Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.112
Peer-review started: April 29, 2015
First decision: July 14, 2015
Revised: July 29, 2015
Accepted: October 13, 2015
Article in press: October 13, 2015
Published online: January 7, 2016
Processing time: 248 Days and 11.6 Hours
Cirrhotic cardiomyopathy has been defined as a chronic cardiac dysfunction in patients with cirrhosis characterized by impaired contractile responsiveness to stress and/or altered diastolic relaxation with electrophysiological abnormalities in the absence of other known cardiac disease. Non-invasive cardiovascular imaging modalities play a major role in unmasking systolic and diastolic dysfunction in patients with cirrhosis. Echocardiography has been the most commonly used modality for assessing myocardial function in these patients. Conventional echocardiographic indices rely on several assumptions that may limit their applicability in patients with a hyperdynamic circulation. Newer imaging modalities may contribute to a more accurate diagnosis of cardiovascular abnormalities in cirrhotic patients, thereby influencing clinical management. We aimed to review the different non-invasive imaging technologies currently used for assessing left ventricular systolic and diastolic function in cirrhosis, as well as to describe new imaging modalities with potential clinical applicability in the near future.
Core tip: Cardiac dysfunction has been documented in cirrhosis. Conventional non-invasive methods are frequently used to detect abnormalities. Newer imaging techniques have been developed and can contribute to a more accurate diagnosis of cirrhotic cardiomyopathy. However, it is essential to understand the strengths and limitations of every modality in order to correctly interpret the results. Currently applied methods for assessing left ventricular myocardial function as well as future perspectives are reviewed.
- Citation: Sampaio F, Pimenta J. Left ventricular function assessment in cirrhosis: Current methods and future directions. World J Gastroenterol 2016; 22(1): 112-125
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.112
Cardiovascular dysfunction in patients with cirrhosis has been recognized for more than sixty years[1,2]. These abnormalities were initially attributed exclusively to the effects of alcohol; however findings from animal and clinical studies performed during the last decades, lent support to the existence of a specific cardiomyopathy in cirrhosis, irrespective of its etiology[3]. Based on these reports, in 2005, cirrhotic cardiomyopathy was defined as a “chronic cardiac dysfunction in patients with cirrhosis characterized by impaired contractile responsiveness to stress and/or altered diastolic relaxation with electrophysiological abnormalities in the absence of other known cardiac disease”. Several criteria for diagnosing this entity were proposed, relying mostly on non-invasive assessment of myocardial function[4-6].
In the last years, the role of cardiac imaging in the management of cardiovascular diseases has been increasingly important. The development of newer imaging modalities resulted in an improvement in diagnostic accuracy and prognostic information thereby influencing clinical management of several cardiac disorders. Although the role of these methods in the diagnostic work-up of cirrhotic cardiomyopathy is still uncertain, several recent studies suggested their utility in unmasking myocardial dysfunction in this population[7,8]. The aim of this paper is to review the different non-invasive imaging technologies currently used for assessing left ventricular systolic and diastolic function in cirrhosis, as well as to describe new imaging modalities with potential clinical applicability in the near future.
Ejection fraction (EF) is the most widely used parameter of global left ventricular systolic function. It is calculated from end-systolic and end-diastolic volumes, which can be estimated by different methods.
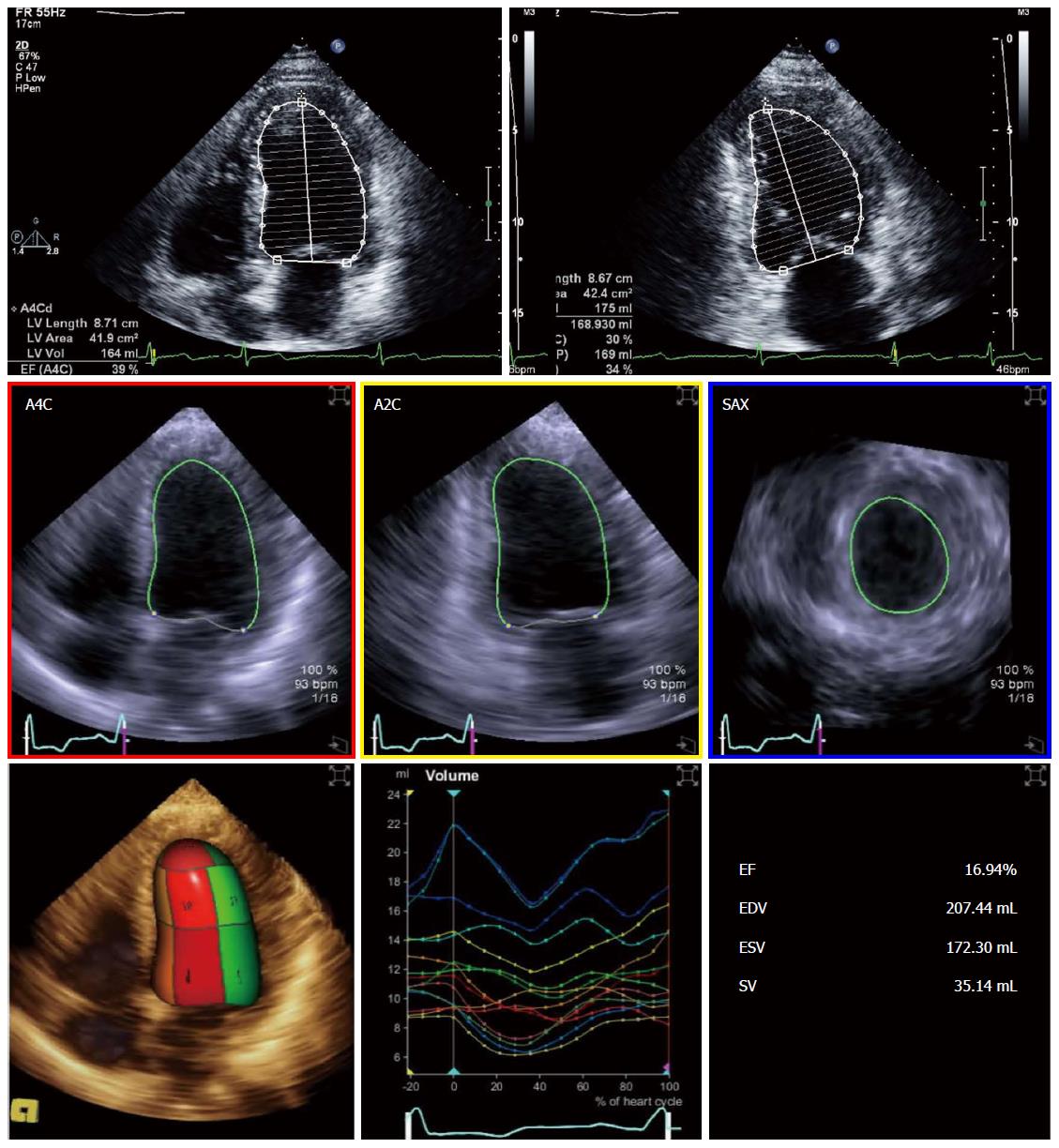
Echocardiography was the most commonly used modality for assessing EF in studies in cirrhosis[9-11]. The disk summation method in two orthogonal planes (modified Simpson’s rule; Figure 1) is still the method of choice for calculating ejection fraction in recent recommendations[12]. Volumes and EF derived from linear dimensions may be very inaccurate in several conditions and should not be used. Three-dimensional echocardiography (3DE) is becoming more available and increasingly used in clinical practice (Figure 1). Left ventricular volumes derived from 3DE do not rely on geometrical assumptions and may be more accurate and reproducible, when compared to cardiac magnetic resonance (CMR)[13-16]. Fully automated software is commercially available allowing fast online measurements and better reproducibility. However, 3DE is highly dependent on image quality and has lower temporal resolution than two-dimensional echocardiography (2DE); some ultrasound systems still require electrocardiogram gating and breath hold making it more prone to artifacts. These issues may limit its applicability in cirrhotic patients with tachycardia and/or unable to hold their breath[17]. Volumes obtained with 3DE are larger than 2DE-derived volumes and should not be used inter-changeably in serial measurements[12]. To the best of our knowledge, there are no studies comparing 3DE with 2DE or CMR in cirrhosis; hence, its validity in this specific setting remains unproven.
Cardiac magnetic resonance has evolved into the reference standard methodology for assessment of cardiac morphology and volumes[18,19]. Such as echocardiography it provides morphological and functional information, does not use ionizing radiation and has a better spatial resolution than 2DE. Volumes obtained with CMR are also larger than echocardiography-derived values[16,20-22]. The widespread use of this method is limited by availability and cost. Its use in cirrhotic patients may also be hampered by the need of repeated end-expiratory apneas for image acquisition and tachycardia (decreasing temporal resolution) or irregular heart rates. Current development of improved free breathing and short breath-hold sequences may soften some of these problems in the near future[23,24].
Computed tomography and single photon emission computed tomography (SPECT) are alternative modalities for calculating EF and some studies in cirrhosis have used SPECT to quantify LV function[25,26]. However, these methods are limited by low temporal resolution and radiation.
According to the current consensus, an EF of less than 52% in men and 54% in women, using 2DE, suggests systolic dysfunction. Reference values for 3DE may be different since there is less published data on normal subjects[12]. Higher cut-off values may also apply to CMR[27]. In the 2005 World Congress of Gastroenterology, a resting EF < 55% was proposed as a diagnostic criteria of systolic dysfunction. However, since EF is highly dependent on loading conditions, a higher cut-off value may need to be considered in patients with cirrhosis due to the peripheral vasodilatation and decreased afterload. This probably explains the finding of normal resting ejection fraction in the majority of the studies in cirrhosis[5,28-30].
Although widely used in systolic function assessment, EF has several limitations. Ejection fraction is not an index of contractility and depends on loading conditions, heart rate and valvular function[31]. Besides, EF relies on accurate tracing of endocardial borders and the inter-observer agreement in different measurements can be modest[32].
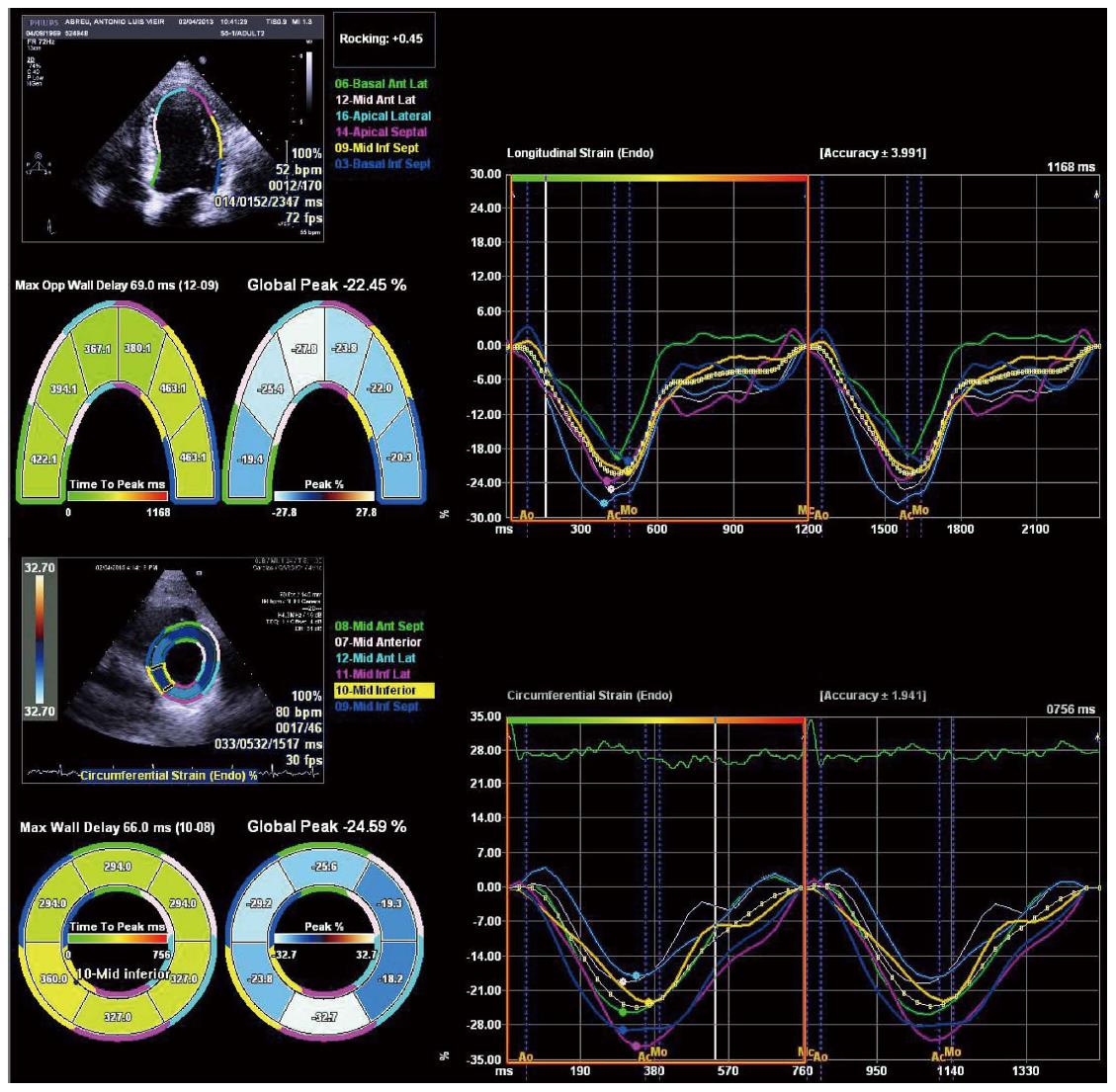
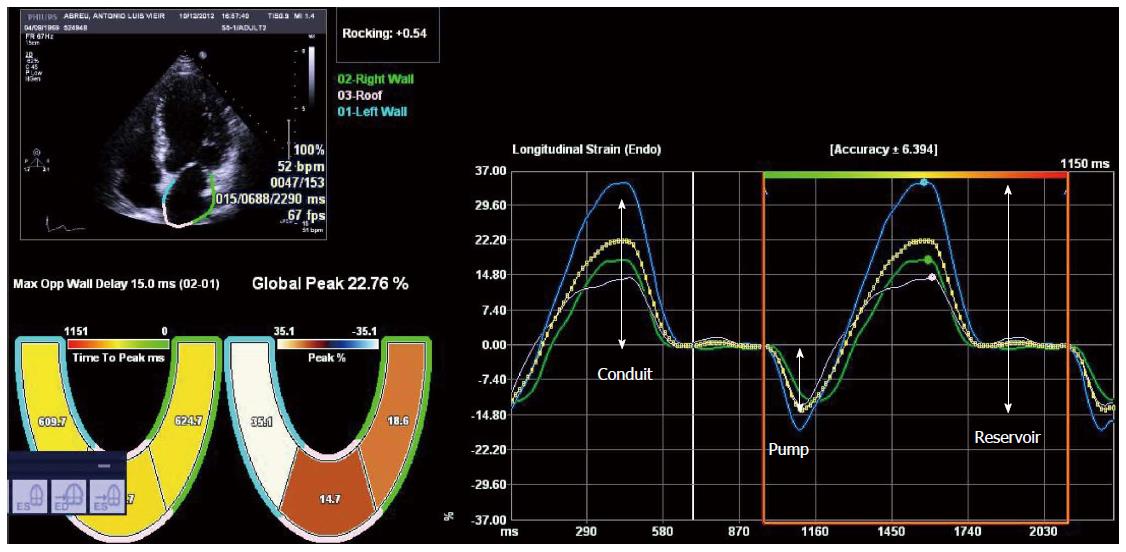
Tissue Doppler imaging (TDI) and speckle tracking echocardiography are newer imaging modalities able to objectively quantify regional and global LV function. Deformation is computed from the spatial gradients of myocardial velocities (using TDI) or from the relative position of “speckles” within a myocardial region, along the cardiac cycle (speckle tracking). Strain (ε) is the fractional change in length of a myocardial segment relative to its original dimension, and is expressed as a percentage (%). Strain rate (SR) is the change in strain over a period of time and is usually expressed as 1/s or s-1. Both TDI and speckle tracking have strengths and weaknesses, that are described in detail elsewhere[33,34]. Briefly, TDI is mainly limited by angle-dependency (making it unsuitable to assess circumferential motion) and by artifacts, while speckle tracking has lower temporal resolution, which may limit its use in patients with higher heart rates (such as patients with decompensated cirrhosis) or in the assessment of short-lived events. Speckle tracking has the advantage of being able to quantify all the components of myocardial mechanics (longitudinal, circumferential and radial motion/deformation as well as rotation and torsion; Figure 2) within the image plane. It may also be more reproducible than TDI[35-39]. There is a large body of evidence supporting the clinical utility of myocardial deformation analysis in cardiovascular disease. These methods were successfully used in identifying subclinical myocardial dysfunction in different settings, in the improvement of the performance of stress echocardiography for diagnosing coronary artery disease, in the assessment of therapeutic interventions in cardiomyopathies and in the prediction of outcomes[40-48]. Left ventricular longitudinal dysfunction has also been previously documented in patients with cirrhosis, at rest[7,8]. Longitudinally oriented subendocardial fibers are more susceptible to damage than the radial fibers of the middle myocardium layer and this probably accounts for these findings. Loading conditions also influence strain and strain rate[49-51] and this should be taken into account when interpreting strain results in patients with cirrhosis, since the decreased afterload secondary to peripheral vasodilatation improves strain values. This may explain previous findings of strain values within the normal range in patients with decompensated cirrhosis as well as similar strain between hospitalized and ambulatory patients[7,8,52].
The presence of an abnormal response to exercise or pharmacological stress has been reported as a feature of cirrhotic cardiomyopathy[26,53-58]. Limitations of classical non-invasive parameters of systolic function also apply under stress (particularly pharmacological stress). Deformation may also be assessed during stress and abnormalities in the response of myocardial deformation to exercise have been found in heart failure patients, using speckle tracking[59]. The improvement in longitudinal strain under low-dose dobutamine may be lower in patients with cirrhosis when compared to a control group suggesting that longitudinal dysfunction can contribute to the inotropic incompetence previously documented in these patients[60].
A major limitation to the widespread use of speckle tracking in routine clinical practice is the significant variability that exists among vendors and software packages that prevent the definition of normal reference values. Likewise, abnormal strain variations in follow-up echocardiographic studies cannot be safely determined when using different analysis software[61,62]. An effort to implement standardization in strain imaging is currently underway and will hopefully reduce intervenor variability of strain[63].
Diastolic dysfunction (DD) has been reported as a common finding in patients with cirrhosis. Abnormalities in membrane receptor function and intracellular signaling pathways, as well as changes in contractile proteins and extracellular matrix composition are probably involved in the pathogenesis of DD in cirrhosis[64-68].
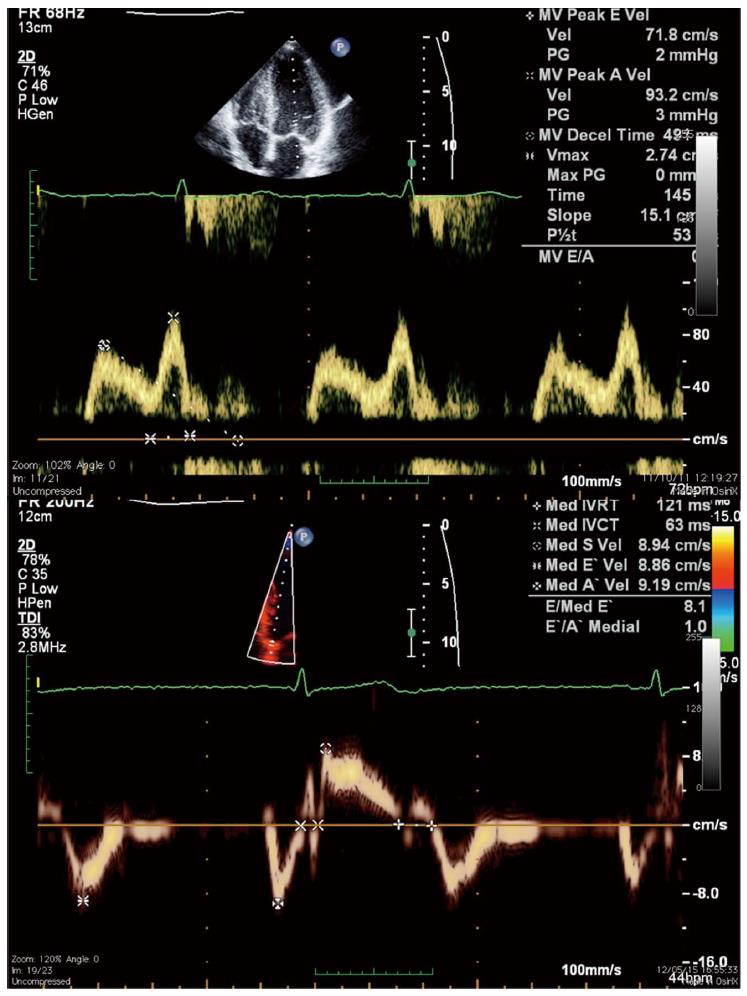
Non-invasive assessment of DD has classically relied on the echocardiographic analysis of mitral inflow pattern using pulsed-wave Doppler (Figure 3). In the presence of mild DD, early diastolic filling is decreased as a result of delayed LV relaxation and atrial contraction becomes a more important contributor to left ventricular filling. This impaired relaxation pattern (grade I DD) is characterized by a decrease in E wave velocity, prolongation of E-wave deceleration time, and an increase in A wave velocity resulting in an inverted E/A ratio (< 1). With worsening DD, the increase in left atrial pressure restores the early diastolic pressure gradient, increasing E wave velocity; on the other hand, LV pressure at the end of diastole is higher, so that the contribution of atrial contraction (A wave) is reduced. So, in grade II DD, mitral flow is similar to that in the normal individual (hence called pseudonormal pattern), with the E wave again greater than the A wave. The Valsalva maneuver, which decreases preload, can be used to differentiate a normal from a pseudonormal pattern since the latter is changed to an impaired relaxation pattern during the maneuver. With even more severe DD (grade III), there is a marked elevation in LA and LV pressures and most filling occurs during early diastole. In this restrictive pattern, E wave velocity is increased, E wave deceleration time is very short (< 160 mseg) and the E/A ratio is > 1.5.
Diastolic dysfunction, as defined in the 2005 World Congress of Gastroenterology (E/A ratio < 1.0, deceleration time > 200 ms and isovolumetric relaxation time > 80 ms, is highly prevalent in patients with cirrhosis[10,69,70]. An association between liver disease severity and DD and an improvement in DD after paracentesis has also been reported. Earlier studies also suggested that DD was related to the liver disease severity and improved after paracentesis[10,71]. More importantly, an association between an E/A ratio < 1 and increased mortality and slower mobilization of ascites after transjugular intrahepatic portosystemic shunt (TIPS) insertion has been suggested[72,73].
However, mitral inflow-based assessment of diastolic function has several limitations that should be taken into account[74-78]. Different loading and heart rate can significantly change the E/A ratio and DT, even in normal subjects[79-81]. This can be a major issue in cirrhotic patients, due to the blood pooling in the splanchnic bed and reduced preload which, along with faster heart rates, may result in lower E/A ratios, regardless of the presence of impaired relaxation. This can also contribute to the association of this pattern with mortality since there is usually a direct relation between hyperdynamic circulation and disease severity[82]. Besides, impaired relaxation has a better prognosis than more advanced stages of DD, both in the general population and in heart failure patients[83,84].
Acknowledging the pitfalls of mitral-flow variables, recommendations for evaluating left ventricular diastolic function by echocardiography have been issued in 2009[80]. According to this consensus document, tissue-Doppler diastolic velocities of the mitral annulus play a major role on DD assessment (Figure 3). Early diastolic annular velocity (E’) is a sensitive parameter of myocardial relaxation[85-87]. E’ is also a surrogate marker of the volume that enters the left ventricle during early diastole. Since E wave velocity reflects the pressure gradient between the left atrium and left ventricle, the E/E’ ratio represents the volume that enters the ventricle for a given LA-LV pressure gradient. A high E/E’ ratio means that there is a small volume change between the two chambers despite a high pressure gradient, reflecting diastolic dysfunction with an increase in left ventricular filling pressure[88,89].
Using a tissue-Doppler based approach, some studies have found a lower prevalence of DD in cirrhotic patients; furthermore, the prevalence of DD did not differ between disease stages[8,52,90,91]. The association between DD and prognosis also remains controversial, with conflicting results reported in different studies[52,92-94].
Although a clear advance in the diagnostic workup of DD, tissue-Doppler based parameters are not flawless, and E/E’ may not adequately reflect left ventricular filling pressures in different settings. Hence, the diagnosis of DD should not rely on a single measurement and a multi-parameter approach (including tissue-Doppler mitral annulus velocities, pulsed-waved Doppler mitral inflow and pulmonary veins velocities, and left atrial volume) is usually recommended[80,95]. Unfortunately, the use of these more complex algorithms results in some variability in the classification of DD, even among expert echocardiographers[96,97]. This probably explains some of the differences in the prevalence of DD among more recent studies in cirrhosis.
Speckle tracking echocardiography can also provide information regarding diastolic function, through the analysis of strain rate during isovolumic relaxation, early filling and late diastole[33,85]. However, the lower temporal resolution of speckle tracking can limit the analysis of fast events (such as those during diastole), and there is no evidence to support its superiority over the already established TDI parameters[98].
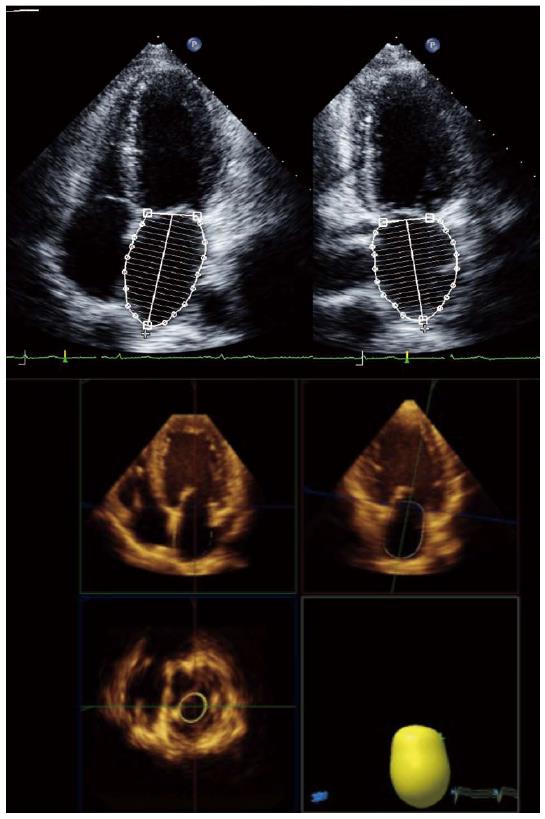
Left atrial volume, preferably indexed to body surface area, is a mandatory measurement for the assessment of diastolic function. Left atrial volume index (LAVI) is strongly associated with the severity and duration of DD reflecting the cumulative effects of elevated filling pressures over time[83]. As for LV volumes and EF, the disk summation algorithm is the recommended method for calculating LAVI (Figure 4)[12]. An increase in LAVI has also been reported in cirrhosis, which has been interpreted as a marker of DD in these patients[29]. However, dilated atria may also be seen in patients with volume overload, anemia or other high-output states such as cirrhosis. We have previously found that, in a cohort of patients with cirrhosis of several etiologies, LAVI was associated with stroke volume, LV end-diastolic volume and hemoglobin and not with diastolic dysfunction, suggesting that atrial enlargement in cirrhosis may be related to loading conditions and should not be used as a marker of DD[99]. Speckle tracking echocardiography can also be used in the assessment of left atrial phasic volumes and deformation, providing information about left atrial function (Figure 5)[100,101]. Atrial dysfunction may be involved in the pathophysiology of heart failure with preserved ejection fraction and may be associated with symptom onset[102-105]. Left atrial longitudinal strain correlates better with LV filling pressures than LA volume or other echocardiographic indices such as the E/E’ ratio[106,107]. A decrease in LA longitudinal strain has also been reported in patients with cirrhosis, and this seems to relate to abnormal relaxation in these patients[99].
Magnetic resonance can be used to study diastolic function[108]. Both mitral inflow and pulmonary vein velocities can easily be determined and a good correlation with echocardiography-derived indices has been demonstrated[109]. However, like echocardiographic variables, mitral inflow velocities calculated by magnetic resonance are heavily dependent on loading conditions and heart rate. Availability issues, costs, and lower temporal resolution (when compared to echocardiography) also hamper cardiac magnetic resonance limiting its use to patients in which echocardiography is non-diagnostic.
Magnetic resonance can also be used to detect myocardial fibrosis through the quantification of late gadolinium enhancement (LGE). A diffuse pattern of intramyocardial LGE has been previously described in patients with cirrhosis; the authors did not analyze a possible relation between LGE extent and DD indices[110]. The use of gadolinium-based contrast agents should be very cautious in patients with renal failure due to case-reports of nephrogenic systemic fibrosis induced by older linear gadolinium chelates[111]. This may be an issue in patients with advanced cirrhosis and hepatorenal syndrome.
Three-dimensional echocardiography will improve to allow the acquisition of single-heartbeat full-volume data sets with higher temporal and spatial resolution. Chamber volume quantification will become quicker, more simple and reproducible with the use of fully automated software (Figures 1 and 4). Three-dimensional speckle tracking can analyze the deformation of the heart from a single data set. Although limited by a lower temporal resolution, 3D speckle tracking use will result in a more complete and more accurate analysis of myocardial function[112].
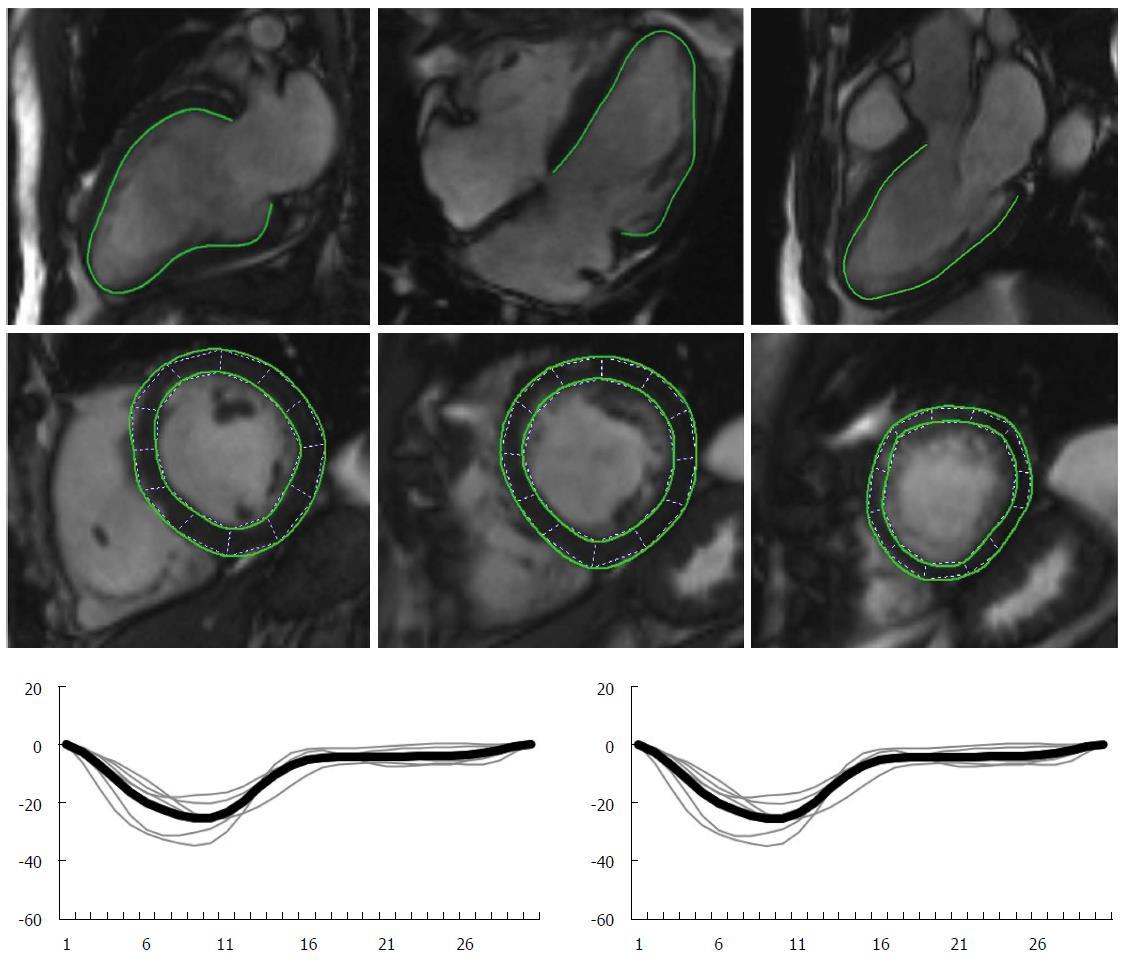
Myocardial deformation can also be quantified with CMR. Systolic and diastolic strain rate, atrial deformation parameters and twisting/untwisting are indices of myocardial function that can be assessed with CMR[113-115]. Newer technologies, like feature tracking (a technique analogous to speckle tracking, which tracks tissue voxel motion of CMR cine images) will result in faster scans and post-processing analysis times (Figure 6).
T1 mapping is a new CMR application that quantifies T1 relaxation times for each myocardial pixel. With the use of gadolinium-based contrast agent, the extracellular volume fraction of the myocardium - a surrogate marker of the size of extracellular matrix - can also be quantified. This may allow for the detection and quantification of diffuse myocardial involvement in different disease processes. A detailed description of technical aspects of these new modalities can be found elsewhere[116,117]. T1 mapping has been a field of intensive research in the last few years. Its usefulness in diagnosing infiltrative diseases (such has amyloidosis, Fabry disease or iron overload) as well as the diagnostic and prognostic value of fibrosis quantification in heart failure, cardiomyopathies, myocarditis or cardiac involvement in systemic diseases has been suggested in several studies[118-132]. Although never previously reported, its use in cirrhosis, conceptually, appears to be very promising. However, issues regarding standardization still preclude clinical use of T1 mapping and this technique is mainly in the research field[116].
In conclusion, these newer CMR technologies, along with classical volume determination, flow analysis and late enhancement quantification will allow a comprehensive evaluation of myocardial function in a single exam, not dependent on a good “acoustic window” for image acquisition and with no radiation.
A large number of parameters derived from different imaging modalities are currently available for the assessment of left ventricular function. Newer technologies will become widely available in the near future, allowing a detailed evaluation of myocardial function, and improving the diagnostic accuracy of the tests. However one must be aware of the limitations of every parameter in order to correctly interpret the results. On the other hand, the use of these new methods in cirrhosis has been limited to a few studies and further work is needed to evaluate their diagnostic performance and, more importantly, the impact on clinical management of this specific group of patients.
P- Reviewer: Hollingsworth KG S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 398] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Shorr E, Zweifach BW, Furchgott RF, Baez S. Hepatorenal factors in circulatory homeostasis. IV. Tissue origins of the vasotropic principles, VEM and VDM, which appear during evolution of hemorrhagi and tourniquet shock. Circulation. 1951;3:42-79. [PubMed] |
| 3. | Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, Afeltra A, Sanyal AJ. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Alqahtani SA, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Semin Liver Dis. 2008;28:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Kazankov K, Holland-Fischer P, Andersen NH, Torp P, Sloth E, Aagaard NK, Vilstrup H. Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int. 2011;31:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Sampaio F, Pimenta J, Bettencourt N, Fontes-Carvalho R, Silva AP, Valente J, Bettencourt P, Fraga J, Gama V. Systolic and diastolic dysfunction in cirrhosis: a tissue-Doppler and speckle tracking echocardiography study. Liver Int. 2013;33:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Keller H, Bezjak V, Stegaru B, Buss J, Holm E, Heene DL. Ventricular function in cirrhosis and portasystemic shunt: a two-dimensional echocardiographic study. Hepatology. 1988;8:658-662. [PubMed] |
| 10. | Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, Bolla GB, Roffi L, Failla M, Grassi G. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro S, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3897] [Cited by in RCA: 5487] [Article Influence: 548.7] [Reference Citation Analysis (0)] |
| 13. | Mor-Avi V, Jenkins C, Kühl HP, Nesser HJ, Marwick T, Franke A, Ebner C, Freed BH, Steringer-Mascherbauer R, Pollard H. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging. 2008;1:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Dorosz JL, Lezotte DC, Weitzenkamp DA, Allen LA, Salcedo EE. Performance of 3-dimensional echocardiography in measuring left ventricular volumes and ejection fraction: a systematic review and meta-analysis. J Am Coll Cardiol. 2012;59:1799-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 15. | Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Schmidt F, Galuschky C, Schummers G, Lang RM. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 18. | Attili AK, Schuster A, Nagel E, Reiber JH, van der Geest RJ. Quantification in cardiac MRI: advances in image acquisition and processing. Int J Cardiovasc Imaging. 2010;26 Suppl 1:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Lima JA, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Gutiérrez-Chico JL, Zamorano JL, Pérez de Isla L, Orejas M, Almería C, Rodrigo JL, Ferreirós J, Serra V, Macaya C. Comparison of left ventricular volumes and ejection fractions measured by three-dimensional echocardiography versus by two-dimensional echocardiography and cardiac magnetic resonance in patients with various cardiomyopathies. Am J Cardiol. 2005;95:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004;44:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 412] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 22. | Lee D, Fuisz AR, Fan PH, Hsu TL, Liu CP, Chiang HT. Real-time 3-dimensional echocardiographic evaluation of left ventricular volume: correlation with magnetic resonance imaging--a validation study. J Am Soc Echocardiogr. 2001;14:1001-1009. [PubMed] |
| 23. | Steeden JA, Atkinson D, Hansen MS, Taylor AM, Muthurangu V. Rapid flow assessment of congenital heart disease with high-spatiotemporal-resolution gated spiral phase-contrast MR imaging. Radiology. 2011;260:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Krishnamurthy R, Pednekar A, Atweh LA, Vogelius E, Chu ZD, Zhang W, Maskatia S, Masand P, Morris SA, Krishnamurthy R. Clinical validation of free breathing respiratory triggered retrospectively cardiac gated cine balanced steady-state free precession cardiovascular magnetic resonance in sedated children. J Cardiovasc Magn Reson. 2015;17:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Dahl EK, Møller S, Kjær A, Petersen CL, Bendtsen F, Krag A. Diastolic and autonomic dysfunction in early cirrhosis: a dobutamine stress study. Scand J Gastroenterol. 2014;49:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Krag A, Bendtsen F, Mortensen C, Henriksen JH, Møller S. Effects of a single terlipressin administration on cardiac function and perfusion in cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775-782. [PubMed] |
| 28. | Gassanov N, Caglayan E, Semmo N, Massenkeil G, Er F. Cirrhotic cardiomyopathy: a cardiologist’s perspective. World J Gastroenterol. 2014;20:15492-15498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 30. | Møller S, Hove JD, Dixen U, Bendtsen F. New insights into cirrhotic cardiomyopathy. Int J Cardiol. 2013;167:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3411] [Cited by in RCA: 3554] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 32. | Pickett CA, Cheezum MK, Kassop D, Villines TC, Hulten EA. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: a meta-analysis. Eur Heart J Cardiovasc Imaging. 2015;16:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 714] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 34. | Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007;5:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 35. | Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur Heart J. 2007;28:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, Abraham TP, Belohlavek M. Two-dimensional strain--a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005;18:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 37. | Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 499] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 38. | Ng AC, Tran da T, Newman M, Allman C, Vidaic J, Kadappu KK, Boyd A, Thomas L, Leung DY. Comparison of myocardial tissue velocities measured by two-dimensional speckle tracking and tissue Doppler imaging. Am J Cardiol. 2008;102:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351-369; quiz 453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 789] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 40. | Andersen NH, Poulsen SH, Eiskjaer H, Poulsen PL, Mogensen CE. Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with Type II diabetes mellitus: a Doppler tissue tracking and strain rate echocardiography study. Clin Sci (Lond). 2003;105:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Bjork Ingul C, Rozis E, Slordahl SA, Marwick TH. Incremental value of strain rate imaging to wall motion analysis for prediction of outcome in patients undergoing dobutamine stress echocardiography. Circulation. 2007;115:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Cardim N, Oliveira AG, Longo S, Ferreira T, Pereira A, Reis RP, Correia JM. Doppler tissue imaging: regional myocardial function in hypertrophic cardiomyopathy and in athlete’s heart. J Am Soc Echocardiogr. 2003;16:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Faber L, Prinz C, Welge D, Hering D, Butz T, Oldenburg O, Bogunovic N, Horstkotte D. Peak systolic longitudinal strain of the lateral left ventricular wall improves after septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: a follow-up study using speckle tracking echocardiography. Int J Cardiovasc Imaging. 2011;27:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Jasaityte R, Dandel M, Lehmkuhl H, Hetzer R. Prediction of short-term outcomes in patients with idiopathic dilated cardiomyopathy referred for transplantation using standard echocardiography and strain imaging. Transplant Proc. 2009;41:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Jurcut R, Wildiers H, Ganame J, D’hooge J, De Backer J, Denys H, Paridaens R, Rademakers F, Voigt JU. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Poulsen SH, Andersen NH, Heickendorff L, Mogensen CE. Relation between plasma amino-terminal propeptide of procollagen type III and left ventricular longitudinal strain in essential hypertension. Heart. 2005;91:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Voigt JU, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, Platsch G, Kuwert T, Daniel WG, Flachskampf FA. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107:2120-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 268] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Weidemann F, Jung P, Hoyer C, Broscheit J, Voelker W, Ertl G, Störk S, Angermann CE, Strotmann JM. Assessment of the contractile reserve in patients with intermediate coronary lesions: a strain rate imaging study validated by invasive myocardial fractional flow reserve. Eur Heart J. 2007;28:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Burns AT, La Gerche A, D’hooge J, MacIsaac AI, Prior DL. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr. 2010;11:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 50. | Burns AT, La Gerche A, Prior DL, Macisaac AI. Left ventricular torsion parameters are affected by acute changes in load. Echocardiography. 2010;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Bijnens BH, Cikes M, Claus P, Sutherland GR. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiogr. 2009;10:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Nazar A, Guevara M, Sitges M, Terra C, Solà E, Guigou C, Arroyo V, Ginès P. LEFT ventricular function assessed by echocardiography in cirrhosis: relationship to systemic hemodynamics and renal dysfunction. J Hepatol. 2013;58:51-57. [PubMed] |
| 53. | Bernardi M, Rubboli A, Trevisani F, Cancellieri C, Ligabue A, Baraldini M, Gasbarrini G. Reduced cardiovascular responsiveness to exercise-induced sympathoadrenergic stimulation in patients with cirrhosis. J Hepatol. 1991;12:207-216. [PubMed] |
| 54. | Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, Hayes PC. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326-332. [PubMed] |
| 55. | Kelbaek H, Rabøl A, Brynjolf I, Eriksen J, Bonnevie O, Godtfredsen J, Munck O, Lund JO. Haemodynamic response to exercise in patients with alcoholic liver cirrhosis. Clin Physiol. 1987;7:35-41. [PubMed] |
| 56. | Laffi G, Barletta G, La Villa G, Del Bene R, Riccardi D, Ticali P, Melani L, Fantini F, Gentilini P. Altered cardiovascular responsiveness to active tilting in nonalcoholic cirrhosis. Gastroenterology. 1997;113:891-898. [PubMed] |
| 57. | Limas CJ, Guiha NH, Lekagul O, Cohn JN. Impaired left ventricular function in alcoholic cirrhosis with ascites. Ineffectiveness of ouabain. Circulation. 1974;49:754-760. [PubMed] |
| 58. | Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268-275. [PubMed] |
| 59. | Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 60. | Sampaio F, Lamata P, Bettencourt N, Alt SC, Ferreira N, Kowallick JT, Pimenta J, Kutty S, Fraga J, Steinmetz M. Assessment of cardiovascular physiology using dobutamine stress cardiovascular magnetic resonance reveals impaired contractile reserve in patients with cirrhotic cardiomyopathy. J Cardiovasc Magn Reson. 2015;17:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Nelson MR, Hurst RT, Raslan SF, Cha S, Wilansky S, Lester SJ. Echocardiographic measures of myocardial deformation by speckle-tracking technologies: the need for standardization? J Am Soc Echocardiogr. 2012;25:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Marwick TH. Consistency of myocardial deformation imaging between vendors. Eur J Echocardiogr. 2010;11:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 63. | Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 846] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 64. | Ceolotto G, Papparella I, Sticca A, Bova S, Cavalli M, Cargnelli G, Semplicini A, Gatta A, Angeli P. An abnormal gene expression of the beta-adrenergic system contributes to the pathogenesis of cardiomyopathy in cirrhotic rats. Hepatology. 2008;48:1913-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Gerbes AL, Remien J, Jüngst D, Sauerbruch T, Paumgartner G. Evidence for down-regulation of beta-2-adrenoceptors in cirrhotic patients with severe ascites. Lancet. 1986;1:1409-1411. [PubMed] |
| 66. | Glenn TK, Honar H, Liu H, ter Keurs HE, Lee SS. Role of cardiac myofilament proteins titin and collagen in the pathogenesis of diastolic dysfunction in cirrhotic rats. J Hepatol. 2011;55:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Lee SS, Marty J, Mantz J, Samain E, Braillon A, Lebrec D. Desensitization of myocardial beta-adrenergic receptors in cirrhotic rats. Hepatology. 1990;12:481-485. [PubMed] |
| 68. | Ma Z, Miyamoto A, Lee SS. Role of altered beta-adrenoceptor signal transduction in the pathogenesis of cirrhotic cardiomyopathy in rats. Gastroenterology. 1996;110:1191-1198. [PubMed] |
| 69. | Finucci G, Desideri A, Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Left ventricular diastolic function in liver cirrhosis. Scand J Gastroenterol. 1996;31:279-284. [PubMed] |
| 70. | Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 71. | Wong F, Liu P, Lilly L, Bomzon A, Blendis L. Role of cardiac structural and functional abnormalities in the pathogenesis of hyperdynamic circulation and renal sodium retention in cirrhosis. Clin Sci (Lond). 1999;97:259-267. [PubMed] |
| 72. | Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, Meregaglia D, Nicolini A. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 73. | Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 74. | Cahill JM, Horan M, Quigley P, Maurer B, McDonald K. Doppler-echocardiographic indices of diastolic function in heart failure admissions with preserved left ventricular systolic function. Eur J Heart Fail. 2002;4:473-478. [PubMed] |
| 75. | Caruana L, Davie AP, Petrie M, McMurray J. Diagnosing heart failure. Eur Heart J. 1999;20:393. [PubMed] |
| 76. | Palmieri V, Innocenti F, Pini R, Celentano A. Reproducibility of Doppler echocardiographic assessment of left ventricular diastolic function in multicenter setting. J Am Soc Echocardiogr. 2005;18:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Petrie MC, Hogg K, Caruana L, McMurray JJ. Poor concordance of commonly used echocardiographic measures of left ventricular diastolic function in patients with suspected heart failure but preserved systolic function: is there a reliable echocardiographic measure of diastolic dysfunction? Heart. 2004;90:511-517. [PubMed] |
| 78. | Thomas MD, Fox KF, Wood DA, Gibbs JS, Coats AJ, Henein MY, Poole-Wilson PA, Sutton GC. Echocardiographic features and brain natriuretic peptides in patients presenting with heart failure and preserved systolic function. Heart. 2006;92:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Choong CY, Herrmann HC, Weyman AE, Fifer MA. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800-808. [PubMed] |
| 80. | Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1513] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 81. | Thomas JD, Choong CY, Flachskampf FA, Weyman AE. Analysis of the early transmitral Doppler velocity curve: effect of primary physiologic changes and compensatory preload adjustment. J Am Coll Cardiol. 1990;16:644-655. [PubMed] |
| 82. | Møller S, Henriksen JH, Bendtsen F. Extrahepatic complications to cirrhosis and portal hypertension: haemodynamic and homeostatic aspects. World J Gastroenterol. 2014;20:15499-15517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 83. | Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 84. | Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation. 1994;90:2772-2779. [PubMed] |
| 85. | Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278-285. [PubMed] |
| 86. | Oki T, Tabata T, Yamada H, Wakatsuki T, Shinohara H, Nishikado A, Iuchi A, Fukuda N, Ito S. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol. 1997;79:921-928. [PubMed] |
| 87. | Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474-480. [PubMed] |
| 88. | Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, Nagueh SF. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 352] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 89. | Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788-1794. [PubMed] |
| 90. | Karagiannakis DS, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas SD. Frequency and severity of cirrhotic cardiomyopathy and its possible relationship with bacterial endotoxemia. Dig Dis Sci. 2013;58:3029-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Merli M, Calicchia A, Ruffa A, Pellicori P, Riggio O, Giusto M, Gaudio C, Torromeo C. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med. 2013;24:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Alexopoulou A, Papatheodoridis G, Pouriki S, Chrysohoou C, Raftopoulos L, Stefanadis C, Pectasides D. Diastolic myocardial dysfunction does not affect survival in patients with cirrhosis. Transpl Int. 2012;25:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Ruíz-del-Árbol L, Achécar L, Serradilla R, Rodríguez-Gandía MÁ, Rivero M, Garrido E, Natcher JJ. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology. 2013;58:1732-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 94. | Sampaio F, Pimenta J, Bettencourt N, Fontes-Carvalho R, Silva AP, Valente J, Bettencourt P, Fraga J, Gama V. Systolic dysfunction and diastolic dysfunction do not influence medium-term prognosis in patients with cirrhosis. Eur J Intern Med. 2014;25:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Little WC, Oh JK. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation. 2009;120:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 96. | Chapman CB, Ewer SM, Kelly AF, Jacobson KM, Leal MA, Rahko PS. Classification of left ventricular diastolic function using American Society of Echocardiography Guidelines: agreement among echocardiographers. Echocardiography. 2013;30:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Unzek S, Popovic ZB, Marwick TH. Effect of recommendations on interobserver consistency of diastolic function evaluation. JACC Cardiovasc Imaging. 2011;4:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Kasner M, Gaub R, Sinning D, Westermann D, Steendijk P, Hoffmann W, Schultheiss HP, Tschöpe C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure-volume loop analysis. Eur J Echocardiogr. 2010;11:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 99. | Sampaio F, Pimenta J, Bettencourt N, Fontes-Carvalho R, Silva AP, Valente J, Bettencourt P, Fraga J, Gama V. Left atrial function is impaired in cirrhosis: a speckle tracking echocardiographic study. Hepatol Int. 2014;8:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Vieira MJ, Teixeira R, Gonçalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. 2014;27:463-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 101. | Todaro MC, Choudhuri I, Belohlavek M, Jahangir A, Carerj S, Oreto L, Khandheria BK. New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging. 2012;13:973-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 102. | Senni M, Paulus WJ, Gavazzi A, Fraser AG, Díez J, Solomon SD, Smiseth OA, Guazzi M, Lam CS, Maggioni AP. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 103. | Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 104. | Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, Haverkamp W, Ozcelik C. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 105. | Sanchis L, Gabrielli L, Andrea R, Falces C, Duchateau N, Perez-Villa F, Bijnens B, Sitges M. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 107. | Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, Narita H, Kimura G. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 108. | Duarte R, Fernandez-Perez G, Bettencourt N, Sampaio F, Miranda D, França M, Portugal P. Assessment of left ventricular diastolic function with cardiovascular MRI: what radiologists should know. Diagn Interv Radiol. 2012;18:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 109. | Rathi VK, Doyle M, Yamrozik J, Williams RB, Caruppannan K, Truman C, Vido D, Biederman RW. Routine evaluation of left ventricular diastolic function by cardiovascular magnetic resonance: a practical approach. J Cardiovasc Magn Reson. 2008;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 110. | Lossnitzer D, Steen H, Zahn A, Lehrke S, Weiss C, Weiss KH, Giannitsis E, Stremmel W, Sauer P, Katus HA. Myocardial late gadolinium enhancement cardiovascular magnetic resonance in patients with cirrhosis. J Cardiovasc Magn Reson. 2010;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Bruder O, Schneider S, Nothnagel D, Pilz G, Lombardi M, Sinha A, Wagner A, Dill T, Frank H, van Rossum A. Acute adverse reactions to gadolinium-based contrast agents in CMR: multicenter experience with 17,767 patients from the EuroCMR Registry. JACC Cardiovasc Imaging. 2011;4:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Jasaityte R, Heyde B, D’hooge J. Current state of three-dimensional myocardial strain estimation using echocardiography. J Am Soc Echocardiogr. 2013;26:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 113. | Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ, Ferro CJ, Young AA, Townend JN, Leyva F. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging. 2015;41:1000-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 114. | Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg-Buchwald C. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 2014;16:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 115. | Kowallick JT, Lamata P, Hussain ST, Kutty S, Steinmetz M, Sohns JM, Fasshauer M, Staab W, Unterberg-Buchwald C, Bigalke B. Quantification of left ventricular torsion and diastolic recoil using cardiovascular magnetic resonance myocardial feature tracking. PLoS One. 2014;9:e109164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 795] [Cited by in RCA: 856] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 117. | Bulluck H, Maestrini V, Rosmini S, Abdel-Gadir A, Treibel TA, Castelletti S, Bucciarelli-Ducci C, Manisty C, Moon JC. Myocardial T1 mapping. Circ J. 2015;79:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 118. | Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 119. | Banypersad SM, Sado DM, Flett AS, Gibbs SD, Pinney JH, Maestrini V, Cox AT, Fontana M, Whelan CJ, Wechalekar AD. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 120. | Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 121. | Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 122. | Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenböck A, Jakowitsch J, Bangert C, Laimer D. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 123. | Pica S, Sado DM, Maestrini V, Fontana M, White SK, Treibel T, Captur G, Anderson S, Piechnik SK, Robson MD. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014;16:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 124. | Sado DM, Maestrini V, Piechnik SK, Banypersad SM, White SK, Flett AS, Robson MD, Neubauer S, Ariti C, Arai A. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J Magn Reson Imaging. 2015;41:1505-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 125. | Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 126. | Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL, Tseng WY. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc Imaging. 2014;7:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 127. | Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 368] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 128. | Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, Carr-White G, Sangle S, Schaeffter T, Nagel E. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging. 2013;6:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 129. | Florian A, Ludwig A, Rösch S, Yildiz H, Sechtem U, Yilmaz A. Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 130. | Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, Robson MD, Moon J, Wordsworth PB, Neubauer S. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis--a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson. 2014;16:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 131. | Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 132. | Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |