Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2693
Peer-review started: August 6, 2014
First decision: August 27, 2014
Revised: September 22, 2014
Accepted: November 18, 2014
Article in press: November 19, 2014
Published online: March 7, 2015
Processing time: 215 Days and 19.4 Hours
AIM: To evaluate the clinical usefulness of endoscopic ultrasonography (EUS) for the diagnosis of the invasion depth of ulcerative colitis-associated tumors.
METHODS: The study group comprised 13 patients with 16 ulcerative colitis (UC)-associated tumors for which the depth of invasion was preoperatively estimated by EUS. The lesions were then resected endoscopically or by surgical colectomy and were examined histopathologically. The mean age of the subjects was 48.2 ± 17.1 years, and the mean duration of UC was 15.8 ± 8.3 years. Two lesions were treated by endoscopic resection and the other 14 lesions by surgical colectomy. The depth of invasion of UC-associated tumors was estimated by EUS using an ultrasonic probe and was evaluated on the basis of the deepest layer with narrowing or rupture of the colonic wall.
RESULTS: The diagnosis of UC-associated tumors by EUS was carcinoma for 13 lesions and dysplasia for 3 lesions. The invasion depth of the carcinomas was intramucosal for 8 lesions, submucosal for 2, the muscularis propria for 2, and subserosal for 1. Eleven (69%) of the 16 lesions arose in the rectum. The macroscopic appearance was the laterally spreading tumor-non-granular type for 4 lesions, sessile type for 4, laterally spreading tumor-granular type for 3, semi-pedunculated type (Isp) for 2, type 1 for 2, and type 3 for 1. The depth of invasion was correctly estimated by EUS for 15 lesions (94%) but was misdiagnosed as intramucosal for 1 carcinoma with high-grade submucosal invasion. The 2 lesions treated by endoscopic resection were intramucosal carcinoma and dysplasia, and both were diagnosed as intramucosal lesions by EUS.
CONCLUSION: EUS provides a good estimation of the invasion depth of UC-associated tumors and may thus facilitate the selection of treatment.
Core tip: The results of large studies evaluating endoscopic ultrasonography (EUS) for the diagnosis of ulcerative colitis (UC)-associated tumors have not been reported previously. The aim of this study was to evaluate the usefulness of EUS for UC-associated tumors. The study group comprised 16 UC-associated tumors for which the depth of invasion was preoperatively estimated by EUS. The depth of invasion was correctly estimated by EUS for 15 lesions (94%). The 2 mucosal lesions treated by endoscopic resection were both diagnosed correctly. EUS provides a good estimation of the invasion depth of UC-associated tumors and may thus facilitate the selection of treatment.
- Citation: Kobayashi K, Kawagishi K, Ooka S, Yokoyama K, Sada M, Koizumi W. Clinical usefulness of endoscopic ultrasonography for the evaluation of ulcerative colitis-associated tumors. World J Gastroenterol 2015; 21(9): 2693-2699
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2693
Ulcerative colitis (UC) is a chronic, refractory, inflammatory bowel disease with repeated flare-ups occurring mainly in young persons. Patients with long-term UC are known to be at increased risk for UC-associated tumors (carcinoma and dysplasia) arising from inflamed mucosa[1,2]. UC-associated carcinomas are often detected at an advanced stage and accompanied by intestinal obstruction, leading to a poor prognosis. Early detection and appropriate treatment are therefore required to improve the outcomes of patients with UC-associated tumors.
In the management of colorectal disease, endoscopic ultrasonography (EUS) is used to evaluate the invasion depth of carcinomas, select treatment[3-6], and qualitatively diagnose submucosal tumors[7,8]. Because surgery has conventionally been indicated for the treatment of UC-associated tumors, including dysplasia, EUS has not played an important role in the selection of treatment[9]. However, recent treatment guidelines for UC proposed in Europe and North America recommend endoscopic resection as a treatment option for protruding-type, adenoma-like dysplasia with no evidence of cancer or dysplasia of the surrounding mucosa[10,11]. However, as the decision to perform endoscopic resection must be based on an accurate preoperative estimation of the depth of tumor invasion, EUS is expected to play an increasingly more important role in the management of UC-associated tumors. Here, we retrospectively evaluate the diagnostic usefulness of EUS for the assessment of the depth of tumor invasion in a series of patients with UC-associated tumors.
The study group comprised 13 patients with 16 UC-associated tumors for which the depth of invasion was preoperatively estimated by EUS from August 1995 through June 2014 at Kitasato University East Hospital. All tumors were then treated by endoscopic resection or surgical colectomy and were diagnosed histopathologically. The mean age of the patients at the time of UC-associated tumor diagnosis was 48.2 ± 17.1 years (range: 27-70), and the mean duration of UC was 15.8 ± 8.3 years (range: 7-38). The extent of UC was pancolitis in 11 patients and left-sided colitis in 2. Clinically, UC was characterized by repeated flare-ups and remissions in 10 patients and chronic persistent disease in 3. One patient had previously undergone a total colectomy with ileorectostomy. Endoscopic examination at the time of EUS showed signs of mild inflammation of the mucosa surrounding 7 UC-associated tumors (44%). All of these lesions predominantly showed mucosal redness, edema, and erosion, with no signs of severe inflammation, such as deep ulcers or spontaneous bleeding. The mucosa surrounding the 9 other lesions (56%) appeared to be in a remission phase. Of the 16 UC-associated tumors, 14 were treated by surgical colectomy and 2 by endoscopic resection [endoscopic mucosal resection (EMR), 1 lesion; endoscopic submucosal dissection (ESD), 1 lesion].
To evaluate the invasion depth of UC-associated tumors by EUS, the involved portion of the intestine was filled with de-aerated water after the completion of conventional endoscopy. EUS was performed using a 20 MHz ultrasonic probe (UM-DP20-25R or UM3R, Olympus, Tokyo, Japan) for 15 lesions and a 12 MHz ultrasonic probe (UM2R, Olympus, Tokyo, Japan) for 1 lesion. All EUS examinations of UC-associated tumors were performed by endoscopists who had at least 10 years of experience in colonoscopy. Prior to colonoscopy and EUS, the patients were given an adequate explanation about the examination objectives and procedures, and written informed consent was obtained.
The normal wall of the large intestine is visualized as a 5-layer structure. The hyperechoic first layer from the luminal side and the hypoechoic second layer correspond to the boundary echo and the mucosa; the hyperechoic third layer corresponds to the submucosa (submucosal layer), the hypoechoic fourth layer to the muscularis propria, and the hyperechoic fifth layer to the subserosa or serosa (adventitia)[12]. The depth of invasion of the UC-associated tumors was estimated by EUS on the basis of tumor-induced narrowing of the wall layer structure or the deepest layer with disruption, similar to sporadic colorectal cancer. On EUS, the degree of submucosal invasion by cancer that invaded the wall of the large intestine (submucosal cancer) was classified as low-grade submucosal invasion for lesions with mild tumor-induced narrowing of the superior margin of the third layer (submucosa) and as high-grade submucosal invasion for lesions with severe narrowing or disruption of the third layer, with no changes of the fourth (muscularis propria) or fifth layers[4]. The internal echo level of the UC-associated tumors was evaluated as hyperechoic if it was equivalent to the echo level of the third layer of the normal colorectal wall, as hypoechoic if it was equivalent to the echo level of the fourth layer, and as isoechoic if it was between the echo levels of the third and fourth layers.
After resection, the UC-associated tumors were examined histopathologically. The depth of invasion of the UC-associated tumors according to EUS was compared with that according to histopathological examination of the resected specimens to evaluate the diagnostic accuracy of EUS. Our institutional review board approved the study protocol. Continuous data are presented as the mean ± SD.
The number of lesions detected by EUS in patients with UC-associated tumors was 1 in 11 patients, 2 in 1 patient, and 3 in 1 patient. Eleven lesions (69%) originated in the rectum, 2 in the sigmoid colon, 2 in the transverse colon, and 1 in the ascending colon. The macroscopic type of the UC-associated tumors according to the Paris Endoscopic Classification[13] and the Japanese Classification of Colorectal Carcinoma[14] was laterally spreading tumor-non-granular type (LST-NG) for 4 lesions, sessile type (Is) for 4, laterally spreading tumor-granular type (LST-G) for 3, semi-pedunculated type (Isp) for 2, type 1 for 2, and type 3 for 1. The mean tumor diameter was 3.4 ± 1.8 cm (range: 1.2-8.0).
The histologic diagnosis of the tumors was carcinoma for 13 lesions and dysplasia for 3. The depth of invasion of the carcinomas was intramucosal (pTis) for 8 lesions, submucosal (pT1) for 2, the muscularis propria (pT2) for 2, and subserosal (pT3) for 1. On the basis of the risk of metastasis, tumors with a submucosal vertical invasion depth of less than 1000 μm were defined as cancer with low-grade submucosal invasion, whereas tumors with a submucosal vertical invasion depth of 1000 μm or greater were defined as cancer with high-grade submucosal invasion[15]. The 2 submucosal carcinomas both exhibited high-grade submucosal invasion. The histopathological diagnosis of the carcinomas was well-differentiated tubular adenocarcinoma for 9 lesions, moderately differentiated tubular adenocarcinoma for 3, and a mixture of mucinous carcinoma and poorly differentiated adenocarcinoma for 1. The grade of atypia of the 3 dysplastic lesions was high-grade dysplasia for 2 lesions and low-grade dysplasia for 1.
The UC-associated tumors were depicted as a well-demarcated mass on EUS, similar to sporadic colorectal cancer and adenoma. The internal echo was isoechoic for 15 lesions and hypoechoic for 1 and was relatively homogeneous. The depth of invasion was accurately diagnosed by EUS for 15 lesions (94%); in contrast, the depth of invasion was misdiagnosed by EUS as intramucosal for 1 LST-NG lesion arising in the rectum (Table 1). On biopsy, this lesion was diagnosed as well-differentiated tubular adenocarcinoma, and surgery was performed. Histopathologically, the submucosal invasion depth was 1600 μm, indicating high-grade submucosal invasion.
| uM | uSM-H | uMP | uSS | |
| pM | 11 | |||
| pSM-H | 1 | 1 | ||
| pMP | 2 | |||
| pSS | 1 |
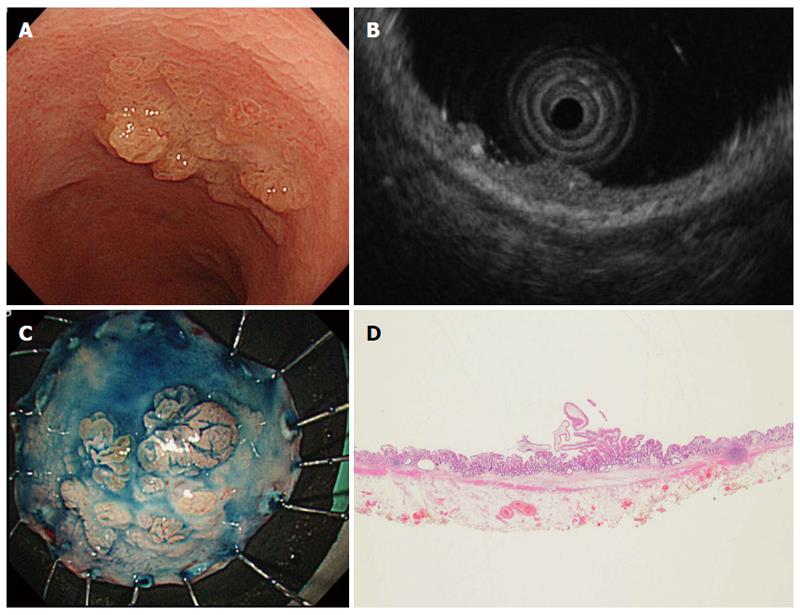
Two of the UC-associated tumors diagnosed by EUS were endoscopically resected. The macroscopic types were LST-G and Is (sessile), and the tumor diameters were 18 mm and 12 mm, respectively. The depth of invasion on EUS was evaluated to be intramucosal for both lesions. The histopathological diagnosis was low-grade dysplasia (Figure 1) and intramucosal cancer.
Among colorectal diseases, EUS is most often used to evaluate colorectal cancer, as the depth of invasion of colorectal cancer can be objectively evaluated on the basis of changes in the layer structure of the large intestinal wall using this technique. We previously reported that EUS facilitates the selection of treatment for early colorectal cancer[4].
In patients with UC, the thickness of the large intestinal wall and changes in the layer structure at inflamed sites on EUS are closely related to disease severity, as evaluated both clinically and endoscopically[16]. EUS has also been reported to be useful for predicting a response to medical treatment and the risk of flare-ups[16-19]. In contrast to Crohn’s disease, the intestinal inflammation associated with UC is usually confined to the mucosa and submucosa of the colorectal wall. In severe cases of UC requiring surgical treatment, however, intestinal inflammation may extend to the muscularis propria or deeper, occasionally leading to toxic megacolon. We previously performed basic studies of surgical specimens of UC and reported that the depth of intestinal inflammation according to EUS was usually consistent with that revealed by histopathological examination[16]. We also reported that many patients with UC in whom intestinal inflammation extends to the muscularis propria or deeper layers on EUS do not clinically respond to medical therapy and require surgery[16].
In the present study, we evaluated the diagnostic accuracy of EUS to assess the invasion depth of UC-associated tumors. Although this was a small study performed in a single center, our results suggest that EUS is useful for estimating the invasion depth of UC-associated tumors, similar to sporadic cases of colorectal cancer, and can thus facilitate the selection of treatment. To our knowledge, the only previous study that used EUS to evaluate UC-associated tumors was a case report by Shimizu et al[20], reporting that EUS was useful for the detection and diagnosis of UC-associated invasive carcinoma arising in the rectum. Results of large studies evaluating EUS for the diagnosis of UC-associated tumors have not yet been reported. Although we studied only 16 UC-associated tumors and used criteria designed to evaluate the invasion depth of sporadic colorectal cancer to estimate the depth of invasion of UC-associated tumors by EUS, the depth of invasion was correctly diagnosed by EUS for all but 1 lesion. A retrospective review of the EUS images of the lesion for which high-grade submucosal invasion was not correctly diagnosed showed evidence suggesting invasion of the submucosa by cancer.
With regard to the treatment strategy for UC-associated tumors, surgical therapy has been indicated for the treatment of not only invasive cancer but also high-grade dysplasia[21]. However, low-grade dysplasia repeatedly detected by endoscopic biopsy has a high risk of being associated with cancer or high-grade dysplasia at other sites of the large intestine, and surgery has therefore been recommended[22]. Blackstone et al[9] reported that lesions referred to as a dysplasia-associated lesion or mass (DALM) in patients with UC are frequently associated with cancer and that surgery should therefore be considered. However, Odze[23] classified DALM into well-demarcated adenoma-like DALM and poorly demarcated non-adenoma-like DALM and claimed that the former can be resected endoscopically, irrespective of the grade of atypia, provided that there is no evidence of cancer or dysplasia in other parts of the large intestine. Recent guidelines for the management of UC in Western countries[10,11] have also begun to acknowledge endoscopic resection as a treatment option for adenoma-like DALM unaccompanied by dysplasia or findings suggestive of cancer in other sites of the large intestine. The difficulty in distinguishing protruding adenoma-like DALM from sporadic adenoma by endoscopic examination and the recent progress in techniques for endoscopic resection such as ESD most likely also contributed to this revision of the guidelines. After endoscopic resection of adenoma-like DALM associated with UC, close endoscopic surveillance is mandatory, even though the risk of cancer developing in other sites of the colon has been reported to be low[24-26]. Surgery for UC is often associated with postoperative complications such as bowel dysfunction, negatively affecting the quality of life of an appreciable proportion of patients. Thus, an accurate preoperative endoscopic diagnosis is essential, and the number of lesions for which endoscopic resection is indicated may be relatively small. However, we believe that endoscopic therapy will play an increasingly important role in the treatment of UC-associated tumors in the future.
The ability to detect and qualitatively diagnose UC-associated tumors using endoscopy has been enhanced by combining conventional endoscopic examinations with the evaluation of tumor pit patterns on chromoendoscopy and magnifying endoscopy[27-30]. The invasion depth of UC-associated tumors is currently evaluated on the basis of the findings of conventional endoscopy and biopsy. However, an accurate estimation of the invasion depth of UC-associated tumors is sometimes precluded by the presence of unevenness or ulceration of the surrounding mucosa caused by the chronic inflammation associated with UC. Compared with sporadic colorectal cancer, UC-associated cancer is more often associated with poorly differentiated adenocarcinoma and may thus deeply invade the colon, with fewer changes of the surface mucosa. Accordingly, the invasion depth of such lesions may be difficult to estimate solely on the basis of conventional endoscopic findings, and EUS would most likely be helpful.
In our study, many lesions evaluated by EUS were found to be dysplasia or cancer limited to the mucosa, and the depth of invasion was accurately diagnosed by EUS, with the exception of 1 carcinoma with high-grade submucosal invasion. Therefore, even in patients with UC-associated tumors, combining EUS with conventional endoscopy may facilitate the selection of treatment and help to decide whether endoscopic resection is indicated for the management of adenoma-like DALM. Smith et al[31] performed EUS using an ultrasonic probe before EMR of an adenoma-like DALM and confirmed that there was no submucosal or deeper invasion or lymph-node metastasis.
Our study has several important limitations. It was a small, retrospective study performed in a single center. To our knowledge, however, the results of EUS in a series of patients with UC-associated tumors have not been reported previously. On the basis of our results, we conclude that EUS provides a good estimation of the invasion depth of UC-associated tumors. Although none of the patients in our study had severe UC requiring surgery, the presence of deep ulcers or inflammation can obscure the structure of the colonic wall on EUS[16]. Deep invasion by carcinomas also leads to a narrowing or rupture of the colonic wall structure on EUS, which can be difficult to distinguish from histologic changes caused by inflammation accompanying UC-associated tumors. Nonetheless, UC-associated tumors are depicted as a localized mass on EUS, and this feature may facilitate differential diagnosis from changes associated with diffuse intestinal inflammation. Further studies of a larger number of patients are needed to draw firm conclusions. The histopathological findings of resected specimens obtained from patients undergoing surgery for UC-associated tumors should be strictly compared with the findings according to EUS to analyze histopathological changes related to the apparent invasion depth of UC-associated tumors by EUS.
Patients with long-term ulcerative colitis (UC) are well known to be at increased risk for UC-associated tumors arising from inflamed mucosa. Conventionally, total colectomy was performed when UC-associated tumors were diagnosed, and the role of endoscopic ultrasonography (EUS) was limited. However, recent treatment guidelines for UC proposed in Europe and North America recommend that the presence or absence of tumorous lesions in other parts of the colorectum should be rigorously evaluated on endoscopy before treatment and have begun to recommend endoscopic resection as a treatment option for protruding-type adenoma-like dysplasia.
In the management of colorectal disease, EUS is useful to evaluate the invasion depth of sporadic carcinomas and adenomas, select treatment, and qualitatively diagnose submucosal tumors. The research hotspot of this article is to clarify the diagnostic usefulness of EUS for the assessment of the depth of tumor invasion in a series of patients with UC-associated tumors.
The only previous study that used EUS to evaluate UC-associated tumors was a case report. Results of large studies evaluating EUS for the diagnosis of UC-associated tumors have not yet been reported. This is the first study to show that EUS can be used for diagnosis of the depth of invasion as well as for the selection of treatment in relatively many patients with UC-associated tumors.
This study results suggest that EUS is useful for estimating the invasion depth of UC-associated tumors, similar to sporadic cases of colorectal cancer. Therefore, even in patients with UC-associated tumors, combining EUS with conventional endoscopy may facilitate the selection of treatment and help to decide whether endoscopic resection is indicated for the management of protruding-type adenoma-like dysplasia.
The diagnostic criteria used to evaluate the invasion depth of UC-associated tumors by EUS in our study were similar to those used for sporadic colorectal cancer. In our series, the invasion depth of all but 1 UC-associated tumor was correctly assessed by EUS. This finding suggests that the diagnostic criteria used to estimate the invasion depth of sporadic colorectal cancer by EUS can be used to some extent to evaluate UC-associated tumors. However, in the presence of severe active inflammation or fibrosis of the intestine surrounding UC-associated tumors, which was not found in our series, the diagnostic criteria might not be able to be used. Further studies of larger numbers of cases are needed.
In this paper, Kobayashi et al evaluated the clinical usefulness of EUS for diagnosis of the invasion depth of UC-associated tumors, and concluded that EUS provides a good estimation of the invasion depth of UC-associated tumors. This paper is well written and the content of the paper is interesting.
P- Reviewer: Caviglia RD, Iizuka M, Moussata D, Sipos F S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2077] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 2. | Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Saitoh Y, Obara T, Einami K, Nomura M, Taruishi M, Ayabe T, Ashida T, Shibata Y, Kohgo Y. Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc. 1996;44:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Kobayashi K, Kida M, Katsumata T, Yoshizawa S, Yokoyama K, Sada M, Igarashi M, Saigenji K. Clinical usefulness of endoscopic ultrasonography for the diagnosis of early colorectal cancer and selecting the treatment procedure. Dig Endosc. 2003;15:298-305. |
| 5. | Akasu T, Kondo H, Moriya Y, Sugihara K, Gotoda T, Fujita S, Muto T, Kakizoe T. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Hizawa K, Suekane H, Aoyagi K, Matsumoto T, Nakamura S, Fujishima M. Use of endosonographic evaluation of colorectal tumor depth in determining the appropriateness of endoscopic mucosal resection. Am J Gastroenterol. 1996;91:768-771. [PubMed] |
| 7. | Kobayashi K, Katsumata T, Yoshizawa S, Sada M, Igarashi M, Saigenji K, Otani Y. Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum. 2005;48:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Kameyama H, Niwa Y, Arisawa T, Goto H, Hayakawa T. Endoscopic ultrasonography in the diagnosis of submucosal lesions of the large intestine. Gastrointest Endosc. 1997;46:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [PubMed] |
| 10. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-4; quiz e12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 11. | Biancone L, Michetti P, Travis S, Escher JC, Moser G, Forbes A, Hoffmann JC, Dignass A, Gionchetti P, Jantschek G. European evidence-based Consensus on the management of ulcerative colitis: Special situations. J Crohns Colitis. 2008;2:63-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, Silverstein FE. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology. 1989;96:433-441. [PubMed] |
| 13. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1325] [Article Influence: 60.2] [Reference Citation Analysis (4)] |
| 14. | Japanese Society for Cancer of the Colon and rectum. Japanese Classification of Colorectal Carcinoma 2nd edition. Tokyo: Kanehara & Co 2009; 5-6. |
| 15. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 486] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 16. | Yoshizawa S, Kobayashi K, Katsumata T, Saigenji K, Okayasu I. Clinical usefulness of EUS for active ulcerative colitis. Gastrointest Endosc. 2007;65:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Shimizu S, Tada M, Kawai K. Endoscopic ultrasonography in inflammatory bowel diseases. Gastrointest Endosc Clin N Am. 1995;5:851-859. [PubMed] |
| 18. | Higaki S, Nohara H, Saitoh Y, Akazawa A, Yanai H, Yoshida T, Okita K. Increased rectal wall thickness may predict relapse in ulcerative colitis: a pilot follow-up study by ultrasonographic colonoscopy. Endoscopy. 2002;34:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Tsuga K, Haruma K, Fujimura J, Hata J, Tani H, Tanaka S, Sumii K, Kajiyama G. Evaluation of the colorectal wall in normal subjects and patients with ulcerative colitis using an ultrasonic catheter probe. Gastrointest Endosc. 1998;48:477-484. [PubMed] |
| 20. | Shimizu S, Myojo S, Nagashima M, Okuyama Y, Sugeta N, Sakamoto S, Kutsumi H, Otsuka H, Suyama Y, Fujimoto S. A patient with rectal cancer associated with ulcerative colitis in whom endoscopic ultrasonography was useful for diagnosis. J Gastroenterol. 1999;34:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 22. | Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 362] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol. 2003;16:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Rubin PH, Friedman S, Harpaz N, Goldstein E, Weiser J, Schiller J, Waye JD, Present DH. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288-1294; discussion 1488-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Hurlstone DP, Sanders DS, Atkinson R, Hunter MD, McAlindon ME, Lobo AJ, Cross SS, Thomson M. Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut. 2007;56:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 28. | Matsumoto T, Nakamura S, Jo Y, Yao T, Iida M. Chromoscopy might improve diagnostic accuracy in cancer surveillance for ulcerative colitis. Am J Gastroenterol. 2003;98:1827-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Sada M, Igarashi M, Yoshizawa S, Kobayashi K, Katsumata T, Saigenji K, Otani Y, Okayasu I, Mitomi H. Dye spraying and magnifying endoscopy for dysplasia and cancer surveillance in ulcerative colitis. Dis Colon Rectum. 2004;47:1816-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Nishiyama S, Oka S, Tanaka S, Hayashi N, Hayashi R, Nagai K, Ueno Y, Shimamoto F, Arihiro K, Chayama K. Is it possible to discriminate between neoplastic and nonneoplastic lesions in ulcerative colitis by magnifying colonoscopy? Inflamm Bowel Dis. 2014;20:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Smith LA, Baraza W, Tiffin N, Cross SS, Hurlstone DP. Endoscopic resection of adenoma-like mass in chronic ulcerative colitis using a combined endoscopic mucosal resection and cap assisted submucosal dissection technique. Inflamm Bowel Dis. 2008;14:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |