INTRODUCTION
Hepatitis B is an infectious disease caused by hepatitis B virus (HBV), which infects the liver of Hominoidea, including humans. The infection of hepatocytes by HBV causes an inflammatory response and the subsequence damage of hepatocytes. Although there is an effective anti-HBV vaccine, chronic infection poses still a huge health burden all over the world[1]. In some hyperendemic areas (such as sub-Saharan Africa and East Asia), the prevalence of hepatitis B is over 8%[2]. Moreover, the people infected with HBV are nearly 3.7% of the global population (more than 240 million people). Of these, the annual number of deaths from HBV is estimated 786000[3]. Current USFDA approved therapies for HBV infections including immune modulators (interferon-alpha and pegylated interferon-alpha) and nucleoside analogues (lamivudine, entercavir, adefovir dipivoxil, telbivudine and tenofovir) have also been approved for the treatment of chronic HBV infection. However, the above-mentioned anti-HBV drugs have their limitations. For example, interferon-alpha has limited efficacy and high incidence of adverse reactions; whereas nucleoside analogues are very efficient HBV DNA inhibitors, but long-term use of these agents may result in drug resistance in a significant number of patients and does not result in the clearance of HBV genome from the infected liver[4]. Therefore, there is an obvious and urgent need to develop novel and efficient anti-HBV agents.
Natural products are important sources of new drugs and leads besides tailored synthesis[5]. To meet the pressing need, the development of naturally derived antiviral agents may be a promising approach[6]. In this review, anti-HBV natural products are reviewed and discussed according to their chemical classes. Attempts are made to cover as many reports as possible on compounds of natural origin that inhibit HBV replication giving special emphasis to new discoveries including novel structures and targets.
TERPENOIDS
Terpenoids are the largest class of natural products basd on a various but definite number of five-carbon isoprene units and play a variety of roles in mediating antagonistic interactions especially in traditional herbal remedies. Many natural terpenes possess promising anti-HBV activity. Betulinic acid was isolated from Pulsatilla chinensis and exhibited an inhibitory effect on HBV replication by down-regulating expression of manganese superoxide dismutase (SOD2) in HBV transgenic mice, which was followed by mitochondrial reactive oxygen species (ROS) generation and dysfunction[7]. Further investigation showed that SOD2 expression was repressed by betulinic acid-induced dephosphorylation of cAMP-response element-binding protein (CREB) at Ser133, a critical transcription factor for SOD2 transcription. The inhibition of HBV replication by betulinic acid was blocked by SOD2 overexpression, and that SOD2 knockdown validated this effect, indicating that the anti-HBV effect of betulinic acid may be achieved by modulating the balance of mitochondrial redox. Betulinic acid suppressed SOD2 expression, with enhanced ROS generation in the liver followed by the significant decline of HBV replication in HBV transgenic mice, suggesting that betulinic acid could be a good anti-HBV candidate[7].
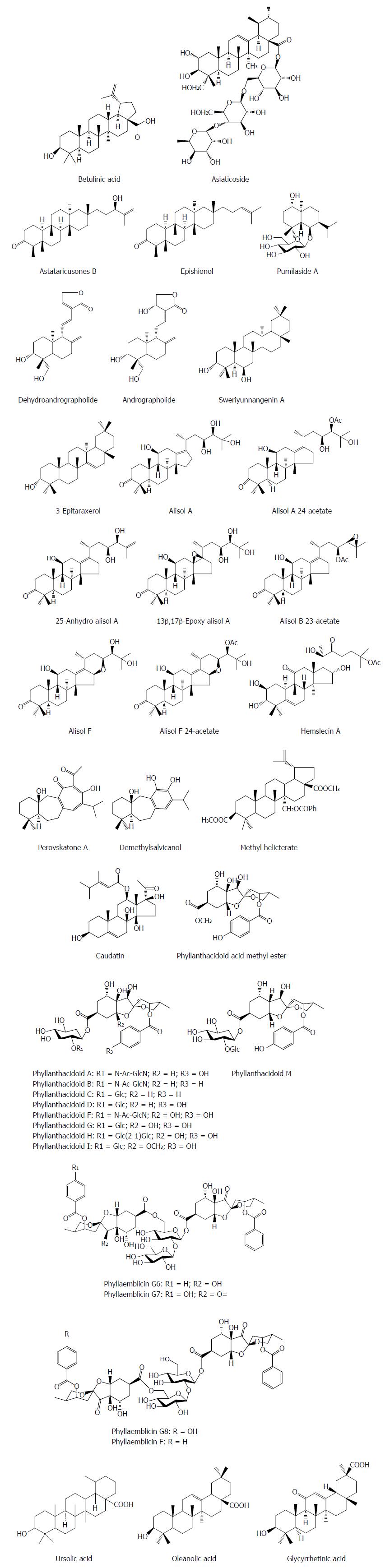
Asiaticoside from Hydrocotyle sibthorpioides inhibited effectively the HBV surface antigen (HBsAg), HBV e antigen (HBeAg), HBV DNA and covalently closed circular DNA (cccDNA) levels in a dose dependent manner[8]. Moreover, asiaticoside significantly decreased the transcription and replication of viral DNA by suppressing the core, S1, S2, and X gene promoter activities. Additionly, asiaticoside reduced markedly duck hepatitis B virus (DHBV) replication. On the third day after drug withdrawal, the DHBV DNA, DHBsAg and DHBeAg levels were enhanced in serum, but the rebound levels in the asiaticoside-treated groups are milder compared with the 3TC-treated group. Furthermore, asiaticoside could alleviate liver damage[8]. Astataricusones B and epishionol were obtained from the roots and rhizomes of Aster tataricus L. f. Astataricusones B showed an inhibitory effect on HBsAg secretion with a 50% inhibition concentration (IC50) value of 23.5 μmol/L, while astataricusones B and epishionol exhibited inhibitory effects on HBeAg secretion with IC50 values of 18.6 and 40.5 μmol/L, respectively. Astataricusones B and epishionol also exhibited inhibitory activities on HBV DNA replication with IC50 values of 2.7 and 30.7 μmol/L, respectively[9]. Pumilaside A from Artemisia capillaris could inhibit not only the secretion of HBsAg and HBeAg in HepG 2.2.15 cell line, but also HBV DNA replication with IC50 values of 15.02 μmol/L (SI = 111.3), 9.00 μmol/L (SI = 185.9) and 12.01 μmol/L (SI = 139.2), respectively[10,11]. Dehydroandrographolide and andrographolide from Andrographis paniculata possessed activity against HBV DNA replication with IC50 values of 22.58 and 54.07 μmol/L and low SI values of 8.7 and 3.7 μmol/L, respectively[12]. Sweriyunnangenin A and 3-epitaraxerol from Swertia yunnanensis showed activities against the secretion of HBsAg (IC50 values of 0.28 and 0.70 mmol/L) and HBeAg (IC50 values of 0.29 and 1.41 mmol/L), respectively[13]. Alisol A from the rhizomes of Alisma orientalis exhibited potent activities against HBsAg (IC50 = 0.039 mM) and HBeAg (IC50 > 0.028 mmol/L) secretion with remarkable selective indices (SIHBsAg = 1.6, SIHBeAg < 0.03), respectively[14].
