Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2816
Peer-review started: April 16, 2014
First decision: May 13, 2014
Revised: October 7, 2014
Accepted: November 18, 2014
Article in press: November 19, 2014
Published online: March 7, 2015
Processing time: 327 Days and 12 Hours
We report a case of intravenous (IV) amiodarone drug induced liver injury (DILI). The patient received IV N-acetylcysteine (NAC) which resulted in a rapid improvement in liver enzymes. While the specific mechanisms for the pathogenesis of IV amiodarone DILI and the therapeutic action of IV NAC are both unknown, this case strongly implies at least some commonality. Because IV amiodarone is indicated for the treatment of serious cardiac arrhythmias in an intensive care unit setting, some degree of ischemic hepatitis is likely a cofactor in most cases.
Core tip: Intravenous (IV) amiodarone drug induced liver injury (DILI) is uncommon, and difficult to distinguish from ischemic hepatitis or congestive hepatopathy. Further, the pathophysiology is uncertain. IV N-acetylcysteine (NAC) is often used empirically as a treatment for idiopathic hepatitis or DILI. We report a case of rapid improvement of liver enzymes in a suspected case of IV amiodarone DILI with IV NAC, suggesting at least some shared features between the pathogenic mechanisms of the former and the therapeutic actions of the latter.
- Citation: Mudalel ML, Dave KP, Hummel JP, Solga SF. N-acetylcysteine treats intravenous amiodarone induced liver injury. World J Gastroenterol 2015; 21(9): 2816-2819
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2816.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2816
Intravenous (IV) amiodarone drug induced liver injury (DILI) is uncommon, and difficult to distinguish from ischemic hepatitis or congestive hepatopathy. Further, the pathophysiology is uncertain. IV N-acetylcysteine (NAC) is often used empirically as a treatment for idiopathic hepatitis or DILI. We report a case of rapid improvement of liver enzymes in a suspected case of IV amiodarone DILI with IV NAC, suggesting at least some shared features between the pathogenic mechanisms of the former and the therapeutic actions of the latter.
A 65-year old woman presented to her primary physician with fatigue and shortness of breath. She was treated with ciprofloxacin and oral prednisone for presumptive upper respiratory tract infection and asthma, respectively. Five days later, her symptoms worsened. She presented to the emergency room, was found to be hypotensive and hypoxic, and was admitted to an intensive care unit. Aside from the asthma, her past medical history included hypothyroidism and remote breast cancer/mastectomy. There was no history of alcohol use and she was non-obese.
On admission, chest X-ray revealed marked cardiomegaly and right lower lobe infiltrate and she was treated with antibiotics for community acquired pneumonia and presumed sepsis. Echocardiogram revealed severe global left ventricular dysfunction with ejection fraction 15%. The patient was hypotensive on presentation due to mixed septic and cardiogenic shock and required aggressive fluid resuscitation and intermittent pressor support. Lactate levels were elevated (2.5 mmol/L), as were alanine transaminase (ALT) and aspartate transaminase (AST) (439 U/L and 305 U/L, respectively). Lactate levels would later normalize and remain largely unremarkable. Transaminases remained elevated (ALT 602 U/L and AST 212 U/L) over the next 2 d.
On hospital day 4, the patient developed atrial fibrillation with rapid ventricular response which was poorly tolerated hemodynamically. She received a 150 mg bolus of amiodarone which resulted in worsening hypotension requiring pressor support, but with no other signs of drug reaction. No further amiodarone was given. Following this episode, there was an acute elevation of ALT to 1730 U/L and AST to 1650 U/L, which was believed secondary to transient hypoperfusion.
Over the next 12 d, liver enzymes spontaneously decreased to ALT and AST of 204 U/L and 56 U/L respectively. An evaluation including a viral hepatitis panel, anti-nuclear antibodies, smooth muscle antibody, serum electrophoresis, anti-liver kidney microsomal 1 and duplex ultrasound were negative. Cardiac catheterization revealed no significant obstructive coronary disease.
