Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2840
Peer-review started: September 23, 2014
First decision: October 14, 2014
Revised: November 7, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: March 7, 2015
Processing time: 168 Days and 14.6 Hours
We present a female patient with preterm labor, severe viral hepatitis B of acute phase, hepatic encephalopathy stage III and coma. After delivery, the illness was exacerbated and the patient presented with clinical signs of vital organ dysfunctions such as acute respiratory distress syndrome, cerebral edema and hypoxemia that needed mechanical ventilation. Emergency liver transplantation was recommended after multidisciplinary panel consultations. The donor, her mother, consented to donate her right liver. Auxiliary partial orthotopic living donor liver transplantion (APOLDLT) was performed. After operation, the patient was on triple medication of tacrolimus plus mofetil mycophenolate and prednisone for immunosuppression. The combination of anti-hepatitis B virus (HBV) immunoglobulin and entecavir was initiated for anti-HBV therapy. Both the patient and the donor recovered well without any complications. The patient was followed up regularly. Her liver function, clinical signs and symptoms improved significantly. Until now, the recipient has been living for more than 78 mo free of any complications. The APOLDLT is a life-saving modality for rescuing patients with high-risk acute liver failure following HBV infection without available donor and hence is recommended under standardized antiviral therapy coverage as stated above.
Core tip: Auxiliary partial orthotopic living donor liver transplantation (APOLDLT) was performed on a female patient with preterm labor, severe viral hepatitis B of acute phase, hepatic encephalopathy and coma. The patient was treated with triple medication for immunosuppression, and combination of anti-hepatitis B virus (HBV) immunoglobulin and Entecavir for anti-HBV therapy after operation. The patient has been living for more than 78 mo free of any complications. The APOLDLT is a life-saving modality for rescuing patients with high-risk acute liver failure following HBV infection without available donor.
- Citation: Li CY, Lai W, Lu SC. Retrospective observation of therapeutic effects of adult auxiliary partial living donor liver transplantation on postpartum acute liver failure: A case report. World J Gastroenterol 2015; 21(9): 2840-2847
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2840.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2840
Auxiliary partial orthotopic liver transplantation (APOLT) refers to the surgical procedure that preserves the partial liver of the recipient and the partial liver of the donor implanted orthotopically into the recipient. APOLT was first successfully performed by Gubernatis et al[1] in 1989. Up to date, not more than 100 cases of adult APOLT have been reported worldwide. The clinical indications for this procedure include acute hepatic failure and congenital metabolic hepatopathy[2-6]. In the late 1990s, living donor graft was used in APOLT and subsequently described as orthotopic living donor liver transplantation (APOLDLT)[7]. Currently, its indication has extended to living related liver transplantations for those with excessive small liver volume graft (ratio of implant to body weight of recipient, GRWR < 0.8) and ABO blood group incompatibility[8]. To the best of our knowledge, there has been no report of adult APOLDLT for rescuing patients with postpartum acute liver failure following severe hepatitis B virus (HBV) infection. We performed APOLDLT on a female patient with preterm labor, severe HBV infection of acute phase, hepatic encephalopathy and coma. The recipient has been living for more than 78 mo free of any complications.
A 26-year-old female patient diagnosed as HBV carrier for the past four years was admitted to the Obstetric Department of our hospital at 2 am on May 6, 2008. She had been experiencing menolipsis for more than eight mo and paroxysmal abdominal pain for one hour. Laboratory examinations showed the following results: blood group B, Rh positive; hepatitis B surface antigen (HBsAg) (+), hepatitis B e antigen (+), hepatitis B core antibody (+) with a HBV-DNA load of 2.06 × 107 copies/mL, hepatitis C virus (-); prothrombin time 26.3 s, prothrombin time activity percentage 29%, prothrombin international normalized ratio 2.05; alanine transaminase 3019.1 IU/L, aspartate aminotransferase 15.6 IU/L, total serum bilirubin 174.4 μmol/L, conjugated bilirubin 78.2 μmol/L and blood ammonia 154 μg/dL. The patient was diagnosed with G3P1G33+3 left occiput anterior preterm labor, severe viral hepatitis B of acute phase, hepatic encephalopathy stage III and coma. She delivered a male infant weighing 1750 g at 3:30 am of May 6, 2008. But after 34 h of delivery, the liver function, coagulation function and model for end-stage liver disease score of the patient did not improve and she was transferred to the Surgical Intensive Care Unit at 1:00 pm of May 7, 2008. In spite of intensive care, the illness was exacerbated and the patient presented with clinical signs of vital organ dysfunctions such as acute respiratory distress syndrome, cerebral edema, and hypoxemia that needed mechanical ventilation. Emergency liver transplantation was recommended after multidisciplinary panel consultations. The patient was referred to the liver transplantation intensive-care unit at 10:00 pm of May 8, 2008 for preoperative preparation. The donor, her 52-year-old mother with blood group B, Rh positive, voluntarily consented to donate her right liver. She was evaluated physically and psychologically to be eligible for the donation procedure. The operation was approved by the Ethics Committee of our hospital. The surgery was performed from 8:40 am of May 9, 2008 to 1:00 am on May 10, 2008. Parameters on the donor and recipient are shown in Table 1.
| Height (cm) | Weight (kg) | Estimated standard liver volume (mL) | CT measured liver volume (mL) | Maximum donated liver volume (mL) | Minimum liver volume needed (mL) | Actual donated liver volume (mL) | GRWR | GW/ESLV1 | |
| Recipient | 166 | 75.0 | 1306.05 | - | - | 750 | - | 0.80 | 49% |
| Donor | 165 | 62.5 | 1277.55 | 1334.124 | 821.809 | 350 | 600 | - | 50% |
APOLDLT was performed under general anesthesia. The right liver lobe of the recipient without the middle hepatic vein was routinely excised and the right hepatic portal vein was preserved to the best of our ability for the impending reconstruction of the portal. The right liver lobe of the donor without the middle hepatic vein was precisely obtained under ultrasound guidance. The dilated donor right hepatic vein was end-to-side anastomosed to recipient vena cava oval opening, which was longitudinally extended down from the right hepatic vein orifice. In the right liver lobe graft, the end-to-end anastomosis was performed for corresponding portal veins, right hepatic arteries and right hepatic ducts. The duration of the operation was 16 h. The warm and cold ischemia time of the right liver graft was 2 and 50 min, respectively. The time interval between donor liver implantation and reperfusion was 55 min. The blood loss of the recipient was 2000 mL.
The patient was on mechanical ventilation for 178 h and regained consciousness 80 h later. She was on continuous renal replacement therapy for 72 h and treated with triple medication of tacrolimus plus mofetil mycophenolate and prednisone for immunosuppression. The combination of anti-HBV immunoglobulin (HBIG) and entecavir was initiated for anti-HBV treatment. The patient recovered well without any complications and was discharged on the 40th day after operation. The donor also recovered well without any complications and was discharged on the 15th day after operation.
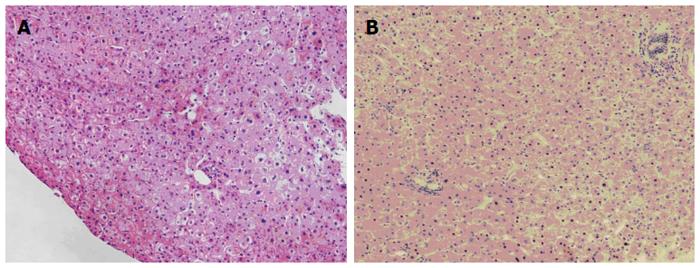
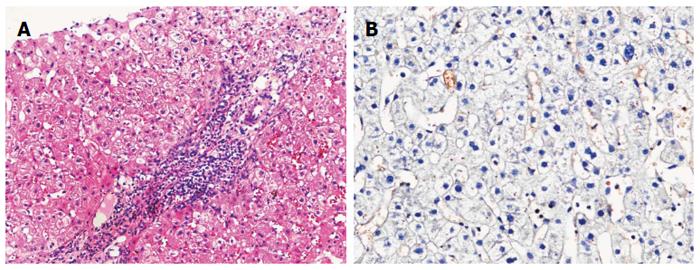
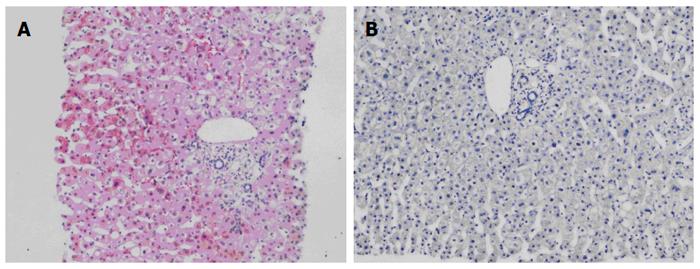
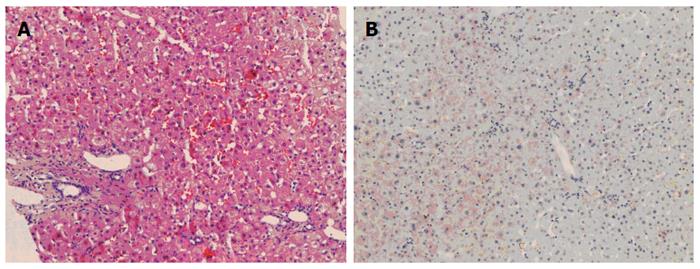
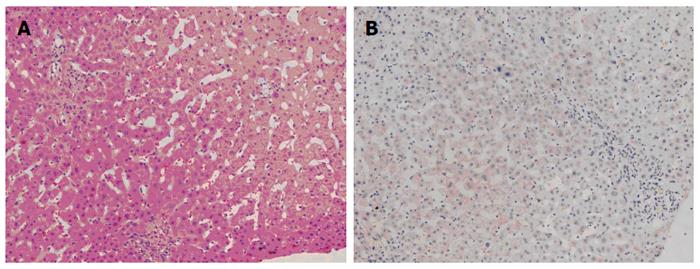
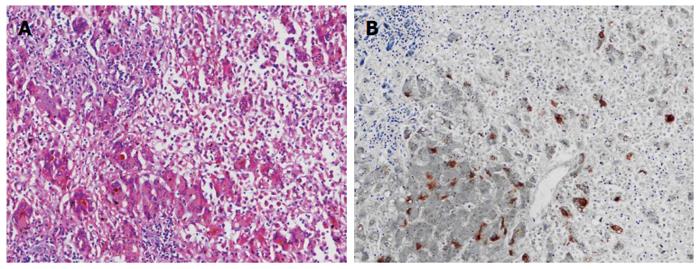
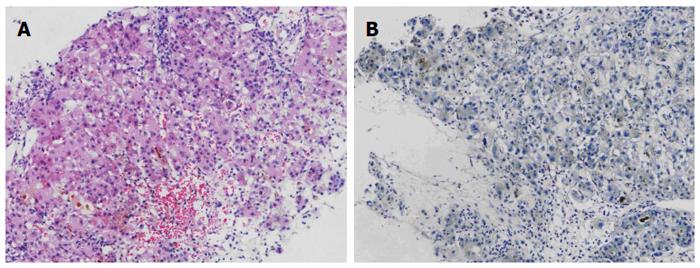
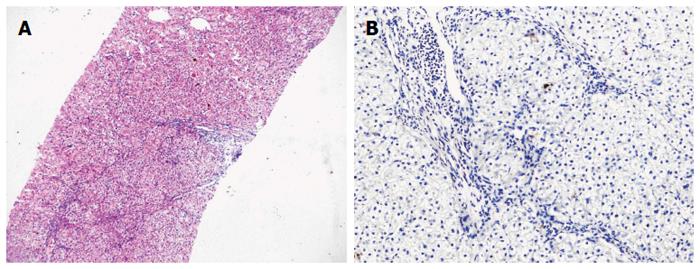
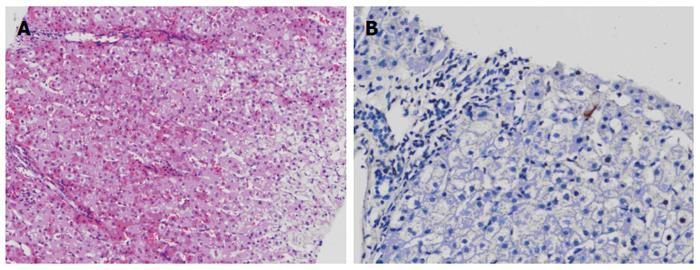
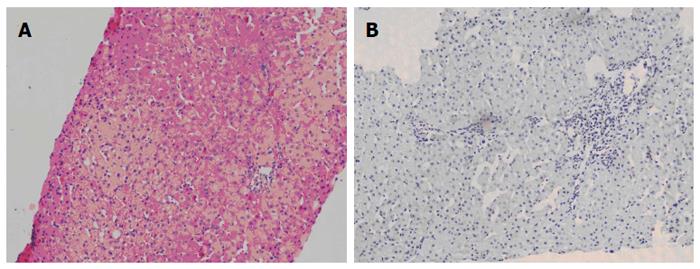
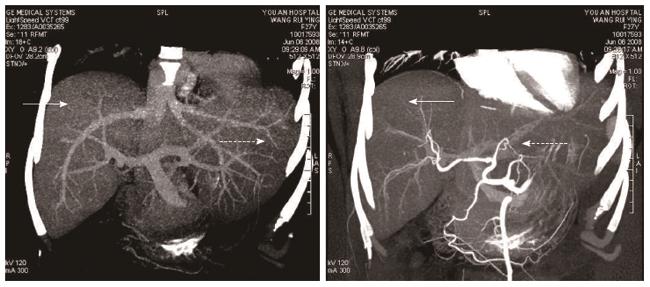
The patient was followed up regularly according to the China Liver Transplant Registry hosted in Hong Kong University. Indices of blood flow hemodynamics of the left and right liver lobes, liver function, blood biochemistry, HBV-M, and serum drug concentration were detected and programmed liver biopsy was performed. The programmed liver biopsy enabled us to know the dynamic changes of hepatic regeneration and HBV status in both the donor’s graft and the recipient’s residual native liver lobe. The pathological and immunohistochemical staining results are shown in Table 2 and Figures 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. The donor liver graft presented a typical pathological change after implantation. The recipient residual liver regenerated rapidly after operation and more than 90% of the necrotized hepatocytes regenerated within two weeks. The liver function, clinical signs and symptoms improved significantly. Color Doppler flow image study revealed transient competing influence of the portal vein blood flow between the donor and the recipient portal vein branch, which were stabilized one month after the surgery, while the blood flow competing influence between the right and left hepatic arteries was unremarkable. CT scanning was performed one month, six months and 12 mo respectively after operation and revealed that the portal vein, hepatic artery as well as implanted liver lobe had fused well with recipient native hepatic lobe (Figure 11). Until now, the recipient has been living for more than 78 mo free of any complications.
| Time point | Serum HBV-M | Degree of hepatocyte necrosis | Hepatic tissue HBV-M | |||
| Donor | Recipient | Donor liver | Recipient residual liver | Donor liver | Recipient residual liver | |
| Preoperation | (-) | HBV-DNA(+)1, HBsAg(+), HBeAg(+), HBcAg(+) | 0% | - | HBsAg(-) | HBsAg(+++) |
| HBcAg(-) | HBcAg(+++) | |||||
| Intraoperation | (-) | HBsAg(+) | Few | 90% | HBsAg(-) | HBsAg(+++) |
| HBcAg(-) | HBcAg(+++) | |||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | 0% | 10% | HBsAg(-) | HBsAg(+) |
| 10 d | HBcAg(+) | HBcAg(-) | ||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | 0% | 0% | HBsAg(-) | HBsAg(-) |
| 30 d | HBcAg(-) | HBcAg(-) | ||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | 0% | 0% | HBsAg(-) | HBsAg(+) |
| 90 d | HBcAg(-) | HBcAg(-) | ||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | 0% | 0% | HBsAg(-) | Fail to sample |
| 180 d | HBcAg(-) | |||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | 0% | 0% | HBsAg(-) | HBsAg(-) |
| 1 yr | HBcAg(-) | HBcAg(-) | ||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | - | - | - | - |
| 2 yr | ||||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | - | - | - | - |
| 3 yr | ||||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | - | - | - | - |
| 4 yr | ||||||
| Postoperation | (-) | HBV-DNA(-)2, HBsAg(+) | - | - | - | - |
| 5 yr | ||||||
Up to date, there has been no report of adult APOLDLT for rescuing patients with postpartum acute hepatic failure following severe HBV infection. In the treatment of acute hepatic failure by liver transplantation, compared with the conventional living related and cadaver liver transplantations, APOLDLT makes not only the regeneration of the residual liver, but also the feasibility of discontinuation of immunosuppressors possible[7]. Moreover, due to the timeliness of living related donor liver, the procedure can be performed in the treatment of acute liver failure[10]. The graft-to-recipient weight ratio (GRWR) in this case was 0.8, lower than the criteria of GWRW ≥ 1.0. Therefore, we selected the APOLDLT procedure in order to avoid the small-for-size syndrome. It consequently proved that the implanted donor liver graft promoted the rapid regeneration potential of the residual necrotized liver, and the regenerated residual native liver supported mutually the implanted liver graft to resume its functions. Postoperative CT scan findings and biochemical indices revealed the anatomically well fused donor and recipient liver lobes with coordinated functions (Figure 11).
HBV markers in the serum of the recipient and in the hepatic tissues of both liver lobes changed rapidly under combined entecavir and HBIG prophylactic protocol, with the features as follows: (1) HBV virus load in recipient serum decreased rapidly from 2.06 × 107 copies/mL to < 5 × 102 copies/mL within nearly two weeks; (2) the donor liver graft presented with detectable HBcAg positive transiently, which was eliminated in a short time (< 30 d); (3) HBV-M in the recipient residual native left liver lobe was eliminated rapidly; and (4) HBsAg was persistently present in the recipient serum with the HBV DNA concentration of less than 5 × 102 copies/mL. The latest programmed liver biopsy showed that HBV markers by immunohistochemical stainning in both the donor liver graft and recipient residual native left liver lobe were all negative. These protective mechanisms against HBV are still unclear, and were postulated to be related to the protective period of the newly implanted liver as reported by Lu et al[11] and Dai et al[12]. Although HBV markers in recipient serum decreased by a magnitude of 105 and disappeared in native residual liver, demonstrating that the HBV elimination may be achieved under short-term effective antiviral therapy protocol in this case, its long-term progression remains to be determined. We must admit, from a long-term perspective, the viral carrier status of the recipient would significantly influence the fate of the recipient and the donor liver graft implanted as well as the native residual liver. Therefore, in contrast to the acute liver failure not caused by HBV, HBV carrier status of native residual liver and HBV allograft re-infection are not only the major concerns of immunosuppressive agent weaning as mentioned previously, but also the key factors deciding long-term survival of both living allograft and recipient, which remain a great challenge to clinicians and need to be further clarified by future follow-up studies. However, despite the concerns mentioned above, APOLDLT is still a life-saving modality for rescuing patients with high-risk acute liver failure following HBV infection without available donor and hence is recommended under standardized antiviral therapy protocol.
A 26-year old female patient diagnosed as hepatitis B virus (HBV) carrier for the past four years had been experiencing menolipsis for more than eight months and paroxysmal abdominal pain for one hour.
The patient was diagnosed with G3P1G33+3 left occiput anterior preterm labor, acute severe HBV infection, hepatic encephalopathy and coma.
Alcoholic liver disease, hepatic cirrhosis, primary carcinoma of liver and hepatic abscess.
Hepatitis B surface antigen (HBsAg)(+), hepatitis B e antigen (+), hepatitis B core antibody (+) with a HBV-DNA load of 2.06 × 107 copies/mL, hepatitis C virus (-); prothrombin time 26.3 s, prothrombin time activity percentage 29%, prothrombin international normalized ratio 2.05; alanine transaminase 3019.1 IU/L, aspartate aminotransferase 15.6 IU/L, total serum bilirubin 174.4 μmol/L, conjugated bilirubin 78.2 μmol/L and blood ammonia 154 μg/dL.
Computed tomography showed liver augmentation.
Acute severe hepatitis with massive necrosis, and hepatocyte immunohistochemical staining for HBsAg positive.
Auxiliary partial orthotopic living donor liver transplantion (APOLDLT) was performed under general anesthesia.
There have been less than 100 cases of adult APOLDLT reported worldwide; but no report of adult APOLDLT for rescuing patients with postpartum acute hepatic failure following severe HBV infection.
Orthotopic living donor liver transplantion refers to the surgical procedure that preserves the partial liver of the recipient and the partial liver of the donor implanted orthotopically into the recipient.
APOLDLT is a life-saving modality for rescuing patients with high-risk acute live failure following HBV infection without available donor and hence is recommended under standardized antiviral therapy.
The authors have described adult auxiliary partial living donor liver transplantation on a case of postpartum acute liver failure following HBV infection, and showed that APOLDLT is a life-saving modality for rescuing patients with high-risk acute liver failure following HBV infection without available donor and hence is recommended under standardized antiviral therapy protocol.
P- Reviewer: Boucek C, Dehghani SM, Hatipoglu S, Ince V, Sugawara Y, Xia Q S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 1991;15:660-665; discussion 660-65;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Rela M. Technique of hepatic arterial anastomosis in living donor pediatric auxiliary partial orthotopic liver transplantation. Liver Transpl. 2013;19:1046-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ohno Y, Mita A, Ikegami T, Masuda Y, Urata K, Nakazawa Y, Kobayashi A, Terada M, Ikeda S, Miyagawa S. Temporary auxiliary partial orthotopic liver transplantation using a small graft for familial amyloid polyneuropathy. Am J Transplant. 2012;12:2211-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Ohno Y, Kobayashi A, Ikegami T, Masuda Y, Mita A, Urata K, Nakazawa Y, Terada M, Ikeda S, Miyagawa S. A new procedure for temporary auxiliary partial liver transplantation using living donor graft for patients with familial amyloid polyneuropathy. Liver Transpl. 2012;18:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Kim BS, Joo SH, Lee SH, Lee JI, Kim HC, Nam DH, Park HC. Auxiliary partial orthotopic liver transplantation for adult onset type II citrullinemia. J Korean Surg Soc. 2011;80 Suppl 1:S51-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Gong N, Chen X. Partial liver transplantation. Front Med. 2011;5:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Kasahara M, Takada Y, Egawa H, Fujimoto Y, Ogura Y, Ogawa K, Kozaki K, Haga H, Ueda M, Tanaka K. Auxiliary partial orthotopic living donor liver transplantation: Kyoto University experience. Am J Transplant. 2005;5:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Inomata Y, Kiuchi T, Kim I, Uemoto S, Egawa H, Asonuma K, Fujita S, Hayashi M, Tanaka K. Auxiliary partial orthotopic living donor liver transplantation as an aid for small-for-size grafts in larger recipients. Transplantation. 1999;67:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Urata K, Hashikura Y, Ikegami T, Terada M, Kawasaki S. Standard liver volume in adults. Transplant Proc. 2000;32:2093-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Shi-Chun L, Meng-Long W, Ning L, Wei L, Ping C, Jin-Ning L, Jun D, Zhen Z, Ju-Shan W, Dong-Dong L. Emergent right lobe adult-to-adult living-donor liver transplantation for high model for end-stage liver disease score severe hepatitis. Transpl Int. 2010;23:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lu SC, Yan LN, Li B, Ma YK, Liu C, Wen TF, Lin QY, Zhao JC, Wang XB, Li XD. Dynamic alteration of HBV markers on active HBV replicative recipients after liver transplantation: a preliminary report. Hepatobiliary Pancreat Dis Int. 2003;2:196-201. [PubMed] |
| 12. | Dai J, Lu SC, Yan LN, Li B, Lai W, Liu J, Zhao J, Wen TF, Gui M, Lin QY. [Investigation on the alteration of hepatitis B virus (HBV) markers in liver allograft of HBV related recipients in perioperative period]. Zhonghua Gan Zang Bing Za Zhi. 2004;12:331-333. [PubMed] |