Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2786
Peer-review started: September 11, 2014
First decision: October 14, 2014
Revised: October 24, 2014
Accepted: December 1, 2014
Article in press: December 1, 2014
Published online: March 7, 2015
Processing time: 180 Days and 5.1 Hours
AIM: To determine the resistance patterns of Helicobacter pylori (H. pylori) strains isolated from patients in Beijing and monitor the change of antibiotic resistance over time.
METHODS: In this prospective, serial and cross-sectional study, H. pylori cultures were successfully obtained from 371 and 950 patients (never receiving eradication) during 2009-2010 and 2013-2014, respectively. Resistance to amoxicillin, clarithromycin, metronidazole, levofloxacin, tetracycline, and rifampicin was determined by Epsilometer test.
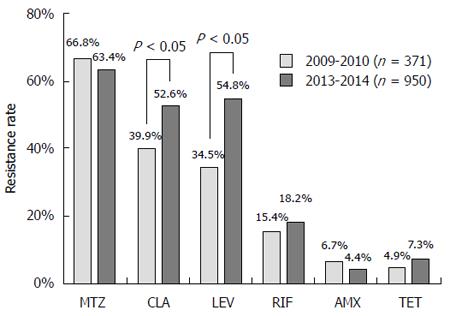
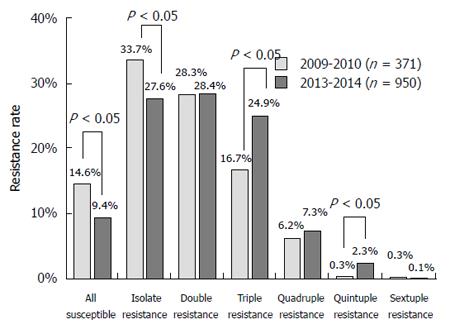
RESULTS: The resistance rates of isolates obtained during 2009-2010 were 66.8%, 39.9%, 34.5%, 15.4%, 6.7%, and 4.9% to metronidazole, clarithromycin, levofloxacin, rifampicin, amoxicillin and tetracycline, respectively; and the corresponding rates for isolates during 2013-2014 were 63.4%, 52.6%, 54.8%, 18.2%, 4.4% and 7.3%, respectively. The resistance rates to clarithromycin and levofloxacin were significantly increased after four years. In 2009-2010, 14.6% of H. pylori isolates were susceptible to all tested antibiotics, with mono (33.7%), double (28.3%), triple (16.7%), quadruple (6.2%), quintuple (0.3%) and sextuple resistance (0.3%) also being detected. In 2013-2014, 9.4% were susceptible to all tested antibiotics, and mono (27.6%), double (28.4%), triple (24.9%), quadruple (7.3%), quintuple (2.3%) and sextuple resistance (0.1%) was also observed. More multiple resistant H. pylori isolates were found during 2013-2014. Gender (to levofloxacin and metronidazole), age (to levofloxacin) and endoscopic findings (to clarithromycin) were independent factors influencing antibiotic resistance.
CONCLUSION: H. pylori resistance to commonly used antibiotics in Beijing is high with increased multiple antibiotic resistance.
Core tip: Because the antimicrobial susceptibility of Helicobacter pylori (H. pylori) strains continues to change over time, it is very important to obtain updated information on antibiotic resistance by dynamic monitoring and serial detection, which is critical to the selection of the most optimal therapeutic regimens for eradication of H. pylori infection. This study provided us the comprehensive and up-to-date data about antibiotic resistance of H. pylori. It was showed that H. pylori resistance to commonly used antibiotics in China is high with increased multiple antibiotic resistances. Therefore, the eradication of H. pylori infection remains a challenge in China.
-
Citation: Zhang YX, Zhou LY, Song ZQ, Zhang JZ, He LH, Ding Y. Primary antibiotic resistance of
Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: A prospective serial study. World J Gastroenterol 2015; 21(9): 2786-2792 - URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2786.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2786
Although studies on Helicobacter pylori (H. pylori) have been carried out since thirty years ago, eradication of H. pylori infection remains a challenge, mainly because of the significantly increasing prevalence of its resistance to antibiotics[1,2]. A suitable therapeutic regimen for H. pylori infection should be based on the specific conditions of patients, especially the antibiotic resistance, in order to achieve better long-term efficacy. In China, there is a high prevalence of H. pylori infection and gastric cancer[3,4].
Because the antimicrobial susceptibility of H. pylori strains continues to change over time, it is very important to obtain updated information on antibiotic resistance by dynamic monitoring and serial detection, which is critical to the selection of the most optimal therapeutic regimens for eradication of H. pylori infection. However, the recent information on such studies conducted in Chinese patients is lacking and most studies have investigated only a few types of antibiotics[5]. Therefore, further studies with large sample sizes and serial observations are urgently needed to provide comprehensive and up-to-date information on the antibiotic resistance of H. pylori.
The aim of this study was to determine the resistance rates and patterns of H. pylori strains isolated from patients with dyspepsia and to monitor the change of antibiotic resistance over time.
This is a prospective, single-centre, serial, cross-sectional observational study.
From August 2009 to February 2010 and from April 2013 to March 2014, patients undergoing upper endoscopy due to dyspeptic symptoms in a tertiary hospital in Beijing, China were enrolled in the study. Patients were included by clinical gastroenterologists if they were of 18 to 70 years old and had not received treatment for eradication of H. pylori infection prior to study entry.
Exclusion criteria were: (1) patients who had received proton pump inhibitors, H2 receptor antagonists, bismuth salts or antibiotics during the four weeks prior to study entry; (2) patients with malignant tumors of the digestive system; (3) patients who had undergone gastrointestinal tract surgery; (4) patients whose conditions were complicated by severe heart, lung, blood, liver or kidney dysfunction; and (5) patients who would not comply to the study.
The study was approved by the independent Ethics Committee of Peking University Health Science Center (IRB00001052-0709) and was carried out in accordance with the ethical guidelines of the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained from each patient prior to study enrollment.
After obtaining general information, a gastric mucosal biopsy specimen from the antrum was collected from each patient during upper endoscopy to check the presence of H. pylori by rapid urease test (HPUT-H102, San Qiang Biological and Chemical Co., Fujian, China). If a patient tested positive, two mucosal biopsy specimens (one from the autrum and the other form the corpus) were put into the same vial for the culture of H. pylori. Two additional specimens (one from the antrum and the other form the corpus) were obtained for histological haematoxylin and eosin staining, and gastritis was evaluated using the updated Sydney system[6].
Briefly, gastric mucosal biopsy specimens were stored in brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) with 5% glycerin at -80 °C and were transported with dry ice to the laboratory at the National Institute for Communicable Disease Control and Prevention, Beijing, China. Frozen samples were thawed at room temperature for 30 min, ground and cultivated on a Columbia agar (Oxoid, Basingstoke, United Kingdom) plate supplemented with 5% defibrinated sheep blood, 3 μg/mL trimethoprim, 2.5 μg/mL vancomycin, 2 μg/mL amphotericin B and 2 μg/mL polymyxin B. The cultured plates were incubated in a microaerobic atmosphere (5% oxygen, 10% carbon dioxide and 85% nitrogen). After culture, translucent colonies of about 0.5-2 mm from selective agar plates were Gram-stained and tested for urease, catalase and oxidase activities. The organisms were identified as H. pylori if the isolates demonstrated curved Gram-negative rods, which were similar to Helicobacter, along with positive urease, catalase and oxidase reactions.
The antibiotic resistance of H. pylori isolates was determined by the Epsilometer test (E-test)[7]. According to the clinical breakpoints for H. pylori proposed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST)[8], the resistance breakpoints for amoxicillin, clarithromycin, metronidazole, levofloxacin, tetracycline and rifampicin were defined as > 0.12, > 0.5, > 8, > 1, > 1 and > 1 mg/L, respectively. After storing H. pylori isolates at -80 °C for two weeks, susceptibility testing for amoxicillin was performed again. All the cultures and tests were conducted at the National Institute for Communicable Disease Control and Prevention of Chinese Center for Disease Control and Prevention.
All statistical analyses were performed using the statistical software SPSS (version 18.0; SPSS Inc., Chicago, IL, United States). Frequencies and percentages were used to describe the antimicrobial resistance of H. pylori isolates. Between-group differences were evaluated using Student’s t test for continuous variables and Pearson’s χ2 or Fisher’s exact test for categorical variables. Univariate analyses were performed for the determination of factors that could influence the antibiotic resistance of H. pylori isolates, such as gender (male vs female), age groups (18-35 vs 36-50 vs 51-75 years of age) and endoscopic findings (peptic ulcer disease vs non-ulcer disease). Patients with duodenal or/and gastric ulcer were considered as having peptic ulcer disease; those without ulcers were considered as patients with non-ulcer dyspepsia. Thereafter, binary logistic regression was used to analyze the relationships between independent variables (gender, age groups and endoscopic findings) and dependent variables (antibiotic susceptibility or resistance) with backward likelihood ratio analysis, which determined independent influencing factors for the antibiotic resistance of H. pylori. The odds ratio (OR) and 95% confidence interval (CI) of different variables related to antibiotic resistance were calculated. Differences with P-values less than 0.05 from a 2-tailed test were considered statistically significant.
In 2009-2010, 450 patients were H. pylori positive as determined by the rapid urease test and bacterial cultures were successfully obtained for 371 (82.4%) patients. In 2013-2014, 1105 patients were H. pylori positive and bacterial cultures were successfully obtained for 950 (86.0%) patients. No significant differences were observed in the rates of positive culture, general characteristics and endoscopic and histological findings of the study population between the two groups (Table 1).
| Characteristic | 2009-2010(n = 371) | 2013-2014(n = 950) | P value |
| Gender (male/female), n | 185/186 | 485/465 | 0.698 |
| Age (yr), mean ± SD | 41.9 ± 13.4 | 42.9 ± 13.2 | 0.215 |
| Endoscopic findings, n | |||
| Peptic ulcer | 60 | 184 | 0.320 |
| Gastric erosions | 75 | 200 | |
| No macroscopic abnormality | 236 | 566 | |
| Histological findings, n | |||
| Non-atrophic gastritis | 245 | 664 | 0.174 |
| Atrophic gastritis | 126 | 286 |
In 2009-2010, the resistance rate of 371 H. pylori isolates to metronidazole (66.8%) was highest, followed by clarithromycin (39.9%), levofloxacin (34.5%), rifampicin (15.4%), amoxicillin (6.7%) and tetracycline (4.9%). In 2013-2014, the resistance rate of 950 H. pylori isolates was also highest to metronidazole (63.4%), followed by levofloxacin (54.8%), clarithromycin (52.6%), rifampicin (18.2%), tetracycline (7.3%) and amoxicillin (4.4%). The resistance rates to clarithromycin and levofloxacin in 2013-2014 were significantly higher than those in 2009-2010, while no significant difference was found in the resistance rates to other four antibiotics (Figure 1).
In 2009-2010, 14.6% of H. pylori isolates were susceptible to all tested antibiotics. Monoresistance (33.7%), double resistance (28.3%), triple resistance (16.7%), quadruple resistance (6.2%), quintuple resistance (0.3%) and sextuple resistance (0.3%) were also observed. In 2013-2014, 9.4% of H. pylori isolates were susceptible to all tested antibiotics, and mono resistance (27.6%), double resistance (28.4%), triple resistance (24.9%), quadruple resistance (7.3%), quintuple resistance (2.3%) and sextuple resistance (0.1%) were also observed. Markedly, more multiple resistant H. pylori isolates were found in 2013-2014 (Figure 2). In the clarithromycin-resistant isolates in 2013-2014, 70.8% and 60.8% of isolates were also resistant to metronidazole and levofloxacin, respectively. In the metronidazole-resistant isolates, levofloxacin-resistant isolates were detected in 63.3% of isolates. The multiple resistance patterns are shown in Table 2.
| Multiple resistance pattern | 2009-2010(n = 371) | 2013-2014(n = 950) |
| Double resistance | ||
| CLA + MTZ | 36 (9.7) | 79 (8.3) |
| MTZ + LEV | 29 (7.8) | 94 (9.9) |
| MTZ + RIF | 15 (4.0) | 14 (1.5) |
| CLA + LEV | 13 (3.5) | 40 (4.2) |
| AMX + MTZ | 3 (0.8) | 3 (0.3) |
| MTZ + TET | 3 (0.8) | 2 (0.2) |
| CLA + RIF | 2 (0.5) | 11 (1.2) |
| AMX + CLA | 2 (0.5) | 4 (0.4) |
| CLA + TET | 1 (0.3) | 5 (0.5) |
| AMX + RIF | 1 (0.3) | 2 (0.2) |
| LEV + RIF | 0 | 8 (0.8) |
| LEV + TET | 0 | 5 (0.5) |
| AMX + LEV | 0 | 1 (0.1) |
| Triple resistance | ||
| CLA + MTZ + LEV | 34 (9.2) | 171 (18.0) |
| CLA + MTZ + RIF | 7 (1.9) | 16 (1.7) |
| AMX + CLA + MTZ | 6 (1.6) | 4 (0.4) |
| MTZ + LEV + RIF | 4 (1.1) | 18 (1.9) |
| CLA + MTZ + TET | 4 (1.1) | 1 (0.1) |
| CLA + LEV + RIF | 2 (0.5) | 11 (1.2) |
| AMX + MTZ + LEV | 2 (0.5) | 2 (0.2) |
| AMX + MTZ + RIF | 1 (0.3) | 0 |
| AMX + CLA + LEV | 1 (0.3) | 0 |
| CLA + LEV + TET | 1 (0.3) | 3 (0.3) |
| MTZ + LEV + TET | 0 | 10 (1.1) |
| AMX + CLA + RIF | 0 | 1 (0.1) |
| CLA + TET + RIF | 0 | 1 (0.1) |
| MTZ + TET + RIF | 0 | 1 (0.1) |
| Quadruple resistance | ||
| CLA + MTZ + LEV + RIF | 12 (3.2) | 38 (4.0) |
| AMX + CLA + MTZ + LEV | 4 (1.1) | 9 (0.9) |
| CLA + MTZ + LEV + TET | 4 (1.1) | 8 (0.8) |
| MTZ + LEV + TET + RIF | 1 (0.3) | 8 (0.8) |
| AMX + MTZ + LEV + TET | 1 (0.3) | 0 |
| AMX + MTZ + LEV + RIF | 1 (0.3) | 0 |
| AMX + CLA + MTZ + RIF | 0 | 3 (0.3) |
| CLA + MTZ + TET + RIF | 0 | 2 (0.2) |
| CLA + LEV + TET + RIF | 0 | 1 (0.1) |
| Quintuple resistance | ||
| CLA + MTZ + LEV + TET + RIF | 1 (0.3) | 17 (1.8) |
| AMX + CLA + MTZ + LEV + RIF | 0 | 3 (0.3) |
| AMX + CLA + MTZ + LEV + TET | 0 | 2 (0.2) |
| Sextuple resistance | 1 (0.3) | 1 (0.1) |
Univariate analysis showed that there was a significant difference in the resistance rate to clarithromycin between the patients with peptic ulcer disease and non-ulcer dyspepsia. There were significant differences in the resistance rates to metronidazole and levofloxacin between men and women. Moreover, the significant difference in levofloxacin resistance rate was observed among different age groups. Multivariate analysis demonstrated that the above-mentioned factors were independent factors influencing the antibiotic resistance of H. pylori (Table 3).
| Resistance rate (%) | GenderMale (n = 670),female (n = 651) | Age (yr)18-35 (n = 496),36-50 (n = 378),51-75 (n = 447) | Endoscopic findingsPUD (n = 244),NUD (n = 1077) |
| Amoxicillin | 5.1, 5.1 | 4.2, 5.3, 5.8 | 2.9, 5.6 |
| Clarithromycin | 46.6, 51.6 | 50.8, 48.7, 47.4 | 36.5, 51.9a (OR = 1.822; 95%CI: 1.364-2.433) |
| Metronidazole | 59.4, 69.4a (OR =1.504; 95%CI: 1.195-1.891) | 63.3, 62.2, 67.3 | 58.6, 65.6 |
| Levofloxacin | 43.6, 54.8a (OR =1.518; 95%CI: 1.219-1.891) | 42.9, 53.4, 52.3a (OR =1.199; 95%CI: 1.053-1.365) | 45.1, 50.0 |
| Tetracycline | 7.2, 6.0 | 6.7, 4.5, 8.3 | 6.1, 6.7 |
| Rifampicin | 19.0, 15.8 | 16.7, 16.9, 18.6 | 20.9, 16.6 |
The results from the present study revealed that the treatment of H. pylori infection remains a challenge in China due to the high rates of H. pylori resistance to commonly used antibiotics. Furthermore, multiple antibiotic resistance patterns were also commonly observed in the H. pylori isolates investigated. The rates of clarithromycin and levofloxacin resistance were further significantly increased in the last four years and multi-resistant strains became more common.
In the present study, resistance to clarithromycin in 2013-2014 was identified in 52.6% of H. pylori isolates. According to the Maastricht IV/Florence consensus report[2], the clarithromycin-based standard triple therapy is unsuitable for eradication of H. pylori in China, only if susceptibility testing for H. pylori is performed before treatment to confirm the lack of resistance to this specific antibiotic. Some studies have found that sequential and concomitant therapies are effective treatment options for patients who show clarithromycin resistance. Thus, these therapies can be recommended for patients from regions with high clarithromycin-resistance rates[2,9-12]. Unfortunately, meta-analyses also showed that dual resistance to clarithromycin and metronidazole could reduce the abilities of sequential and concomitant therapies to eradicate H. pylori[13,14], which indicated that these therapies could only be used in regions with high rates of isolated clarithromycin resistance. Our recent findings also confirmed this[15]. The data in this study showed a high rate of resistance to metronidazole (70.8%) in clarithromycin-resistant isolates. Based on these results, it is reasonable to speculate that sequential and concomitant therapies are not suitable for the management of H. pylori infection in China.
In recent years, because of the high resistance to clarithromycin and metronidazole, levofloxacin has been extensively studied in patients with H. pylori infection. Moreover, levofloxacin has been proven to be effective in eradicating H. pylori in some trials[16-18]. Nevertheless, these studies were mainly conduced in regions where the levofloxacin resistance was lower. In this study, the resistance rate of H pylori isolates to levofloxacin was found to be as high as 54.8% with a highly combined resistance to clarithromycin and metronidazole, indicating that levofloxacin-containing therapy might not be a good choice for the initial empiric treatment of H. pylori in China.
In the current study, the H. pylori isolates were found to be relatively susceptible to tetracycline with an overall resistance rate of 4.9%-7.3%, which suggested that tetracycline could be used in the initial treatment of H. pylori infection in China. It is generally thought that the increase in dosage and dosing frequency of metronidazole can reduce high-level resistance to metronidazole[1,19]. Nowadays, bismuth salts have widely been used in clinical practice because they are easily obtained in China. Therefore, the classic quadruple therapy consisting of metronidazole, tetracycline, bismuth and proton pump inhibitors can be suggested as the best empirical first-line regimen for H. pylori eradication in China, which is consistent with the recommendations of the Maastricht IV/Florence consensus report[2]. Unfortunately, tetracycline is not generally available in China, which has affected its clinical application. Although semisynthetic tetracycline derivatives including minocycline are easily obtainable in the clinic, it is still uncertain whether these derivatives can be used as alternatives to tetracycline for the treatment of H. pylori infection.
In this study, the rate of resistance to amoxicillin was relatively low, suggesting that amoxicillin should be fully utilized in the eradication of H. pylori infection. With the general increase in the rate of resistance to commonly used antibiotics, dual therapy (proton pump inhibitor plus amoxicillin) drew attention again, but the study results were not consistent and conclusive[20,21]. Shirai et al[20] have shown a very good eradication efficacy in their study, but rabeprazole and amoxicillin should be administered four times a day. Up to now, no related research has been reported in China.
Rifampicin is rarely used for the treatment of H. pylori infection In China. In the present study, the high rate of resistance (18.2%) to this drug in the H. pylori isolates studied may be due to its frequent use in the treatment of tuberculosis in China, where a high prevalence of tuberculosis infection is observed.
The study results revealed that the clarithromycin resistance rate of H. pylori isolates was significantly higher in the patients with non-ulcer disease than with peptic ulcer disease, which was consistent with the results of previous studies[22,23]. Additionally, resistance to levofloxacin and metronidazole was more frequent in women and in middle-aged and elderly patients. Such a phenomenon may be related to the more frequent use of levofloxacin and metronidazole in these patients, especially in women with gynecological diseases. Antimicrobial resistance is closely dependent on antimicrobial use. In Western countries, the commercialization of quinolones as levofloxacin is more recent than that of clarithromycin, and levofloxacin is strictly limited to use as an antibiotic. Therefore, the resistance rate to levofloxacin of H. pylori is relatively low[2]. However, in China over the past 30 years, quinolones are widely used as the non-prescription drugs even in animal husbandry and aquaculture, which results in such a high resistance rate. Recently, restrictions in the use of levofloxacin were just demanded.
The antimicrobial susceptibility of the H. pylori isolates was determined by the E-test in the current study, primarily because this method was easily performed, especially in a clinical study with large sample size. Although some previous studies have suggested that the rate of metronidazole resistance may be overestimated by E-test[24,25], the results were not consistent or conclusive. A high resistance rate to metronidazole was also found in a recent study conducted in the southeast coastal region of China by the reference agar dilution method[26].
In this study, two gastric mucosal biopsy specimens (one from the autrum and the other from the corpus) were put into the same vial for the culture of H. pylori. Due to the restrictions of research funding and study conditions, the cultures of H. pylori from the mucosal specimens of the antrum and corpus were not obtained and tested. Hence, the difference in the antimicrobial resistance profile of H. pylori isolates obtained according to the location could not be analyzed. This might have underestimated the antimicrobial resistance rates, which was the potential limitation of this study.
The comprehensive and up-to-date information on H. pylori resistance obtained in this study will be very helpful to select the most optimal eradication regimens in both China and other regions with a high prevalence of antibiotic resistance. With the current rates of resistance, the priority would be to get new drugs and/or improved methods of detection of resistance. The use of a more accessible and comfortable method to obtain H. pylori was already suggested[27], and progress in molecular detection of resistance from faecal samples[28] is a very promising line of research.
In conclusion, a prospective serial study was carried out with a large sample size to determine the resistance patterns of H. pylori isolates from Chinese patients and to monitor the changes in antibiotic resistance over time. It was showed that H. pylori resistance rates to commonly used antibiotics in China are high with increased multiple antibiotic resistance.
The eradication of Helicobacter pylori (H. pylori) infection remains a challenge, mainly because of the significantly increasing prevalence of its resistance to antibiotics.
Because the antimicrobial susceptibility of H. pylori strains continues to change over time, it is very important to obtain updated information on antibiotic resistance by dynamic monitoring and serial detection, which is critical to the selection of the most optimal therapeutic regimens for eradication of H. pylori infection.
This study provided us the comprehensive and up-to-date data about antibiotic resistance of H. pylori. It was showed that H. pylori resistance rates to commonly used antibiotics in China are high with increased antibiotic multiple resistances.
This comprehensive and up-to-date information on H. pylori resistance will be very helpful to select the most optimal eradication regimens in clinical settings.
Multiple antibiotic resistance is defined as H. pylori being resistant to two or more kinds of antibiotics simultaneously.
Antibiotic resistance is a main factor with therapeutic effects on patients with H. pylori infection. In this manuscript, the authors reported the resistance pattern of H. pylori to six antibiotics in different periods. It is very helpful to select the most optimal eradication regimens.
P- Reviewer: Niu ZS S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 2. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1591] [Article Influence: 122.4] [Reference Citation Analysis (5)] |
| 3. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 4. | Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Cheng H, Hu FL. [The epidemiology of Helicobacter pylori resistance to antibiotics in Beijing]. Zhonghua Yi Xue Za Zhi. 2005;85:2754-2757. [PubMed] |
| 6. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 7. | Liu G, Xu X, He L, Ding Z, Gu Y, Zhang J, Zhou L. Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter. 2011;16:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Clinical breakpoints-bacteria (v 3. 1). Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf. |
| 9. | Zullo A, Hassan C, Ridola L, De Francesco V, Vaira D. Standard triple and sequential therapies for Helicobacter pylori eradication: an update. Eur J Intern Med. 2013;24:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (95)] |
| 14. | Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5:23-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Zhou L, Zhang J, Chen M, Hou X, Li Z, Song Z, He L, Lin S. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am J Gastroenterol. 2014;109:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Federico A, Nardone G, Gravina AG, Iovene MR, Miranda A, Compare D, Pilloni PA, Rocco A, Ricciardiello L, Marmo R. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143:55-61.e1; quize e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, Sica M, Rocco A, Salerno R, Marmo R. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010;59:1465-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Liou JM, Lin JT, Chang CY, Chen MJ, Cheng TY, Lee YC, Chen CC, Sheng WH, Wang HP, Wu MS. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39:465-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, Ohashi K, Ishizaki T, Hishida A, Furuta T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Graham DY, Javed SU, Keihanian S, Abudayyeh S, Opekun AR. Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: studies from the United States. J Gastroenterol. 2010;45:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | De Francesco V, Ierardi E, Hassan C, Zullo A. Helicobacter pylori therapy: Present and future. World J Gastrointest Pharmacol Ther. 2012;3:68-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Zullo A, Perna F, Hassan C, Ricci C, Saracino I, Morini S, Vaira D. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment Pharmacol Ther. 2007;25:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 25. | McDermott PF, Simala-Grant JL, Taylor DE. Chapter 59: Antimicrobial Resistance in Helicobacter and Campylobacter. Antimicrobial Drug Resistance. Totowa, NJ: Humana Press 2009; 847-863. |
| 26. | Su P, Li Y, Li H, Zhang J, Lin L, Wang Q, Guo F, Ji Z, Mao J, Tang W. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 27. | Perez-Trallero E, Montes M, Larrañaga M, Arenas JI. How long for the routine Helicobacter pylori antimicrobial susceptibility testing? The usefulness of the string test to obtain Helicobacter for culture. Am J Gastroenterol. 1999;94:3075-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Queralt N, Bartolomé R, Araujo R. Detection of Helicobacter pylori DNA in human faeces and water with different levels of faecal pollution in the north-east of Spain. J Appl Microbiol. 2005;98:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |