013;1:1015. |
| 101. | Capasso E, Durzu S, Piras S, Zandieh S, Knoll P, Haug A, Hacker M, Meleddu C, Mirzaei S. Role of (64)CuCl 2 PET/CT in staging of prostate cancer. Ann Nucl Med. 2015;29:482-488. [PubMed] |
| 102. | Qin C, Liu H, Chen K, Hu X, Ma X, Lan X, Zhang Y, Cheng Z. Theranostics of malignant melanoma with 64CuCl2. J Nucl Med. 2014;55:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Kim KI, Jang SJ, Park JH, Lee YJ, Lee TS, Woo KS, Park H, Choe JG, An GI, Kang JH. Detection of increased 64Cu uptake by human copper transporter 1 gene overexpression using PET with 64CuCl2 in human breast cancer xenograft model. J Nucl Med. 2014;55:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |