Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2651
Peer-review started: September 7, 2014
First decision: October 14, 2014
Revised: November 2, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: March 7, 2015
Processing time: 184 Days and 5 Hours
AIM: To investigate the protective effect of clodronate-containing liposomes against severe acute pancreatitis (SAP)-triggered acute gastric mucosal injury (AGMI) in rats.
METHODS: Clodronate- and phosphate-buffered saline (PBS)-containing liposomes were prepared by reverse-phase evaporation. The SAP rat model was established by injecting sodium taurocholate into the pancreatic subcapsular space. Sprague-Dawley rats were randomly divided into three groups: control (C), SAP plus PBS-containing liposome (P) and SAP plus clodronate-containing liposome (T). Serum tumor necrosis factor (TNF)-α levels were estimated by ELISA. Pathological changes in the gastric mucosa and pancreas were observed by hematoxylin and eosin (HE) staining. Apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling staining. The numbers of macrophages in the gastric mucosa were analyzed by CD68 immunohistochemical staining.
RESULTS: The liposomes had a mean diameter of 150 ± 30 nm. The TNF-α levels were significantly higher in the P group than that in the C group (2 h, 145.13 ± 11.50 vs 23.2 ± 2.03; 6 h, 245.06 ± 12.11 vs 30.28 ± 6.07, P < 0.05), and they were significantly lower in the T group than that in the P group (2 h, 93.24 ± 23.11 vs 145.13 ± 11.50; 6 h, 135.18 ± 13.10 vs 245.06 ± 12.11, P < 0.05). The pathological scores of the pancreas were lower in the T group than in the P group (2 h, 1.88 ± 0.83 vs 4.13 ± 0.83; 6 h, 2.87 ± 0.64 vs 6.25 ± 0.88, P < 0.01). The pathological scores of the gastric mucosa were also lower in the T group than in the P group (2 h, 1.12 ± 0.64 vs 2 ± 0.75; 6 h, 1.58 ± 0.53 vs 3 ± 1.31, P < 0.05). In addition, increased CD68 levels were observed in the gastric mucosa of the P group compared with the C group. Clodronate-containing liposomes decreased the CD68 levels in the mucosa of the T group. The apoptotic indexes of the gastric mucosa were higher in the T group than in the P group (2 h, 15.7 ± 0.92 vs 11.5 ± 1.64; 6 h, 21.12 ± 1.06 vs 12.6 ± 2.44, P < 0.01).
CONCLUSION: Gastric macrophages contribute to the pathogenesis of gastric injury in SAP. Clodronate-containing liposomes have protective effects against AGMI in rats with SAP.
Core tip: In this study, we investigated the protective effect of clodronate liposomes against severe acute pancreatitis (SAP)-triggered acute gastric mucosal injury in rats. Our results revealed that gastric macrophages are involved in the pathogenesis of gastric injury in SAP. Moreover, clodronate-containing liposomes have protective effects against gastric mucosal injury in rats with SAP. Therefore, the blockade of macrophage infiltration may represent a novel therapeutic strategy for the treatment of SAP.
- Citation: Dang SC, Wang H, Zhang JX, Cui L, Jiang DL, Chen RF, Qu JG, Shen XQ, Chen M, Gu M. Are gastric mucosal macrophages responsible for gastric injury in acute pancreatitis? World J Gastroenterol 2015; 21(9): 2651-2657
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2651.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2651
Severe acute pancreatitis (SAP) is often complicated by systemic inflammatory response syndrome (SIRS), eventually leading to dysfunction of multiple organs, including the liver, lungs, kidneys, intestine, and stomach[1-4]. Up to 50% of patients with SAP may have stress-related acute gastric mucosal injury (AGMI)[5]. Despite intensive research and clinical investigations, the pathogenesis of AGMI induced by SAP remains unclear. SAP-induced AGMI may be associated with ischemic reperfusion injury, excessive release of inflammatory mediators, microcirculatory disturbance, and oxidative stress[6-11].
Recent research has revealed that activated macrophages secrete inflammatory cytokines such as TNF-α and IL-6 etc., which results in gastric mucosal injury[12-15]. Increased apoptosis in the gastric mucosa is known to be responsible for SAP-associated mucosal dysfunction[16]. Gastric mucosal macrophages secrete inflammatory factors, and therefore play a key role in AGMI. Hence depleting macrophages may reduce gastric mucosal damage in SAP.
Clodronate belongs to the bisphosphonate (BP) family of drugs, and it is a potent inhibitor of osteoporosis and other diseases[17,18]. As with other BPs, clodronate liposomes are readily phagocytosed by macrophages[19]. Van Rooijen et al[20] reported that clodronate could selectively inhibit the viability of macrophages by inducing apoptosis. Once clodronate is incorporated within liposomes, it is phagocytosed by macrophages, resulting in selective depletion of macrophages[21].
In this study, we investigated the effect of clodronate-containing liposomes on AGMI. Our results provide the basis for a new strategy for SAP treatment.
Forty-eight Sprague-Dawley (SD) rats (weight, 350-385 g) were provided by the Jiangsu University. Sodium taurocholate was obtained from Sigma (Sigma, United States). Clodronate was obtained from Wei-jing (Shanghai Wei-jing Technology Enterprise Co., Ltd.). The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit was purchased from Roche (In situ Apoptosis Detection Kit; Cat. No. 11684817910; Roche, Switzerland). The CD68 immunohistochemical kit was purchased from Fuzhou Maxim Company (China), and tumor necrosis factor (TNF)-α was purchased from Invitrogen Corp. (Carlsbad, California, United States).
Clodronate and phosphate-buffered saline (PBS) (control) liposomes were prepared by reverse-phase evaporation, as described previously[20]. The suspensions were stored in an inert atmosphere of nitrogen until use. The concentration of encapsulated clodronate was determined using an ultraviolet spectrophotometer. The liposomes were dissolved in 4 mL of sterilized PBS (5 mg/mL). The suspension was shaken gently before administration (dosage, 20 mg/kg) to the rats, as described by Brigham et al[22] and Zhang et al[23].
The rats were housed in individual cages maintained at 21-23 °C and 60% ± 10% humidity. The rats were acclimatized for 1 wk before commencement of any experimental procedure, and allowed access to standard rat chow and water. All animal experiments were conducted in compliance with the guidelines specified by the Institutional Ethics Board. The rats were randomly divided into three groups: C group (control), P group (SAP + PBS-liposome), and T group (SAP + clodronate-liposome). These groups were further divided into two subgroups each: 2 h and 6 h (n = 8 in each group). The rat model with SAP was established by injecting sodium taurocholate (2 mL/kg body weight) into the pancreatic subcapsular space. PBS-containing liposomes (2 mL/kg body weight) were injected into the rats of the P group very slowly through the tail vein. Similarly, clodronate-containing liposomes were injected into the T-group rats, and normal saline into the C-group rats. At 2 and 6 h after the injections, the animals were sacrificed and the gastric mucosa and pancreas were harvested. No mortalities were observed at 2 and 6 h.
To assess the TNF-α level, blood was collected from the superior mesenteric vein. Serum TNF-α level was estimated using an ELISA kit. The data were expressed as pg/mL.
Pancreatic and gastric tissue samples (4-μm thick) were fixed in 10% buffered formalin for 24 h. The paraffin-embedded tissue sections were then stained with hematoxylin and eosin (HE), and observed by a morphologist who was blinded to the experiments. The pancreatic pathological score was determined according to Kaiser’s scoring criteria[24]. The degree of pathological injury was assessed under a light microscope (each sample was blindly evaluated by a pathologist). The degree of gastric mucosa injury was scored according to the Masuda criteria[25], with slight modifications, as follows: normal, 0; injury in the surface epithelium, 1; congestion and edema in the upper mucosa, 2; congestion, hemorrhage, and edema in the middle and lower mucosa, 3; structural disorder or necrosis in the upper mucosal glands, 4; and deep necrosis and ulceration, 5. The average injury score for each rat was calculated from 10 random fields.
Apoptosis of the tissue samples was detected by TUNEL staining according to the manufacturer’s protocol. Data were presented as the average results of three sections per tissue per rat.
Gastric mucosal macrophage infiltration in the rats was assessed by CD68 immunohistochemical staining using a commercial monoclonal anti-rat CD68 (macrophage marker) antibody.
The Statistical Product and Service Solutions software (version 19.0) was used for the analyses. All values were reported as the mean ± SD. One-way analysis of variance was performed in cases where equal variance was assumed. Otherwise, a nonparametric test (Kruskal-Wallis test) was used. AGMI grading was analyzed using the Mann-Whitney U test. A P value less than 0.05 was considered statistically significant.
The spherical and mainly unilamellar liposomes had similar size distributions, as assessed by transmission electron microscopy (TEM). The liposomes had a mean diameter of 150 ± 30 nm. The encapsulation yield of the liposomes was 26%.
The serum TNF-α levels were higher in the P group than in the C group (2 h, 145.13 ± 11.50 vs 23.2 ± 2.03; 6 h, 245.06 ± 12.11 vs 30.28 ± 6.07, P < 0.05). The serum TNF-α levels were significantly decreased in the T group, compared with the P group (2 h, 93.24 ± 23.11 vs 145.13 ± 11.50; 6 h, 135.18 ± 13.10 vs 245.06 ± 12.11, P < 0.05).
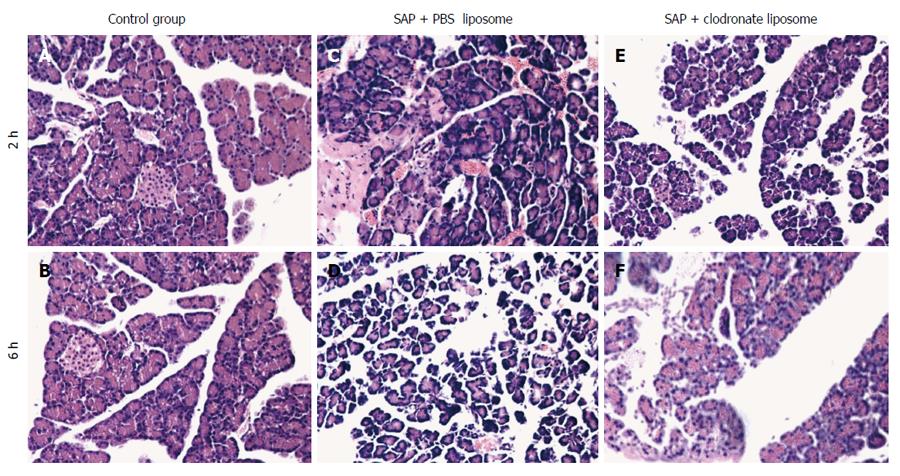
No significant changes were observed in the pancreas of the C group rats upon gross observation. Visible bloody ascites, pancreatic edema, hemorrhage, and necrosis were observed in the abdominal cavity of rats in the P group, and mild morphological changes in the pancreas, small amount of ascites, and visible pancreatic focal hemorrhage were observed in the T group. Microscopically, the rats in the C group showed normal pancreatic histology; the pancreas of the P-group rats were faintly edematous, with some inflammatory cell infiltration (at 2 h). Adipose tissue surrounding the pancreas, moderate hemorrhage, necrosis, and pancreatic acinar cells and tissues were also observed (at 6 h). The rats in the T group had mild pancreatic interstitial edema and neutrophil and mononuclear cell infiltration. However, no significant hemorrhage or necrosis was observed (Figure 1). The pancreatic histological scores were significantly different in the P and T groups, compared with the C group (2 h, 4.13 ± 0.83, 1.88 ± 0.83 vs 1 ± 0.53; 6 h, 6.25 ± 0.88, 2.87 ± 0.64 vs 1.25 ± 0.46, P < 0.01). The pathological change in the T group was less severe than that in the P group (2 h, 1.88 ± 0.83 vs 4.13 ± 0.83; 6 h, 2.87 ± 0.64 vs 6.25 ± 0.88, P < 0.01).
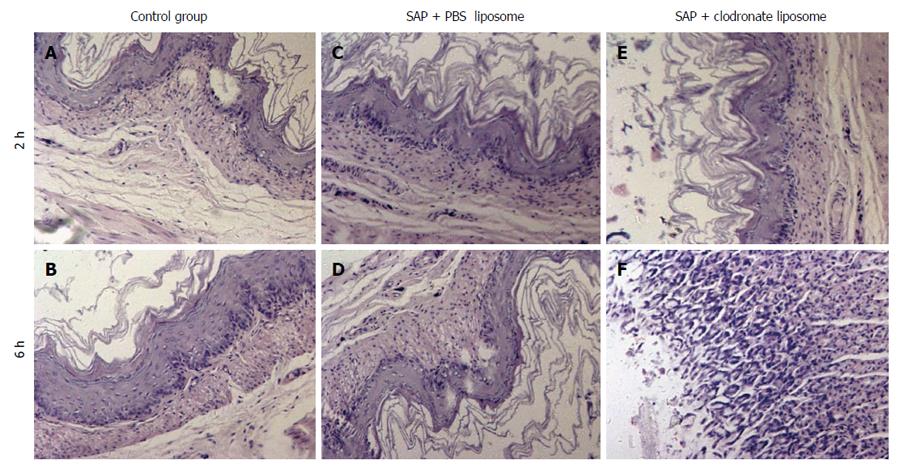
The mucosal surface of the C-group rats was smooth, with no significant abnormality, and the gastric mucosal glands were of consistent size and shape, and were neatly arranged in a single column. No inflammatory cell infiltration into the mucosal layer was detected. In the SAP group, extensive edema, leucocyte infiltration, and disoriented/asymmetrical gastric tubes were observed at 2 h after SAP induction. At 6 h, these pathological changes were even more pronounced. The T-group rats had reduced number of inflammatory cells in the gastric mucous layer, neatly arranged gastric glands, and mucus thickening, and showed recovery of glandular structure. The severity of the pathological scores in the P and T groups were significantly higher than that in the C group (2 h, 2 ± 0.75, 1.12 ± 0.64 vs 0.13 ± 0.35; 6 h, 3 ± 1.31, 1.58 ± 0.53 vs 0.25 ± 0.46, P < 0.05). The severity score of the P group was significantly higher than that of the T group (P < 0.05; Figure 2).
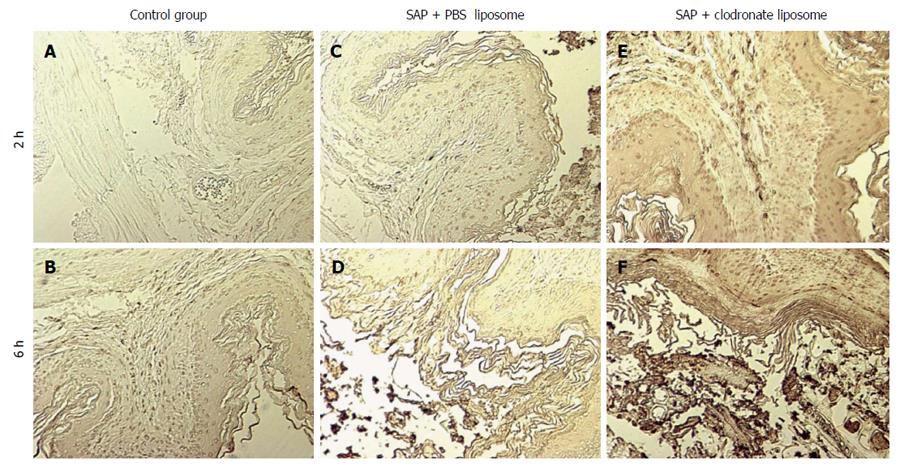
Additional evidence of apoptosis in the gastric mucosa was obtained by a TUNEL assay. There were significant differences regarding the number of apoptotic cells between the C and P groups (2 h, 3.38 ± 2.12 vs 11.5 ± 1.64; 6 h, 4 ± 2.9 vs 12.6 ± 2.44, P < 0.05). The apoptotic cell index of the mucosal macrophages in the T group at 2 and 6 h was significantly higher than that in the P group (2 h, 15.7 ± 0.92 vs 11.5 ± 1.64; 6 h, 21.12 ± 1.06 vs 12.6 ± 2.44, P < 0.05; Figure 3).
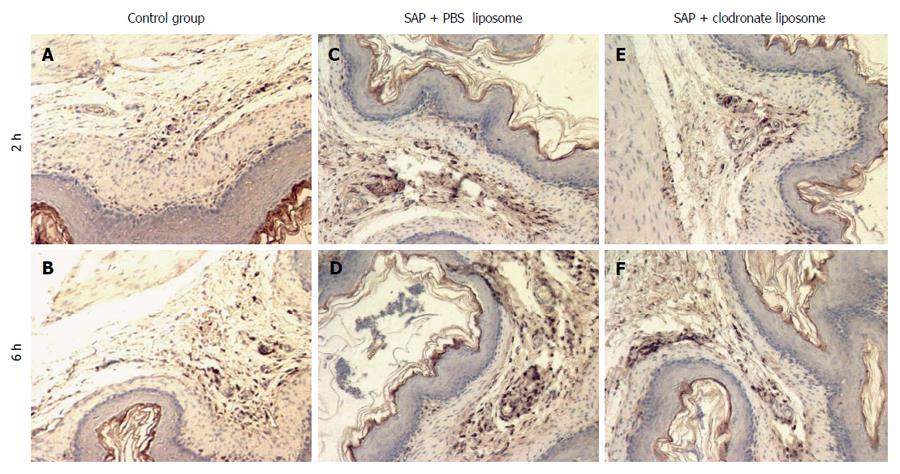
Immunostaining for CD-68 revealed that the gastric tissue was under homeostatic conditions in the C group. Macrophage numbers were decreased in the gastric tissue sections obtained from the T group. Normal mucosa with basal levels of CD68 expression was observed in the C group. Intense staining of CD68-positive cells was observed in the muscularis, submucosa (muscularis mucosa), and mucosa layers of the stomach, particularly in the muscularis mucosa and the vascular system of the P-group rats. Clondronate administration significantly decreased the staining of CD68, particularly in the muscularis mucosa of the T group, compared with the P group (Figure 4).
The results of the present study confirmed that SAP is associated with complications such as SIRS, MODS, AGMI, and local pathogenesis within the pancreas. SAP-induced pathological changes in the stomach worsen over time. Diffused microcirculatory disorders (MCDs) may play a key role in the development of AGMI. However, the underlying mechanism is still unknown. AGMI causes disturbance of microcirculation, which can damage the gastric mucosa and lead to vasoconstriction, shunting, leukocyte adherence, increased blood viscosity, and coagulation. Oxygen free radicals, ischemia-reperfusion injury, and various inflammatory mediators are the principal mediators of the transformation of AGMI from a local inflammatory process to a systemic illness. The excessive proinflammatory cytokine release associated with AGMI is responsible for the deterioration of local and systemic functions[10]. During the last decade, several studies have indicated that macrophages are the initial mediators of SAP, which eventually leads to multiple organ failure[26-29]. Therefore, treating SAP by depleting the infiltrating macrophages has attracted immense interest.
Mediators of inflammation, produced by macrophages, induce an inflammatory cascade, and cause gastric mucosal injury and gastric dysfunction. TNF-α, produced by macrophages, results in injury to multiple organs and causes inflammation, edema, ischemia, hemorrhage, and neutrophilic leucocytes accumulation. The neutrophilic leucocytes in turn secrete various pro-inflammatory factors[30,31]. At the early stage of SAP, interleukin (IL)-1β stimulates the expression of phospholipase A-1 (PLA-2), and triggers vascular migration of monocytes to the site of infection. MCD, nitrous oxide, and reactive oxygen species are crucial mediators of AGMI[32-34].
In this study, we investigated the possible contribution of gastric mucosal macrophages to the severity of induced SAP. TNF-α level was notably increased in the P group, and this increase was suppressed by clodronate in the T group rats (P < 0.01). Less severe gastric mucosal damage was observed in the T group compared with the P group (P < 0.01).
Gastric tissue injury was closely correlated with CD68 expression, indicating that gastric mucosal macrophages are involved in the pathogenesis of gastric mucosa injury in SAP. Depletion of macrophages by liposome-encapsulated clodronate inhibited inflammation and gastric injury in rats with SAP. This might serve as the basis for a new therapeutic strategy for the treatment of SAP and SAP-induced gastric mucosa injury.
In conclusion, our study revealed that macrophages might be the main mediators of SAP-induced AGMI, and that depletion of macrophages can markedly reduce gastric inflammation.
Severe acute pancreatitis (SAP) is often associated with systemic inflammatory response syndrome, and eventually leads to dysfunction of multiple organs. Up to 50% of patients with SAP may have stress-related acute gastric mucosal injury. Recent studies have shown that activated macrophages secrete inflammatory factors that lead to systemic inflammatory response and eventually MODS, including gastric mucosal injury.
In this study, the authors demonstrated that SAP-induced inflammation is mediated by macrophage infiltration in the gastric mucosa, and treatment with clodronate depletes macrophages. The study provides the basis for a novel therapeutic strategy for the treatment of SAP.
In the present study, the authors examined whether liposomes containing clodronate could prevent the development of SAP-induced gastric mucosal injury by modulating the inflammatory process.
This is an experimental study designed to detect the infiltration of macrophages into the gastric mucosa in SAP and to evaluate the effects of clodronate-containing liposomes on gastric mucosal macrophages. The authors concluded that clodronate-containing liposomes protected SAP rats against gastric mucosal injury.
Clodronate belongs to the bisphosphonate family of drugs. Bisphosphonate clodronate could selectively inhibit the viability of macrophages by inducing apoptosis.
The manuscript is interesting and reports important data on acute pancreatitis. In the Discussion, the authors report that clodronate-containing liposomes induce apoptosis in macrophages.
P- Reviewer: Shiotani A S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Rau BM, Krüger CM, Hasel C, Oliveira V, Rubie C, Beger HG, Schilling MK. Effects of immunosuppressive and immunostimulative treatment on pancreatic injury and mortality in severe acute experimental pancreatitis. Pancreas. 2006;33:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Zhang JX, Dang SC, Qu JG, Wang XQ, Chen GZ. Changes of gastric and intestinal blood flow, serum phospholipase A2 and interleukin-1beta in rats with acute necrotizing pancreatitis. World J Gastroenterol. 2005;11:3578-3581. [PubMed] |
| 3. | Zhang JX, Dang SC. Ligustrazine alleviates acute lung injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:605-609. [PubMed] |
| 4. | Shi C, Andersson R, Zhao X, Wang X. Potential role of reactive oxygen species in pancreatitis-associated multiple organ dysfunction. Pancreatology. 2005;5:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Hsu CY, Lee KC, Chan CC, Lee FY, Lin HC. Gastric necrosis and perforation as a severe complication of pancreatic pseudocyst. J Chin Med Assoc. 2009;72:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Dang SC, Zhang JX, Qu JG, Wang XQ, Fan X. Ligustrazine alleviates gastric mucosal injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:213-218. [PubMed] |
| 7. | Soong CV, Lewis HG, Halliday MI, Rowlands BJ. Intramucosal acidosis and the inflammatory response in acute pancreatitis. Am J Gastroenterol. 1999;94:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Dobosz M, Hac S, Mionskowska L, Dymecki D, Dobrowolski S, Wajda Z. Organ microcirculatory disturbances in experimental acute pancreatitis. A role of nitric oxide. Physiol Res. 2005;54:363-368. [PubMed] |
| 9. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Osman MO, Gesser B, Mortensen JT, Matsushima K, Jensen SL, Larsen CG. Profiles of pro-inflammatory cytokines in the serum of rabbits after experimentally induced acute pancreatitis. Cytokine. 2002;17:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Yeo M, Kim DK, Cho SW, Lee SJ, Cho IH, Song GS, Moon BS. New phytopharmaceutical agent CJ-20001 modulates stress-induced inflammatory infiltration into gastric mucosa. Hepatogastroenterology. 2012;59:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Mikami Y, Takeda K, Shibuya K, Qiu-Feng H, Shimamura H, Yamauchi J, Egawa S, Sunamura M, Yagi H, Endo Y. Do peritoneal macrophages play an essential role in the progression of acute pancreatitis in rats? Pancreas. 2003;27:253-260. [PubMed] |
| 13. | Wereszczynska-Siemiatkowska U, Dlugosz JW, Siemiatkowski A, Chyczewski L, Gabryelewicz A. Lysosomal activity of pulmonary alveolar macrophages in acute experimental pancreatitis in rats with reference to positive PAF-antagonist (BN 52021) effect. Exp Toxicol Pathol. 2000;52:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Gutierrez PT, Folch-Puy E, Bulbena O, Closa D. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut. 2008;57:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Dang SC, Zeng YH, Wang PJ, Chen BD, Chen RF, Kumar Singh A, Kumar P, Feng S, Cui L, Wang H. Clodronate-superparamagnetic iron oxide-containing liposomes attenuate renal injury in rats with severe acute pancreatitis. J Zhejiang Univ Sci B. 2014;15:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ercan S, Basaranlar G, Gungor NE, Kencebay C, Sahin P, Celik-Ozenci C, Derin N. Ghrelin inhibits sodium metabisulfite induced oxidative stress and apoptosis in rat gastric mucosa. Food Chem Toxicol. 2013;56:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Yi S, Hawthorne WJ, Lehnert AM, Ha H, Wong JK, van Rooijen N, Davey K, Patel AT, Walters SN, Chandra A. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J Immunol. 2003;170:2750-2758. [PubMed] |
| 18. | Berardi D, Raffaelli L, Perfetti G, Paolantonio M, Trisi P. Clodronate combined with a surfactant (Tween 20) does not improve osseointegration: a rabbit immunohistomorphometric study. Int J Immunopathol Pharmacol. 2009;22:829-835. [PubMed] |
| 19. | Yanai A, Maeda S, Shibata W, Hikiba Y, Sakamoto K, Nakagawa H, Ohmae T, Hirata Y, Ogura K, Muto S. Activation of IkappaB kinase and NF-kappaB is essential for Helicobacter pylori-induced chronic gastritis in Mongolian gerbils. Infect Immun. 2008;76:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93-99. [PubMed] |
| 21. | Frith JC, Mönkkönen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5’-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 317] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Brigham DE, Little G, Lukyanenko YO, Hutson JC. Effects of clodronate-containing liposomes on testicular macrophages and Leydig cells in vitro. J Endocrinol. 1997;155:87-92. [PubMed] |
| 23. | Zhang JX, Dang SC, Qu JG, Wang XQ. Preventive effect of tetramethylpyrazine on intestinal mucosal injury in rats with acute necrotizing pancreatitis. World J Gastroenterol. 2006;12:6386-6390. [PubMed] |
| 24. | Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295-C1304. [PubMed] |
| 25. | Masuda E, Kawano S, Nagano K, Tsuji S, Takei Y, Hayashi N, Tsujii M, Oshita M, Michida T, Kobayashi I. Role of endogenous endothelin in pathogenesis of ethanol-induced gastric mucosal injury in rats. Am J Physiol. 1993;265:G474-G481. [PubMed] |
| 26. | Xu XW, Yang XM, Bai YH, Zhao YR, Shi GS, Zhang JG, Zheng YH. Treatment with ginkgo biloba extract protects rats against acute pancreatitis-associated lung injury by modulating alveolar macrophage. Prz Gastroenterol. 2014;9:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Xue P, Guo J, Yang XN, Huang W, Xia Q. Changes of neuronal acetylcholine receptor alpha 7 of peritoneal macrophage in experimental acute pancreatitis treated by Chaiqin Chengqi Decoction (). Chin J Integr Med. 2014;20:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Gea-Sorlí S, Guillamat R, Serrano-Mollar A, Closa D. Activation of lung macrophage subpopulations in experimental acute pancreatitis. J Pathol. 2011;223:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Campos LM, Omori CH, Lotito AP, Jesus AA, Porta G, Silva CA. Acute pancreatitis in juvenile systemic lupus erythematosus: a manifestation of macrophage activation syndrome? Lupus. 2010;19:1654-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A. Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut. 1994;35:909-915. [PubMed] |
| 31. | Hsu DZ, Chen YW, Chu PY, Periasamy S, Liu MY. Protective effect of 3,4-methylenedioxyphenol (sesamol) on stress-related mucosal disease in rats. Biomed Res Int. 2013;2013:481827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Ueda K, Ueyama T, Yoshida K, Kimura H, Ito T, Shimizu Y, Oka M, Tsuruo Y, Ichinose M. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol. 2008;295:G460-G469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Ortiz-Masiá D, Hernández C, Quintana E, Velázquez M, Cebrián S, Riaño A, Calatayud S, Esplugues JV, Barrachina MD. iNOS-derived nitric oxide mediates the increase in TFF2 expression associated with gastric damage: role of HIF-1. FASEB J. 2010;24:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Chen H, Yang L. Toll-like receptor 4 participates in gastric mucosal protection through Cox-2 and PGE2. Dig Liver Dis. 2010;42:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |