Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2848
Peer-review started: September 3, 2014
First decision: October 14, 2014
Revised: October 18, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: March 7, 2015
Processing time: 188 Days and 17 Hours
The Da Vinci Surgical System may help to overcome some of the difficulties of laparoscopy for complicated abdominal surgery. The authors of this article present a case of robot-assisted, one-stage radical resection of three tumors, including robotic anterior resection for rectal cancer, segmental hepatectomy for liver metastasis, and wedge-shaped excision for lung metastasis. A 59-year-old man with primary rectal cancer and liver and lung metastases was operated upon with a one-stage radical resection approach using the Da Vinci Surgical System. Resection and anastomosis of rectal cancer were performed extracorporeally after undocking the robot. The procedure was successfully completed in 500 min. No surgical complications occurred during the intervention and postoperative period, and no conversion to laparotomy or additional trocars were required. To the best of our knowledge, this is the first case of simultaneous resection for rectal cancer with liver and lung metastases using the Da Vinci Surgery System to be reported. The procedure is feasible and safe and its main advantages for patient are avoiding repeated operation, reducing surgical trauma, shortening recovery time, and early implementation of postoperative adjuvant therapy.
Core tip: This is believed to be the first case of simultaneous resection for rectal cancer with liver and lung metastases using the Da Vinci Surgery System. The procedure is feasible and safe and its main advantages are avoiding repeated operation, reducing surgical trauma, shortening recovery time, and early implementation of postoperative adjuvant therapy.
- Citation: Xu JM, Wei Y, Wang XY, Fan H, Chang WJ, Ren L, Jiang W, Fan J, Qin XY. Robot-assisted one-stage resection of rectal cancer with liver and lung metastases. World J Gastroenterol 2015; 21(9): 2848-2853
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2848
Since 2002, laparoscopic technique has revolutionized the treatment of colorectal cancer, and shows similar oncological results to open surgery[1,2]. The limitations of laparoscopic approaches for treatment of colorectal cancer with resectable distant metastasis include 2D imaging, limited maneuverability of instruments, and an unsteady camera platform.
In an effort to improve standard laparoscopic techniques, the development of robotic surgical systems in surgery has been a recent advance in the past decade. The Da Vinci Surgical System (Intuitive Surgical, Mountain View, CA, United States) is a surgical robot with multiple arms operated remotely from a console, and is capable of seven degrees of freedom and 2two degrees of axial rotation to replicate human wrist-like movements with magnifying 3D visualization.
Comparisons of robotic and laparoscopic colorectal cancer resection demonstrate the feasibility and safety of the robotic platform despite longer operating times[3,4]. In addition, the data indicate that robot-assisted hepatic resection can overcome the limitations of laparoscopic surgery, and open a new treatment strategy for benign and malignant liver tumors[5,6]. In the field of thoracic surgery, the Da Vinci Surgical System is safe and feasible for individual dissection, isolation, and division of the pulmonary hilar structures during video-assisted thoracic surgery lobectomy[7].
We hypothesized that we could use a robotic platform to perform radical resection for patients with colorectal liver and lung metastases. We present our first report of robotic one-stage resection of three tumors, including anterior resection for rectal cancer, segmental hepatectomy for liver metastasis, and wedge-shaped excision for lung metastasis using this device.
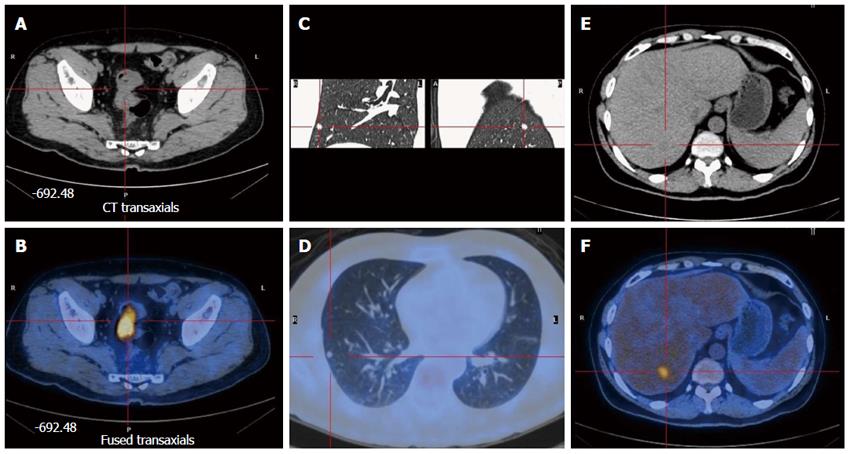
A 59-year-old Chinese man presented with a 4-mo history of intermittent rectal bleeding, with increased stool frequency. A diagnosis of rectal cancer was made after colonoscopy revealed an ulcerative mass 13 cm from the anal verge. Histological examination of the colonoscopy biopsy specimen indicated moderately differentiated adenocarcinoma. Preoperative abdominal computed tomography (CT) and rectal magnetic resonance imaging (MRI) indicated that the stage of the primary tumor was cT3N0. However, chest CT, liver MRI, and positron emission tomography showed rectal cancer with liver and lung metastases (Figure 1). This patient was discussed in a multidisciplinary team, including a colorectal surgeon, thoracic surgeons, liver surgeons, and a radiologist. The decision was that the preoperative stage of this patient was cT3N0M1, and that both liver and lung metastases were considered as a single lesion that was suitable for radical resection. After obtaining written informed consent, we carried out a one-stage resection of the three tumors using the Da Vinci Surgical System.
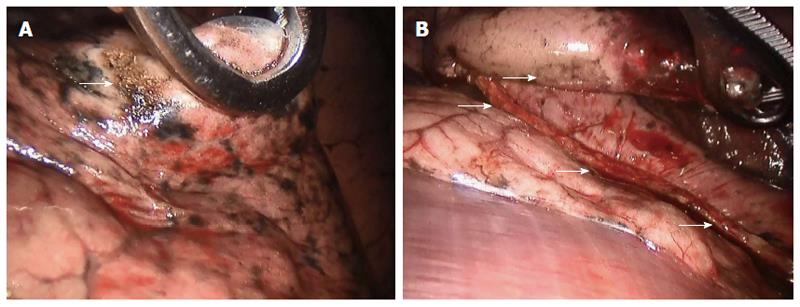
The patient was placed in the left lateral decubitus position under general anesthesia and single-lung ventilation. The robotic arm was established on the operating table anterior to the patient’s thigh. Incisions included an anterior 1.5-cm incision in the eighth intercostal space and a middle 3-cm working incision in the fifth intercostal space. The camera was connected with the robotic arm and introduced through the anterior incision. The ribs were not spread or traumatized. The right lower lobe where the tumor was located was transected with an Endo GIA stapling device (Figure 2). Resection of lung tumor was finished within 30 min.
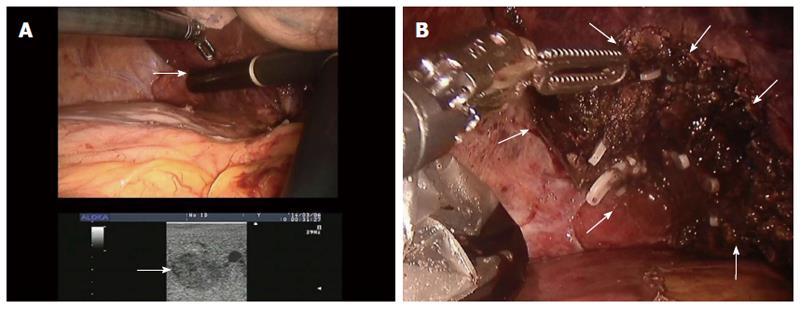
The patient was placed in the supine position under general anesthesia and bilateral-lung ventilation, and four trocars were used. A 12-mm trocar for the robotic camera was placed below the umbilicus by the Hasson method. Two additional 12-mm trocars were placed at the left upper quadrant and epigastrically under laparoscopic guidance. A 12-mm trocar for an assistant was also placed at the right upper quadrant area. Intraoperative ultrasonography was used to examine the liver lesions and obtain adequate surgical resection margins (Figure 3A). The liver was mobilized by dividing the right triangular and round ligaments. Parenchymal division proceeded from the anterior edge of the liver by harmonic scalpel and electrocautery. The small vessels and bile ducts exposed during parenchymal dissection were ligated and divided by clipping. Glisson’s pedicles of Segment VII were clamped and divided by suturing (Figure 3B). A closed suction drain catheter was placed in the subhepatic space. Segmental hepatectomy required 270 min.
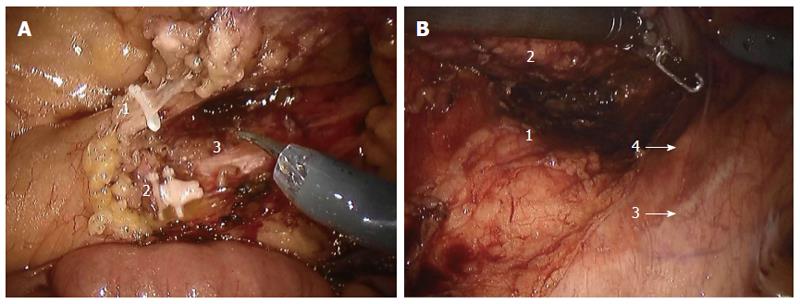
The patient was placed in the modified lithotomy position, with the legs apart, on a beanbag mattress to prevent sliding. The dissection started with incision of the peritoneum at the origin of the inferior mesenteric artery (IMA) using monopolar scissors. The assistant provided gentle traction to the sigmoid colon anteriorly to enable clear identification of the incision line. The IMA and inferior mesenteric vein were carefully skeletonized, clipped, and divided while preserving the periaortic hypogastric nerve plexus (Figure 4A). Medial-to-lateral dissection of the sigmoid colon was carried out between Toldt’s fascia and the left colonic mesentery. Anterior traction allowed dissection of the avascular plane between the mesorectal fascia and presacral fascia, while avoiding injury to the hypogastric nerves posteriorly (Figure 4B). Dissection was carried out to about 5 cm from the anal verge. After adequate pelvic dissection, the robot cart was undocked, and a laparoscopic articulating linear stapler was used to transect the rectum from the left lower quadrant port. Bowel continuity was restored with a circular stapler.
The procedure was successfully completed in 480 min with a docking time of 90 min and a console time of 390 min. Intraoperative blood loss was 600 mL. The chest tube was removed on postoperative day 2 and abdomen tubes were removed on postoperative day 5. Passing of flatus was noted on postoperative day 2. A liquid diet was then allowed and well tolerated. The pathological outcome of the liver and lung tumor was metastatic adenocarcinoma, rectal adenocarcinoma, and the stage was pT3N1M1. The margin of both primary and metastatic lesions was negative. Eighteen lymph nodes were resected, and two of them were positive. No postoperative complications occurred and the patient was discharged from hospital on postoperative day 7. Just 2 wk after surgery, the patient began to receive postoperative adjuvant chemotherapy, followed by radiotherapy at 2 mo. At a follow-up of 90 d, no further complications and tumor recurrence were observed.
Colorectal cancer is the third leading cause of cancer morality worldwide. The 5-year survival rate is currently only 5% for patients with inoperable stage IV disease[8]. For patients with liver or lung metastasis, surgical resection remains the only treatment that is associated with a long survival time, with 40% survival at 5 years and about 25% of patients demonstrating a postoperative survival of up to 10 years in specialized centers[9]. Therefore, the current guidelines recommend that the treatment strategy should be directed toward resectability[10]. However, the best type of surgical procedure is still controversial for patients with resectable primary and metastatic lesions. Research shows that one-stage resection of colorectal cancer and synchronous liver or lung metastases can be performed safely with similar outcomes compared with staged procedures[11]. The focus of this case report was on whether the Da Vinci Surgical System was feasible and safe for one-stage radical resection of rectal cancer and distant metastases.
Robot-assisted laparoscopic abdominal surgery has gained attention because it can compensate for the inherent limitations of conventional laparoscopic surgery[12,13]. The Da Vinci Surgical System improves vision, which becomes 3D, and provides more accurate hand-eye coordination that increases the surgeon’s skill. Furthermore, it also eliminates tremor and increases the degree of freedom for operating instruments. These advantages make it possible to proceed with surgery that would otherwise be difficult or impossible, especially for those who already have experience with laparoscopic surgery. In rectal surgery, robot-assisted surgery for rectal cancer can be carried out safely and in accordance with current oncological principles[14]. Robotic total mesorectal excision may allow for better preservation of urinary and sexual functions, and robotic surgery may attenuate the learning curve for laparoscopic rectal resection[15]. However, to date, the impact of robotic rectal surgery on the long-term oncological outcomes of minimally invasive total mesorectal excision remains undetermined. Large prospective randomized clinical trials, such as the international randomized trial ROLARR, are required to establish the benefits of robotic rectal surgery[16].
Robotic treatment of metastatic colorectal cancer is still at the initial stage. From 2005 to 2013, we performed > 500 cases of traditional operations using one-stage resection for colorectal cancer and liver metastasis in Zhongshan Hospital. This treatment was associated with a long survival time, with 40% survival at 5 years[17,18]. Up to June 2014, 365 cases have been treated with the Da Vinci Surgical System. Our preliminary experience suggests that the Da Vinci Surgical System is safe and feasible for treatment of synchronous colorectal liver metastasis. However, large, prospective randomized trials are urgently needed to evaluate the outcomes of this procedure.
This article is believed to be the first case report of robot-assisted one-stage resection of three tumors, including rectal cancer, liver metastasis and lung metastasis. The main advantages for patient are avoiding repeated operation, and reducing surgical trauma, when compared with the traditional surgery, in which patients need to be admitted three times and accept repeated operations[11]. In addition, the patient was discharged from hospital on postoperative day 7, and accepted adjuvant chemotherapy after 2 wk of surgery. The shortened recovery time will allow the patient early commencement of postoperative adjuvant therapy and lead to improved long-term outcome[19].
A 59-year-old Chinese man presented with a 4-mo history of intermittent rectal bleeding, with increased stool frequency.
A diagnosis of rectal cancer was made after colonoscopy revealed an ulcerative mass 13 cm from the anal verge.
Preoperative abdominal computed tomography (CT) and rectal magnetic resonance imaging (MRI) indicated that the stage of the primary tumor was cT3N0M1.
Histological examination of the colonoscopy biopsy specimen indicated moderately differentiated adenocarcinoma.
Chest CT, liver MRI, and positron emission tomography showed rectal cancer with liver and lung metastases.
The pathological outcome of the liver and lung tumor was metastatic adenocarcinoma, rectal cancer was adenocarcinoma, and the stage was pT3N1M1.
This patient was discussed in a multidisciplinary team, and the decision was that the preoperative stage was cT3N0M1, and both the liver and lung metastases were considered as a single lesion that was suitable for radical resection, and we carried out a one-stage resection of three tumors using the Da Vinci Surgical System.
Large prospective randomized clinical trials such as the international randomized trial ROLARR are required to establish the benefits of robotic rectal surgery.
Multidisciplinary team. All important items related to multidisciplinary team management of liver metastases from colorectal cancer (CRC) were selected prior to the meeting by the coordinator and referred to an expert for presentation at the meeting. Meta-analyses, randomized controlled trials, and studies evaluating clinical practice in the management of liver metastases from CRC were identified and reviewed before and discussed during the meeting. After discussion, specific controversial issues were submitted to a vote of each expert to reflect the state of consensus. Recommendations were formulated when approved by all or a large majority of the panel members.
The procedure is feasible and safe and its main advantages for patient are avoiding repeated operation, reducing surgical trauma, shortening recovery time, and early implementation of postoperative adjuvant therapy.
This paper is the exploratory study. The rectal cancer is aggressive malignant tumor, metastasis to liver or lung is the common appearance in rectal cancer. Simultaneous resection of primary tumor, liver metastasis and lung metastasis is recommended surgical procedure. It is valuable exploration to perform the simultaneous resection by The Da Vinci Site robotic surgery platform.
P- Reviewer: Liu XE S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Wang CH
| 1. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 1816] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 2. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1681] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 3. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 772] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 4. | D’Annibale A, Pernazza G, Morpurgo E, Monsellato I, Pende V, Lucandri G, Termini B, Orsini C, Sovernigo G. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol. 2010;17:2856-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Reggiani P, Antonelli B, Rossi G. Robotic surgery of the liver: Italian experience and review of the literature. Ecancermedicalscience. 2013;7:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Choi SB, Park JS, Kim JK, Hyung WJ, Kim KS, Yoon DS, Lee WJ, Kim BR. Early experiences of robotic-assisted laparoscopic liver resection. Yonsei Med J. 2008;49:632-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg. 2006;131:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 810] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 10. | Cai GX, Cai SJ. Multi-modality treatment of colorectal liver metastases. World J Gastroenterol. 2012;18:16-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, Barbas AS, Abdalla EK, Choti MA, Vauthey JN. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481-3491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Boggi U, Moretto C, Vistoli F, D’Imporzano S, Mosca F. Robotic suture of a large caval injury caused by endo-GIA stapler malfunction during laparoscopic wedge resection of liver segments VII and VIII en-bloc with the right hepatic vein. Minim Invasive Ther Allied Technol. 2009;18:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Giulianotti PC, Sbrana F, Bianco FM, Addeo P. Robot-assisted laparoscopic extended right hepatectomy with biliary reconstruction. J Laparoendosc Adv Surg Tech A. 2010;20:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Pucci MJ, Beekley AC. Use of robotics in colon and rectal surgery. Clin Colon Rectal Surg. 2013;26:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Baek SK, Carmichael JC, Pigazzi A. Robotic surgery: colon and rectum. Cancer J. 2013;19:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Baek SJ, Kim SH, Cho JS, Shin JW, Kim J. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg. 2012;36:2722-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Dexiang Z, Li R, Ye W, Haifu W, Yunshi Z, Qinghai Y, Shenyong Z, Bo X, Li L, Xiangou P. Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol. 2012;19:2860-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 312] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 19. | Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 484] [Article Influence: 34.6] [Reference Citation Analysis (0)] |