Alisol A 24-acetate, 25-anhydro alisol A, 13β,17 β-epoxy alisol A, alisol B 23-acetate, alisol F, and alisol F 24-acetate from the rhizomes of Alisma orientalis exhibited an inhibitory activity on HBsAg secretion of HepG2.2.15 cells with IC50 values of 2.3, 11.0, 15.4, 14.3, 0.6 and 7.7 mmol/L, and on HBeAg secretion with IC50 values of 498.1, 17.6, 41.0, 19.9, 8.5 and 5.1 mmol/L, respectively[15,16]. Hemslecin A from the genus Hemsleya showed an effect against HBV DNA replication (IC50 = 11.2 lM, SI = 5.8) in HepG2.2.15 cells[17]. Perovskatone A and demethylsalvicanol from Perovskia atriplicifolia possessed a superior inhibitory effect on HBsAg (IC50: 0.16 mmol/L and 2.23 mmol/L) and HBeAg (IC50: 1.54 mmol/L and 3.67 mmol/L), respectively[18]. Methyl helicterate (MH) from the Chinese herb Helicteres angustifolia significantly decreased the HBsAg/HBeAg secretion, the HBV DNA/cccDNA levels, and the amount of viral RNA in HepG2.2.15 cells; in DHBV-infected ducklings, MH significantly reduced the serum DHBV DNA, liver total viral DNA, and cccDNA levels, and improved the liver pathological changes[19]. Caudatin from Cynanchum auriculatum had an inhibitory activity against HBsAg secretion and HBV DNA replication with the IC50 values of 142.67 μmol/L (SI = 1.7), 40.62 mmol/L (SI = 6.0), respectively, and 3-O- (3, 4, 5-trimethoxy) cinnamoyl caudatin had the novel mechanism of anti-HBV action by interfering HBV enhancers and promoters[20]. Phyllanthacidoid acid methyl ester and phyllanthacidoids A, B, C, D, F, G, H, I and M from Phyllanthus acidus Skeels displayed potential anti-HBV activities, with IC50 values of 0.8-36 μmol/L against HBsAg and HBeAg, and the results indicated that the 5-ketal group and sugar moieties had contributions to the selectivity of HBsAg and HBeAg[21]. Phyllaemblicins G6-G8 and phyllaemblicin F from Phyllanthus emblica displayed potential anti-HBV activities, especially for phyllaemblicin G6 with IC50 values of 8.53 ± 0.97 and 5.68 ± 1.75 μmol/L towards the HBsAg and HBeAg secretion, respectively[22]. Ursolic acid from the heartwood of Streblus asper showed an anti-HBV effect, with IC50 values of 89.91 μmol/L for HBsAg and IC50 values of 97.61 μmol/L for HBeAg, respectively[23]. Oleanolic acid from Swertia yunnanensis inhibited the secretion of HBsAg with an IC50 value of 1.26 mmol/L and HBeAg with an IC50 value of 0.94 mM, respectively[13]. Glycyrrhizin and its metabolite glycyrrhetinic acid (GA) are the main effective constituents of Licorice root (Glycyrrhizae glabra) for the treatment of chronic hepatitis B. GA exhibited inhibitory activities against HBV DNA replication (IC50 = 39.28 μmol/L), HBV surface antigen secretion (IC50 = 20.86 μmol/L) and HBV e antigen secretion (IC50 = 10.49 μmol/L)[24] (Figure 1).
Figure 1 Chemical structures of representative anti-hepatitis B virus natural terpenes.
LIGNANS
Lignans are a widely distributed phenylpropanoid derivatives in plants and are classified into five main structure types including lignans, oligomeric lignans, hybrid lignans, norlignans and neolignans. Lignan is also an important natural anti-HBV conponent. Helioxanthin (HE-145) was isolated from the heartwood of Taiwania cryptomerioides Hayata, and it was found to inhibit the gene expression and replication of HBV in HCC cells[25]. To elucidate the mode of anti-HBV action of HE-145, the authors examined the effects of HE-145 on HBV promoter activities by the luciferase reporter assay. The rusults showed that HE-145 inhibited selectively core promoter (CP) and surface antigen promoter II (SPII), but had no effect on X gene promoter (Xp) and surface antigen promoter I (SPI). Meanwhile, the inhibition of HE-145 on CP or SPII promoter was not observed in non-liver cells such as 293T and HeLa, suggesting that the inhibitory effects of HE-145 on SPII or CP could be liver-specific. The electrophoretic mobility shift assay indicated that HE-145 reduced the DNA-binding activity of HepA2 cell nuclear extract to several cis elements on CP, including alpha-fetoprotein transcription factor binding site, peroxisome proliferator-activated receptor (PPAR) binding site, and Sp1 binding site. Anomalous expression of PPAR-γ or hepatocyte nuclear factor 4-α reversed partially the HE-145-mediated HBV RNA suppression[25]. In short, HE-145 possess a unique anti-HBV mechanism and could be a novel class of potential anti-HBV agents. Nevertheless, what are its specific mechanisms of action? Down-regulation of these transcription factors by HE-145 or inactivation of a common coactivator? Hence, further studies are required to answer these questions.
A lignan glycoside (+)-cycloolivil-4′-O-β-D-glucopyranoside from Swertia chirayita exhibited an inhibitory effect not only on the HBsAg and HBeAg secretion with IC50 values of 0.31 ± 0.045 mmol/L (SI = 4.29) and 0.77 ± 0.076 mmol/L (SI = 1.75), respectively, but also on the replication of HBV DNA with an IC50 value of 0.29 ± 0.034 mmol/L (SI = 4.66)[26,27]. Schisanwilsonin D, schisantherin C, deoxyschizandrin and (+)-gomisin K3 were isolated from the fruits of Schisandra wilsoniana and showed anti-HBV effects; schisantherin C exhibited the strongest anti-HBV effect by diminishing HBsAg and HBeAg secretion by 59.7% and 34.7%, respectively[28]. Honokiol from Streblus asper showed marked anti-HBV activities with IC50 values of 3.14 μmol/L (SI = 21.47) and 4.74 μmol/L (SI = 14.22) for HBsAg and HbeAg, respectively[29]. Four lignans from the root of Streblus asper, (7’R,8’S,7″R,8″S)-erythro-strebluslignanol G, magnolol, isomagnolol and isolariciresinol, exhibited significant anti-HBV effects in HepG2.2.15 cells with IC50 values of 1.58, 2.03, 10.34 and 3.67 μmol/L, respectively, for HBsAg, and of 3.24, 3.76, 8.83 and 14.67 μmol/L, respectively, for HBeAg. (7’R,8’S,7″R,8″S)-erythro-strebluslignanol G and magnolol showed significant anti-HBV activities to inhibit the replication of HBV DNA with IC50 values of 9.02 and 8.67 μmol/L, respectively[30]. 9-β-xylopyranosyl-isolariciresinol from the stem bark of Streblus asper exhibited a significant anti-HBV activity in Hep G2.2.15 cells with an IC50 value of 6.58 μmol/L for secretion of HBsAg, and of 24.86 μmol/L for secretion of HBeAg[31]. Niranthin and nirtetralin B are two new lignans from Phyllanthus niruri L. (Euphorbiaceae). Niranthin and nirtetralin B inhibited HBV antigen secretion in HepG2.2.15 cells with IC50 values for HBsAg of 15.6 and 17.4 μmol/L, IC50 values for HBeAg of 25.1 and 63.9 μmol/L, respectively. In ducklings with DHBV, niranthin and nirtetralin B significantly decreased the serum HBsAg, HBeAg, DHBV DNA, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, and improved the pathological changes of liver tissue, respectively[32,33]. Three lignans, (+)-(7′S, 7″S, 8′R, 8″R)-4, 4′, 4″-trihydroxy-3, 5′, 3″-trimethoxy-7-oxo-8-ene [8-3′, 7′-O-9″, 8′-8″, 9′-O-7″] lignoid, (1S)-4-hydroxy-3-[2-(4-hydroxy-3-methoxy-phenyl)-1-hydroxymethyl-2-oxo-ethyl] -5-methoxy-benzaldehyde and herpetetrone from the seeds of Herpetospermum caudigerum Wall displayed inhibitory effects on HBsAg secretion with IC50 values of 20.5, 0.34, and 4.89 μmol/L, and on HBeAg secretion with IC50 values of 3.54, 0.048, and 8.02 μmol/L, respectively[34] (Figure 2).
Figure 2 Chemical structures of representative anti-hepatitis B virus natural lignans.
PHENOLIC ACIDS
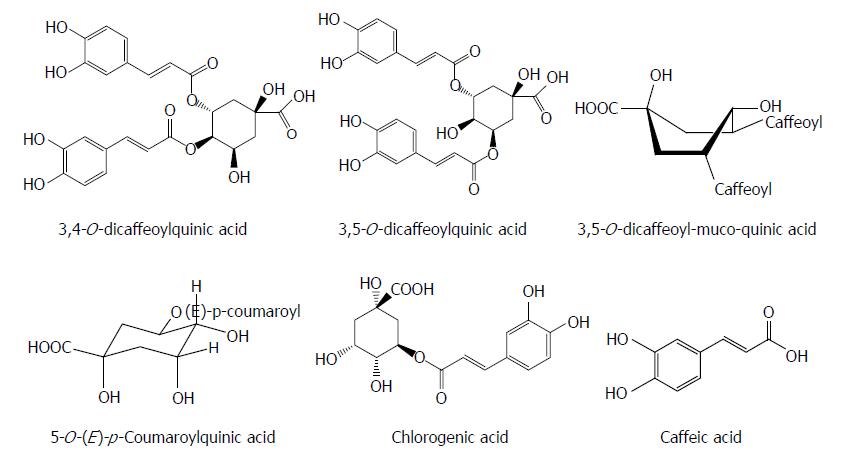
Phenolic acids are the different types of naturally occurring aromatic compounds containing a phenolic ring and a carboxylic acid function. Interestingly, some phenolic acids often have antiviral effects. 3,4-O-dicaffeoylquinic acid and 3,5-O-dicaffeoylquinic acid isolated from Laggera alata significantly suppressed the HBsAg and HBeAg production and their inhibitory rates on the HBsAg and HBeAg expression were 89.96%/86.9% and 81.01%/72.9%, respectively. Meanwhile, 3,4-O-dicaffeoylquinic acid markedly decreased the HBV cccDNA content and significantly increased the heme oxygenase-1 (HO-1) expression in HepG2.2.15 cells and HBV transgenic mice. 3,5-O-dicaffeoylquinic acid showed a similar effect. Because HO-1 can destabilize the HBV core protein, this observation suggests that HO-1 overexpression may contribute to the antiviral activity of the two compounds by decreasing the stability of the HBV core protein, which blocks the refill of nuclear HBV cccDNA[35,36]. Seven quinic acid derivatives, 3,4-O-dicaffeoylquinic acid, 4,5-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid, 3,5-O-dicaffeoyl-muco-quinic acid, 5-O-caffeoylquinic acid, 3-O-caffeoylquinic acid and 5-O-(E)-p-coumaroylquinic acid, isolated from the aerial parts of Lactuca indica L. (Compositae), effectively reduced HBV DNA level in HepG2.2.15 cells, and treatment with 3,4-O-dicaffeoylquinic acid, 3,5-O-dicaffeoyl-muco-quinic acid and 5-O-(E)-p-coumaroylquinic acid led to a marked decline in the extracellular HBV DNA level[37].
Chlorogenic acid and its related compounds are widely distributed in the leaves and fruits of dicotyledonous plants such as coffee beans and have a diverse antiviral activity. Chlorogenic acid, quinic acid and caffeic acid inhibited HBsAg production as well as HBV-DNA replication in HepG2.2.15 cells. Chlorogenic acid and caffeic acid also decreased the serum DHBV level in a DHBV-infected duckling model[38] (Figure 3).
Figure 3 Chemical structures of representative anti-hepatitis B virus natural phenolic acids.
POLYPHENOLS
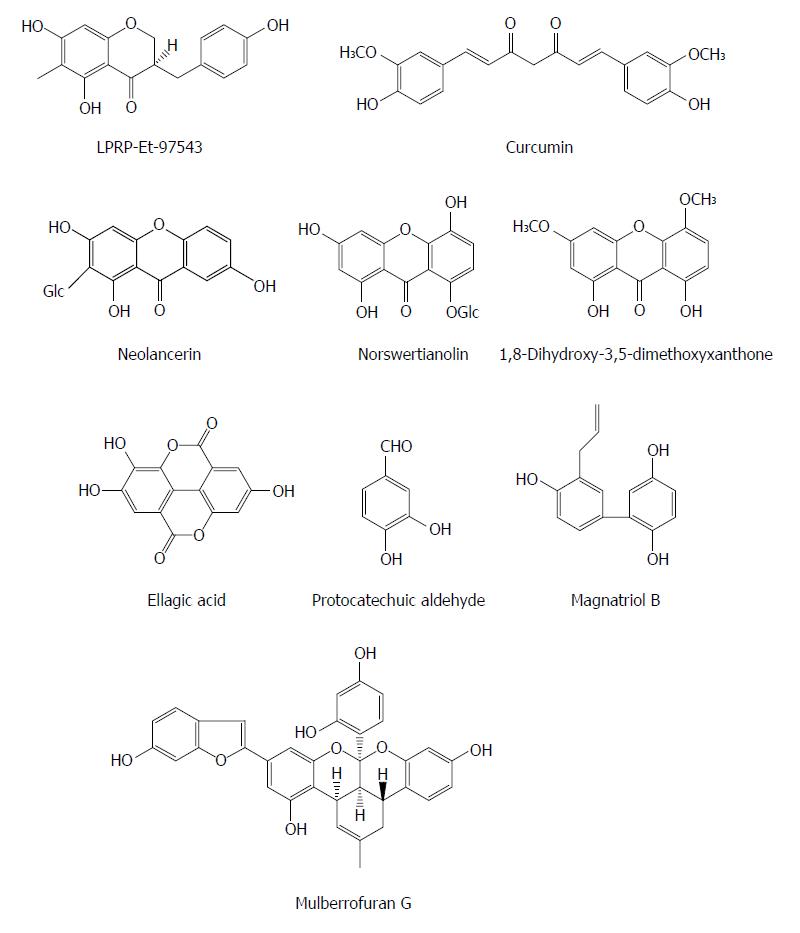
Polyphenols are some secondary metabolites containing two or more phenolic rings and are widely involved in defense against aggression by pathogens such as virus and bacteria. The compound LPRP-Et-97543 from Liriope platyphylla roots was observed to have potential anti-HBV effects in HepG2.2.15 cells. The antiviral mode of LPRP-Et-97543 was further studied using the HBV-transfected Huh7 cells. The effects of LPRP-Et-97543 on the viral precore/pregenomic and S/preS RNA were apparent during HBV gene expression. Analysis of promoter activity indicated that LPRP-Et-97543 markedly decreased S, preS, and Core but not X promoter activities. Further studies showed that LPRP-Et-97543 attenuated the nuclear expression of p65/p50 proteins from nuclear factor-kappaB (NF-κB) family and augmented the protein level of cytoplasmic IκBα without affecting their expression in HBV-nontransfected cells. Furthermore, LPRP-Et-97543 decreased the binding of NF-κB protein to CS1 site of HBV surface gene and suppressed CS1 element containing promoter activity in HBV-expressing cells. Nevertheless, HBV transfection markedly increases CS1 containing promoter activity in cells. Lastly, the inhibitory activity of LPRP-Et-97543 on HBV DNA replication was significantly reversed by transfection with the plasmid expressing p65 in HBV-positive cells. In conclusion, the feedback regulation of HBV gene expression and DNA replication may contribute to the anti-HBV effect of LPRP-Et-97543 by HBV proteins, which interferes with the NF-κB pathway[39].
Curcumin (a natural polyphenol) exhibited an inhibitory effect on HBV gene expression and replication in HBV-expressing cells by down-regulation of peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α), a co-activator of HBV transcription, suggesting that curcumin may be a potential host-targeting agent for the treatment of HBV infection[40]. Additionly, since HBV is very sensitive to the metabolic regulator PGC-1α, targeting PGC-1a could be a potential strategy of anti-HBV therapy.
Neolancerin from Swertia yunnanensis showed an activity against the secretion of HBsAg with an IC50 value of 0.21 mmol/L and HBeAg with an IC50 value of 0.04 mmol/L; and norswertianolin, 1,8-dihydroxy-3,5-dimethoxyxanthone, neolancerin from Swertia yunnanensis showed a significant inhibitory effect on HBV DNA replication with IC50 values of 0.01, 0.07 and 0.09 mmol/L, respectively[13]. Six phenols (including m-hydroxybenzoic acid, phydroxybenzoic acid, m-hydroxy benzenmethanol, 3,4-dihydroxybenzoic acid, ethyl 3,4-dihydroxybenzoate and ethyl 2,5-dihydroxybenzoate) from Swertia mussotii exhibited activities inhibiting the secretion of HBsAg and HBeAg with IC50 values from 0.23 to 5.18 mmol/L, and HBV DNA replication with IC50 values from 0.06 to 2.62 mmol/L[41]. Ellagic acid was isolated from Phyllanthus urinaria and exhibited unique anti-HBV functions by blocking HBeAg secretion in HepG2 2.2.15 cells (IC50 = 0.07 mg/mL), suggesting that it may be a potential agent for treatment of immune tolerance in individuals with HBV infection[42]. Protocatechuic aldehyde (PA) derived from Salvia miltiorrhiza appeared to down-regulate the HBsAg and HBeAg secretion as well as the HBV DNA release in HepG2.2.15 cells in a dose and time dependent manner; PA at doses of 25, 50, or 100 mg/kg (intraperitoneally, twice daily) also decreased viremia in ducks with DHBV infection[43]. Magnatriol B from Streblus asper exhibited moderate anti-HBV activities with IC50 values of 168.18 μmol/L (SI = 1.19) and 34.78 μmol/L (SI = 5.77) on HBsAg and HbeAg, respectively[29]. Mulberrofuran G from the root bark of Morus alba L. showed a moderate activity, inhibiting the replication of HBV DNA with an IC50 value of 3.99 μmol/L in HepG2.2.15 cells[44] (Figure 4).
Figure 4 Chemical structures of representative anti-hepatitis B virus natural polyphenols.
LACTONES
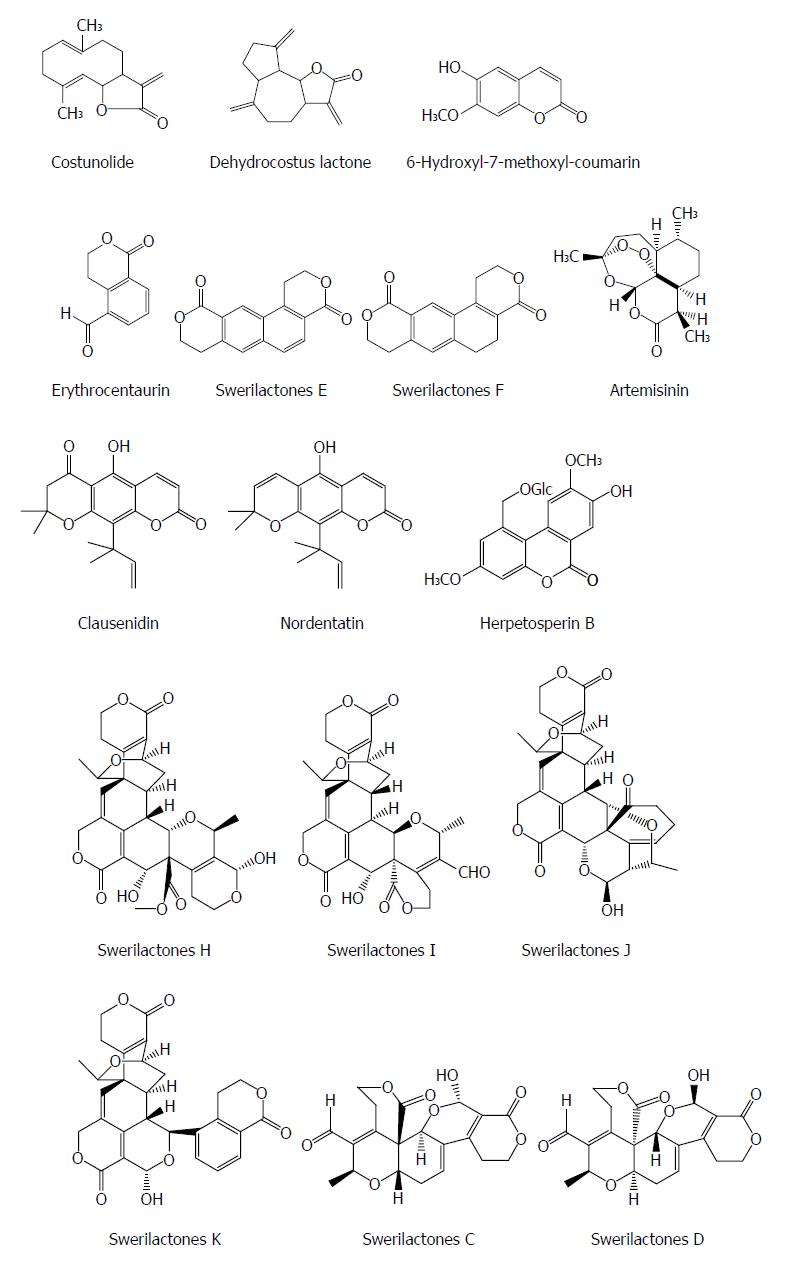
Lactone is a naturaly component containing a cyclic ester of hydroxycarboxylic acid and also exhibits an antiviral activity. Costunolide and dehydrocostus lactone from the root of Saussurea lappa Clarks show a potent inhibitory effect on the HBsAg and HBeAg expression in Hep3B cells with IC50 values of 1.0 and 2.0 μmol/L, respectively. The suppression of HBsAg gene expression by the two compounds was mainly at the level of mRNA. Furthermore, the inhibitory activities of the two compounds on HBsAg and HBeAg were also observed in HepA2 cells[45]. 6-hydroxyl-7-methoxyl-coumarin from the heartwood of Streblus asper showed an anti-HBV effect, with an IC50 value of 29.60 μmol/L for HBsAg and an IC50 value of 46.41 μmol/L for HBeAg, respectively[23]. Erythrocentaurin (ET) as a C10-skeleton secoiridoid from Swertia yunnanensis inhibited the secretion of HBsAg with an IC50 value of 1.30 mmol/L and HBeAg with an IC50 value of 1.14 mmol/L, and HBV DNA replication with an IC50 value of 0.76 mmol/L; and a series of ET analogs with the alteration on the aldehyde group exhibited more potent anti-HBV agents[13,46].
Swerilactones E and F (two novel lactones with a phenyl group) from Swertia mileensis showed significant suppressive effects on the secretion of HBsAg with IC50 values of 0.22 and 0.70 mM and HBeAg with IC50 values of 0.52 and > 6.78 mmol/L in HepG 2.2.15 cells, respectively[47]. Artemisinin from Artemisia annua induced moderate inhibition on HBsAg secretion and HBV DNA replication with IC50 values of 55 and > 100 mmol/L, respectively[48]. Two pyranocoumarins clausenidin and nordentatin from Clausena excavata suppressed HBsAg in HepA2 cells[49]. Herpetosperin B from the seeds of Herpetospermum caudigerum showed an anti-HBV activity with HBsAg secretion by 33.1% at 200 μg/mL[50]. Swerilactones H-K from Swertia mileensis exhibit a potent anti-HBV activity against HBV DNA replication with IC50 values ranging from 1.53 to 5.34 μmol/L[51]. Swerilactones C and D from Swertia mileensis exhibited inhibitory activities in HepG 2.2.15 cells against the secretion of HBsAg (IC50 = 1.24 and 2.96 mmol/L, respectively) and HBeAg (IC50 = 0.77 and 1.47 mmol/L, respectively)[52] (Figure 5).
Figure 5 Chemical structures of representative anti-hepatitis B virus natural lactones.
ALKALOIDS
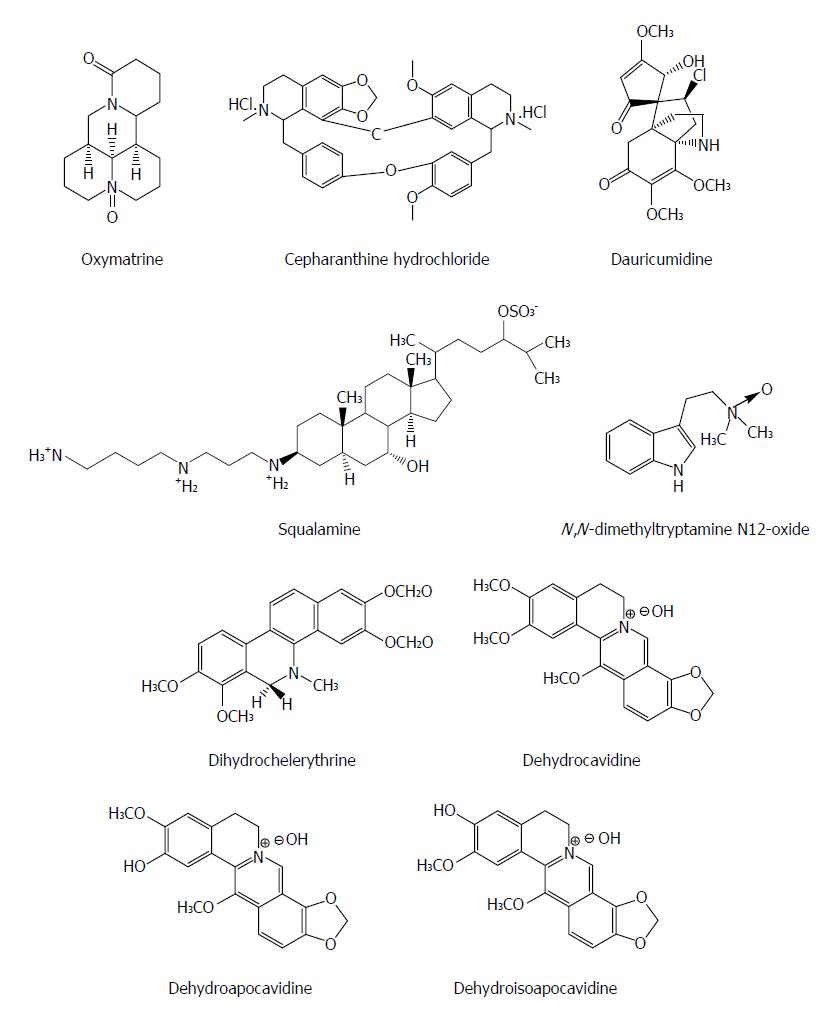
Alkaloids are a class of naturally occurring compounds containing mostly basic nitrogen atoms and also include some related neutral and even weakly acidic compounds. Many alkaloids have potent pharmacological effects, such as quinine, pilocarpine, and atropine. Oxymatrine from the plant Kushen (Sophora japonica) has been used to treat patients with hepatitis B in China for decades with a confirmed safety and often is used to replace the nucleoside analogues in order to decrease the emergence of treatment-resistant HBV mutants[53]. Monotherapy with oxymatrine (orally, for twelve months) decreased blood HBeAg by 70% and HBV DNA by 96% in patients with chronic hepatitis B (CHB) resistant to lamivudine (n = 17), equal to its efficacy in treatment-naïve CHB cohort (n = 20)[54]. Oxymatrine significantly down-regulates the mRNA expression of host heat-stress cognate 70 (Hsc70) at the post transcriptional level through destabilizing Hsc70 mRNA, and then inhibits the replication of HBV and exhibits anti-HBV efficacy[55].
Cepharanthine is a natural biscoclaurine alkaloid extracted from Stephania cepharantha Hayata. Cepharanthine hydrochloride (CH), as a natural alkaloid-derived compound, suppressed HBeAg production and HBV DNA replication by either wild-type or lamivudine-resistant HBV clinical isolates in a dose dependent manner and down-regulated significantly the Hsc70 mRNA levels, suggesting that its activity could be associated with its inhibitory effect on host Hsc70[56]. Squalamine from the sea lamprey (Petromyzon marinus) and the dogfish shark (Squalus acanthias) possesses a broad-spectrum antiviral effect on human viruses including HBV. Enveloped DNA and RNA viruses are proved to be susceptible, suggesting that it has a broad-spectrum antiviral activity. The antiviral mechanism of squalamine is likely based on its capacity to neutralize the negative electrostatic surface charge of intracellular membranes that renders the host cell less effective in the process of viral replication[57]. Dauricumidine from Hypserpa nitida Miers showed an IC50 value of 0.450 mM (SI = 4.13) on HBsAg secretion in HepG2.2.15 cells[58]. N,N-dimethyltryptamine N12-oxide from liver-protective medicinal plant Evodia fargesii Dode (Rutaceae) had a potent suppressive effect on HBV DNA replication (IC50 = 17.6 μmol/L, SI > 5.7) in HepG2.2.15 cells[59]. Dihydrochelerythrine isolated from Corydalis saxicola Bunting exhibited the strongest effect on HBsAg and HBeAg secretion with an IC50 value < 0.05 mM and SI > 3.5, respectively[60]. Dehydrocavidine, dehydroapocavidine and dehydroisoapocavidine from Corydalis saxicola Bunting (Papaveraceae) exhibited a suppressive activity against the secretion of HBsAg and HBeAg in Hep2.2.15 cells[61] (Figure 6).
Figure 6 Chemical structures of representative anti-hepatitis B virus natural alkaloids.
FLAVONOIDS
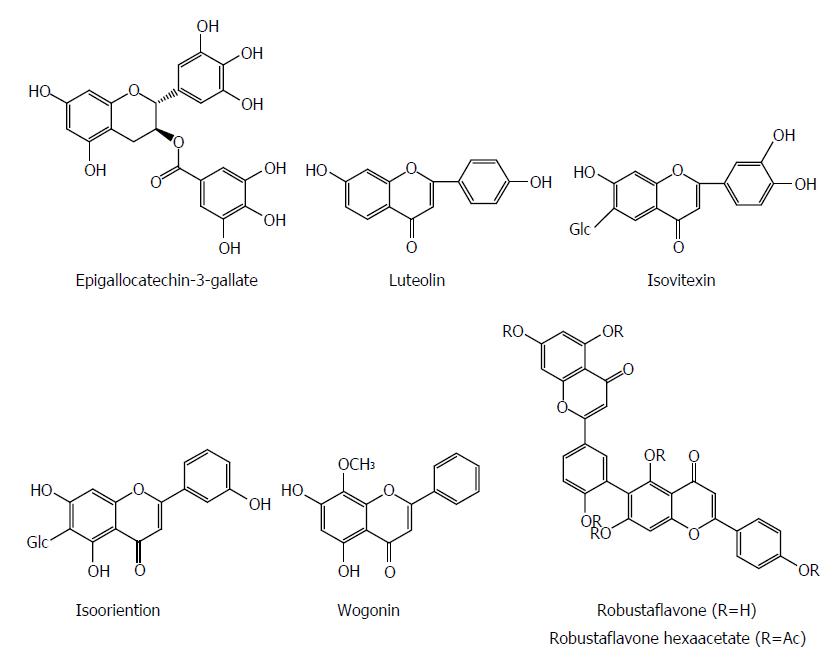
Flavonoids are a group of polyphenolic compounds containing a 15-carbon skeleton (C6-C3-C6), which consists of a heterocyclic ring (C) and two phenyl rings (A and B). Flavonoids have a wide range of pharmacological effects including antiviral activity. Epigallocatechin-3-gallate (EGCG) from green tea suppressed HBV entry into immortalized human primary hepatocytes at a concentration of 50 μmol/L by more than 80%[62]. EGCG effectively inhibited the HBsAg and HBeAg secretion in HepG2 2.2.15 cells in a dose and time dependent manner, whereas its effect was stronger than that of lamivudine; EGCG also reduced the extracellular HBV DNA level[63]. Sodium taurocholate cotransporting polypeptide (NTCP), a new-found receptor for HBV, is expressed in DMSO-differentiated HuS-E/2 cells. During HBV inoculation, EGCG significantly suppressed viral infection in both HA-NTCP-expressing Huh7 cells and DMSO-differentiated HuS-E/2 cells. Meanwhile, EGCG induced clathrin-dependent endocytosis of NTCP from the plasma membrane followed by lysosomal degradation. Additionly, EGCG suppressed the clathrin-mediated endocytosis of transferrin. However, no inhibitory effect of EGCG was observed on HBV virion secretion or genome replication in cells in vitro. Furthermore, EGCG also unaltered the HBV entry factor expression and virion characteristic. Finally, the potent inhibitory effect of EGCG on HBV entry was validated using four different genotypes, A to D, suggesting that it could be a potential agent for prevention of HBV reinfection[62].
Luteolin and isovitexin from Swertia yunnanensis showed inhibitory effects on the secretion of HBsAg (IC50 values of 0.10 and < 0.03 mmol/L) and HBeAg (IC50 values of 1.51 and 0.23 mmol/L), and exhibited a marked suppressive effect on HBV DNA replication (IC50 values < 0.01 and 0.05 mmol/L)[13]. Isooriention from Swertia mussotii displayed significant anti-HBV activities against HBsAg and HBeAg secretion with IC50 values of 0.79 and 1.12 mmol/L, as well as HBV DNA replication with an IC50 value of 0.02 mmol/L[41]. Wogonin from Scutellaria radix effectively inhibited the HBV antigen secretion with an IC50 value of 4 mg/mL for both HBsAg and HBeAg and also reduced HBV DNA level in hepG2.2.15 cells. DHBV DNA polymerase was dramatically suppressed by wogonin with an IC50 value of 0.57 mg/mL. In ducks with DHBV infection, wogonin decreased the plasma level of DHBV DNA with a 50% effective dose (ED50) of 5 mg/kg. In human HBV-transgenic mice, wogonin significantly reduced plasma HBsAg level[64]. Robustaflavone isolated from Rhus succedanea exhibited a potent inhibitory effect on HBV replication in HepG2.2.15 cells, with a 50% effective concentration (EC50) of 0.25 mM, and an SI of 153. Robustaflavone hexaacetate suppressed HBV replication with an EC50 of 0.73 mmol/L[65] (Figure 7).
Figure 7 Chemical structures of representative anti-hepatitis B virus natural flavonoids.
OTHERS
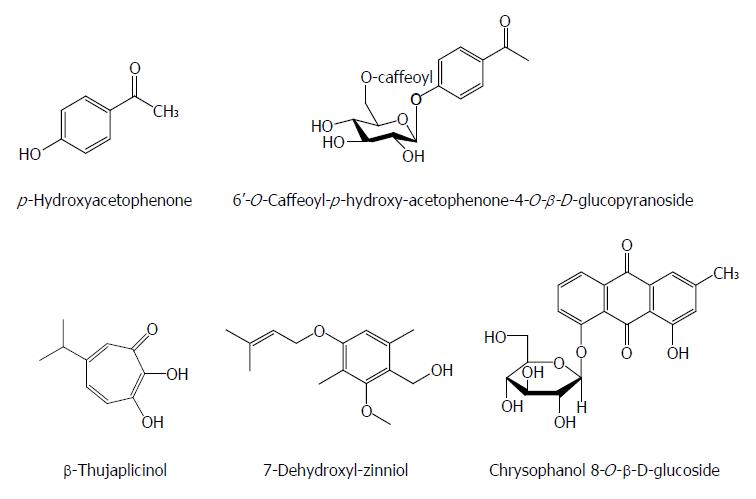
Except for the above-mentioned chemical classes, some other natural components also have anti-HBV activities. p-Hydroxyacetophenone (p-HAP) from Artemisia morrisonensis exhibited an inhibitory activity on HBsAg secretion and HBV DNA replication with IC50 values of 785.7 and 306.4 μmol/L, respectively. Additionly, 6’-O-Caffeoyl-p-hydroxy-acetophenone-4-O-β-D-glucopyranoside from A. capillaris possessed a significant suppressive effect on HBV DNA replication with an IC50 value of 8.0 μmol/L[66]. In the HBV-transfected Huh7 cells, the 2.4 kb preS RNA of HBV surface gene enhanced markedly relative to the 2.1 kb S RNA with p-HAP. p-HAP markedly augmented the promoter activity of HBV preS and specifically reduced endoplasmic reticulum (ER) stress related glucose-regulated protein 78 RNA/protein levels, but not those of glucose-regulated protein 94, in treated Huh7 cells. p-HAP also led to a marked intracellular accumulation of virus. Furthermore, treatment with thapsigargin (ER chaperone inducer) relieved the suppressive effect of p-HAP on the HBV DNA levels in the supernatant of HBV-expressed cells. Hence, the anti-HBV mechanism of p-HAP could involve the regulation of HBV surface gene expression and block the secretion of virion by interference with the ER stress pathway[67].
β-Thujaplicinol from the heartwood of Western Red Cedar trees (Thuja plicata, Thuja occidentalis and Chamaecyparis obtusaI) inhibited RNAseHs from HBV genotypes D and H in biochemical assays with IC50 values of 5.9 and 2.3 μmol/L, respectively. It blocked replication of HBV genotypes A and D in culture by inhibiting the RNAseH activity with an estimated EC50 of 5 μmol/L and a 50% cytotoxic concentration (CC50) of 10.1 mmol/L[68]. Chrysophanol 8-O-β-D-glucoside from Rheum palmatum L. showed a significant inhibitory effect on HBV DNA production and antigen expression with an IC50 value of 36.98 mg/mL on HBV DNA inhibition, and effectively suppressed HBV DNA polymerase activity[69]. 7-Dehydroxyl-zinniol from the culture of Alternaria solani, an endophytic fungal strain residing in the roots of Aconitum transsectum showed a moderate anti-HBV activity[70] (Figure 8).
Figure 8 Chemical structures of other anti-hepatitis B virus natural products.
CONCLUSION
Despite that several anti-HBV drugs and genetic engineering vaccines are available for patients with HBV, HBV infection is still a severe public health problem in the world. In fact, no effective drugs or therapeutic methods can cure hepatitis B so far. Therefore, it is urgent affairs to further intensify research and development of anti-HBV new drugs, especially non-nucleoside agents. Natural products with enormous molecular complexity and diversity offer a large opportunity for finding novel antiviral lead compounds or good candidates with unique anti-HBV mechanisms. In this review, the natural products with anti-HBV activity are classified and analysed according to their structure types including terpenes, lignans, phenolic acids, polyphenols, lactones, alkaloids and flavonoids. These anti-HBV natural products can be cited as promising leads or candidates.
The anti-HBV mechanisms of action of the compounds described above involve multiple aspects, and several natural compounds exhibit special antiviral characteristics and novel mode of action. Betulinic acid (a triterpene) from Pulsatilla chinensis reduces HBV replication by inhibiting the expression of SOD2 with subsequent overgeneration of mitochondrial ROS, with promising HBV clearance both in HBV-infected hepatocytes and in HBV-transgenic mice[7]. Helioxanthin (a lignan) from the heartwood of Taiwania cryptomerioides Hayata is a promising anti-HBV agent and acts through liver-specific transcriptional machinery for viral CP to suppress the gene expression of HBV and the production of viral particles in vitro[25]. The anti-HBV effects of 3,4-O-dicaffeoylquinic acid and 3,5-O-dicaffeoylquinic acid (a phenolic acid) from Laggera alata are probably associated with the up-regulation of HO-1 by decreasing HBV core protein stability, which blocks nuclear HBV cccDNA refill in HepG2.2.15 cells[35,36]. The mechanism of HBV inhibition by LPRP-Et-97543 (a polyphenol) from Liriope platyphylla might involve feedback control of the gene expression and DNA replication of HBV by viral proteins, which interferes with the NF-κB pathway in HBV-transfected Huh7 cells[39]. Curcumin (a polyphenol) suppresses the gene expression and DNA replication of HBV by down-regulation of PGC-1α, a starvation-induced protein that initiates the gluconeogenesis cascade and that can coactivate robustly HBV transcription in HBV expressing cells[40]. β-Thujaplicinol (a polyphenol) from the heartwood of Western Red Cedar trees inhibits HBV replication by blocking the activity of viral ribonuclease H in vitro[68]. Oxymatrine (a alkaloid) from Sophora japonica and Cepharanthine (a alkaloid) from Stephania cepharantha down-regulate Hsc70 mRNA expression at the post transcriptional level through destabilizing Hsc70 mRNA, and then inhibits HBV replication in cells[55,56]. Epigallocatechin-3-gallate (a flavonoid) from green tea, as a new HBV entry inhibitor, induced clathrin-mediated endocytosis of NTCP-HBV from the plasma membrane followed by lysosomal degradation and suppressed the clathrin-mediated endocytosis of transferrin in vitro[62]. The in vitro mechanism of HBV inhibition by p-Hydroxyacetophenone from Artemisia morrisonensis may involve the regulation of HBV surface gene expression and blocking the secretion of virions by interference with the ER stress signaling pathway[67].
In general, there are not many reports of novel mode of action or new targets of anti-HBV natural products. Additionly, although many natural products are reported to exhibit potent anti-HBV effects to date, they are limited to cell-based or transgenic mice-based studies. Unfortunatley, none of these have been added into the existing list of anti-HBV drugs. Given that many of these naturally originated compounds with various skeletons and diverse biological activities discussed above are already available, future efforts should be devoted to optimize and develop these lead compounds into potent anti-HBV agents for clinical application. Continued screening of natural products with anti-HBV activities may find some novel skeletons that possess new mechanism of action, but it may be difficult to directly lead to the discovery of any clinical candidate compounds.
ACKNOWLEDGMENTS
The author is grateful to Prof. Chang-Deng Hu at College of Pharmacy, Purdue University, United States, for kindly performing the revising and English language editing job of this review.