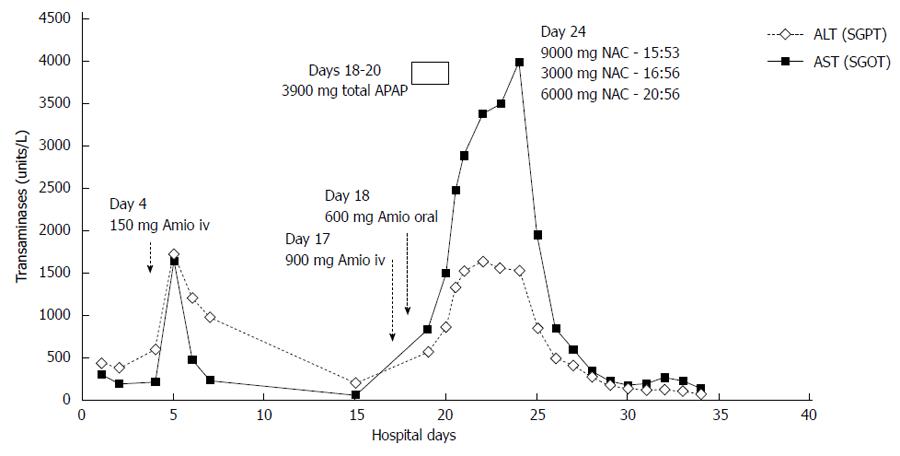
The patient’s respiratory and clinical status improved during this time. However, she remained in atrial fibrillation with rapid ventricular rates refractory to beta blockers and digoxin. On hospital day 17, after a transesophageal echocardiography guided cardioversion, the patient received a slow infusion of amiodarone (0.5 mg/min) without bolus during careful monitoring of her blood pressure. She received approximately 900 mg IV amiodarone overnight and no hypotension was observed. IV amiodarone was then stopped due to nausea, but another 3 doses of 200 mg of oral amiodarone were administered the next day. In the next 5 d, her transaminases again rose quickly and significantly in a stepwise manner. On day 23, her ALT and AST were 1541 U/L and 3994 U/L. Aside from occasional low dose acetaminophen (APAP), total dose 3900 mg in a 48 h period between days 18 and day 20, she was not on any other hepatotoxic medications. No acetaminophen was given after day 20. A liver biopsy was not done.
IV NAC, 300 mg/kg, was then administered over 21 h using the standard United States Food and Drug Administration approved acetaminophen protocol. After 12 h of NAC, her AST fell by 50% to 1958 U/L. Twenty-four hours later the AST fell to 851 U/L, representing an 80% decrease (Figure 1).
Over the next 8 d, the liver enzymes continued to decrease and day 34 (day of death) ALT and AST were 84 U/Land 142 U/L. However, her bilirubin began to increase at hospital day 27, and direct and total bilirubin elevated to 11.75 mg/dL and 16.0 mg/dL respectively after the second IV amiodarone administration. Concurrently, she had developed fungemia and died of multi organ failure.
Acute IV amiodarone toxicity, in contrast to chronic oral use, is relatively rare[1]. Gluck et al[2] have recently even questioned its existence. However, a number of case reports have been described. Because IV amiodarone is often used in seriously ill patients for cardiac indications, it can be challenging to distinguish DILI from ischemic hepatitis from, for example, even transient hypotension or other co-morbid events. Moreover, as is the case in this report, liver histology is frequently not obtained, as these patients are sick and frequently are on anti-platelet agents or anticoagulation. Consistent with prior reports, IV amiodarone DILI, like ischemia, causes an acute and severe hepatitis. We believe IV amiodarone DILI is most likely in our case as there was no significant evidence for hypotension and there was a re-challenge.
The etiology of IV amiodarone DILI is unknown, but is thought to be considerably different from the more common chronic oral hepatotoxicity. NAC is often used off-label for non-acetaminophen acute liver failure, including DILI in both adult and pediatric populations with mixed success[3,4].
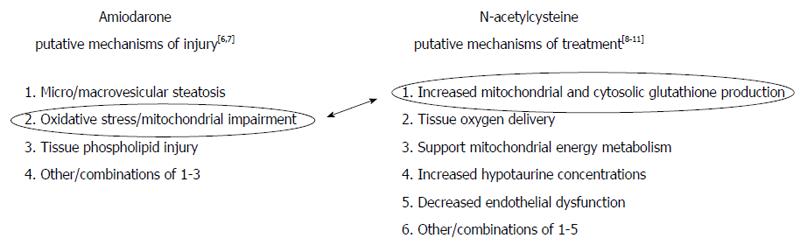
We note in Figure 2[5-11] that there are (1) numerous putative non-exclusive mechanisms by which IV amiodarone might cause acute DILI; and (2) numerous putative non-exclusive mechanisms by which IV NAC might treat DILI[5]. However, recent elegant work by Serviddio et al[6] bridged these terrains. Using a rat model, their research focused on the impact of mitochondrial oxidative stress and respiratory chain dysfunction as key to the pathogenesis of amiodarone hepatotoxicity and showed that NAC specifically abrogates these deleterious effects. In doing so, their work elucidates some of the basic mechanisms of both drugs, at least when co-administered. Although their research used a murine model, it served as the basis for the use NAC in this patient.
Our results, which show a striking improvement in transaminases levels immediately following NAC administration, are consistent with their experience. And while some reports of IV amiodarone DILI have described spontaneous resolution of hepatic injury, we believe this is the first describing improvement attributable to NAC. Using a cell culture model, Durukan et al[12] have also reported that NAC can treat amiodarone toxicity.
In retrospect, our patient may have benefited from additional NAC, as her transaminase improvement stalled somewhat after discontinuation. We used the standard 21 h APAP/NAC protocol, believing this to be adequate. However, others have reported using longer protocols for DILI and/or acute hepatotoxicity. Also, our patient received low dose acetaminophen as an anti-pyretic around the time she received the IV amiodarone. Although this was promptly discontinued as her transaminases were rising, it is possible even these low doses potentiated the IV amiodarone DILI and/or its apparent responsiveness to NAC. The patient ultimately died of multi-organ failure and fungemia; the contribution of her liver toxicity was uncertain but probably small.
Finally, although she did not have any significant known hypotensive episodes around the time of her second amiodarone infusion, we cannot fully exclude the possibility that there could have been some contribution from ischemia. Indeed, the fact that her AST was higher than her ALT on her second flare, this may be likely[13]. Application of the Council for International Organizations of Medical Sciences scale for causality assessment is useful: our patient scores maximally in favor of DILI for each of the seven items of hepatocellular injury with the exception of possible recent hypotension and the interpretation of her second ALT response[14]. As such, she scores as either “highly probable” or only “probable”. We note, however, that since IV amiodarone is indicated in the treatment of serious cardiac arrhythmias, ischemic hepatitis is a universal bedside consideration, which may lead to the under-diagnosis and under-reporting of IV amiodarone DILI.
Some authors have postulated that the diluent polysorbate 80 may be responsible for toxicity seen with IV but not oral amiodarone[15]. While our results do not disagree with this possibility, our apparent NAC responsiveness makes this seem unlikely in the present report. It is noted that while our IV amiodarone did contain polysorbate 80, IV amiodarone is commercially available without this diluent.
In conclusion, we believe that NAC at least partially treated IV amiodarone DILI for our patient. This experience lends support to a shared mitochondrial dysfunction/oxidative stress mechanism for these drugs, with NAC specifically ameliorating the injury from amiodarone in this setting. Earlier and more prolonged NAC administration may have be more beneficial.
Iintravenous (IV) amiodarone drug induced liver injury possibility treated with IV N-acetylcysteine (NAC).
Transaminases fell markedly after IV NAC administration
Ischemic hepatitis was a likely cofactor.
Transaminases rose twice in response to IV amiodarone, but fell precipitously after IV NAC.
IV NAC was the only liver specific treatment applied.
Multiple other reports have described IV amiodarone toxicity, but its incidence, pathogenesis, and ideal treatments remain unknown.
This is an interesting case reports with various clinical issues. The manuscript is interesting and well written.
P- Reviewer: Garcia-Fernandez MI, Teschke R S- Editor: Nan J L- Editor: A E- Editor: Ma S
| 1. | Available from: http://livertox.nlm.nih.gov/Amiodarone.htm. |
| 2. | Gluck N, Fried M, Porat R. Acute amiodarone liver toxicity likely due to ischemic hepatitis. Isr Med Assoc J. 2011;13:748-752. [PubMed] |
| 3. | Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ, Murray NG, McCashland T, Reisch JS. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864, 864.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 4. | Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Rodriguez-Baez N, Olio DD, Karpen S, Bucuvalas J, Lobritto S. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo-controlled clinical trial. Hepatology. 2013;57:1542-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Hadžić N. Challenging the dogmas; the NAC tie. Hepatology. 2013;57:1297-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Serviddio G, Bellanti F, Giudetti AM, Gnoni GV, Capitanio N, Tamborra R, Romano AD, Quinto M, Blonda M, Vendemiale G. Mitochondrial oxidative stress and respiratory chain dysfunction account for liver toxicity during amiodarone but not dronedarone administration. Free Radic Biol Med. 2011;51:2234-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Kannan R, Sarma JS, Guha M, Venkataraman K. Tissue drug accumulation and ultrastructural changes during amiodarone administration in rats. Fundam Appl Toxicol. 1989;13:793-803. [PubMed] |
| 8. | Hein OV, Ohring R, Schilling A, Oellerich M, Armstrong VW, Kox WJ, Spies C. N-acetylcysteine decreases lactate signal intensities in liver tissue and improves liver function in septic shock patients, as shown by magnetic resonance spectroscopy: extended case report. Crit Care. 2004;8:R66-R71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 10. | Zwingmann C, Bilodeau M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology. 2006;43:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6-20. [PubMed] |
| 12. | Durukan AB, Erdem B, Durukan E, Sevim H, Karaduman T, Gurbuz HA, Gurpinar A, Yorgancioglu C. May toxicity of amiodarone be prevented by antioxidants? A cell-culture study. J Cardiothorac Surg. 2012;7:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Henrion J. Hypoxic hepatitis. Liver Int. 2012;32:1039-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Giannattasio F, Salvio A, Varriale M, Picciotto FP, Di Costanzo GG, Visconti M. Three cases of severe acute hepatitis after parenteral administration of amiodarone: the active ingredient is not the only agent responsible for hepatotoxicity. Ann Ital Med Int. 2002;17:180-184. [PubMed] |