Published online Mar 7, 2015. doi: 10.3748/wjg.v21.i9.2645
Peer-review started: September 21, 2014
First decision: October 14, 2014
Revised: November 6, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: March 7, 2015
Processing time: 169 Days and 17.5 Hours
AIM: To investigate angiopoietin (Ang) and vascular endothelial growth factor (VEGF) expression in rats with ulcerative colitis (UC) and colorectal cancer (CRC).
METHODS: Dysplasia and cancer were investigated in rats that received three cycles of 3.5% dextran sulfate sodium (DSS) in drinking water for 7 d followed by distilled water for 14 d after intraperitoneal pretreatment with 20 mg/kg 1,2-dimethylhydrazine (DMH) (CRC group). Colitis was investigated in rats that received three cycles of 3.5% DSS in drinking water for 7 d followed by distilled water for 14 d after intraperitoneal pretreatment with saline (UC group). Rats without DSS or DMH treatment served as controls. Expression of the tyrosine kinase with immunoglobulin-like and EGF-like domains (Tie)-2 and its ligands, Ang-1 and Ang-2, as well as VEGF were evaluated in the colorectum by Western blotting.
RESULTS: Compared with rats in the control group, rats in the CRC and UC groups developed the symptoms of acute colitis with diarrhea, rectal bleeding, wasting, and loss of body weight (P < 0.05). In addition, the mean length of colorectum of CRC and UC rats was significantly shorter than that of control rats (8.29 ± 0.21 and 8.31 ± 0.86, respectively, vs 12.34 ± 0.12 cm; P < 0.05). Furthermore, rats in the CRC group, but not in the UC or control groups, developed multiple tumors in the colorectal region. Western blot analysis revealed that rats in the CRC and UC groups had markedly increased protein levels of Ang-1, Ang-2, Tie-2, and VEGF in the colorectum compared to rats in the control group.
CONCLUSION: Increased expression of Ang-1, Ang-2, Tie-2, and VEGF in ulcerative colitis-derived colorectal cancer might lead to chronic colitis and pathologic angiogenesis in rats.
Core tip: Colorectal cancer is the most severe complication in ulcerative colitis. Angiopoietin (Ang)-1, Ang-2, their receptor, tyrosine kinase with immunoglobulin-like and EGF-like domains (Tie)-2, and vascular endothelial growth factor may play a key role in forming vasculature which is involved in carcinogenesis. In this study, rats with ulcerative colitis and colorectal cancer had increased expression of these proteins, which suggests they may contribute to the development of colon cancer, chronic colitis, and pathologic angiogenesis in the rat.
- Citation: Liu WX, Gu SZ, Zhang S, Ren Y, Sang LX, Dai C. Angiopoietin and vascular endothelial growth factor expression in colorectal disease models. World J Gastroenterol 2015; 21(9): 2645-2650
- URL: https://www.wjgnet.com/1007-9327/full/v21/i9/2645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i9.2645
Colorectal cancer (CRC) is the third most common malignancy and one of the leading causes of cancer-related deaths worldwide[1,2]. Ulcerative colitis (UC) is a chronic inflammatory bowel disease of the large intestine in which the severity and extent of disease can range from mild proctitis to extensive fulminant disease[3,4]. The incidence of CRC has been increasing in patients with UC, and the risk of CRC increases with increased extent and duration[5-8]. However, the mechanisms underlying the frequent occurrence of CRC in patients with UC are still elusive.
Angiogenesis is necessary for the initiation and progression of malignant tumors and thus is considered as a hallmark of cancer[9,10]. In various hypotheses, the formation of new blood vessels from the existing vasculature is essential for the growth and progression from colitis to tumors[11-13]. Both angiopoietin (Ang)-1 and Ang-2 are members of a family of secreted proteins that bind to an endothelium-specific receptor tyrosine kinase, namely the tyrosine kinase with immunoglobulin-like and EGF-like domains (Tie)-2 receptor[14]. Although Ang-1 and Ang-2 bind to Tie-2 with similar affinity, they exert different functions[15]. Ang-1 is constitutively expressed on blood vessels and is thought to induce stabilization by maintaining pericyte and smooth cell muscle coverage, whereas Ang-2 is expressed in regions of vascular remodeling and antagonizes the effects of Ang-1, thereby enabling removal of the periendothelial cells and destabilizing the vessels[16]. Ang-1/Ang-2 play a critical role in the process of inflammation and cancer in various tissues[17]. The vasculature is usually quiescent under the stabilizing influences of Ang1/Tie-2[18]. In tumors, vascular destabilization allows vascular endothelial growth factor (VEGF) and other growth factors to facilitate angiogenesis, with the alteration in Ang-1/Ang-2 ratio in favor of Ang-2 being closely associated with tumor angiogenesis. Moreover, a close correlation has been found between Ang-2 and VEGF expression in tumors[19,20]. In recent years, the effect of these angiogenic factors in carcinogenesis has attracted much attention. However, the exact role of Ang-1, Ang-2, Tie-2, and VEGF in UC and CRC remains largely unknown.
Animal models are important for testing the various hypotheses concerning the etiology and pathogenesis of UC and CRC, thereby providing insights into the underlying mechanisms. The rat model induced by dextran sulfate sodium (DSS) is applicable to study UC, as the clinical and pathologic features in this model are very similar to those in patients with UC. In addition, the 1,2-dimethylhydrazine (DMH)-induced rat model of CRC is widely used in the study of colorectal carcinogenesis, bearing histopathologic and molecular characteristics of tumor genesis that mimic human CRC[21]. In the present study, we investigated the colorectal protein expression of Ang-1, Ang-2, Tie-2, and VEGF in these models to elucidate their potential role in the progression from inflammation to malignant transformation.
All rats in the study were used strictly in accordance with the National Institutions of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats (n = 44) weighing 200-220 g, were obtained from the Experiment Animal Center, China Medical University. Rats were housed in plastic cages containing corn-chip bedding and were maintained on a 12 h light/12 h dark cycle (07:00-19:00 h, light cycle; 19:00-07:00 h, dark cycle) with a room temperature of 22 ± 1 °C and a humidity of 65%-70%. Water and food were available ad libitum. The design of this research was approved by the China Medical University Animals Committee (No. 2011-2362).
Acute colitis was induced by administration of DSS (molecular weight 5000; Sigma Aldrich, St. Louis, MO, United States) at 3.5% (wt/vol) in drinking water for 7 d. Since DSS has a direct toxic effect on epithelial cells and can lead to erosion with a complete loss of surface epithelium, we induced a relatively moderate colitis by administration of 3.5% DSS. The clinical course of colitis was monitored by a daily disease activity index, including the three parameters of weight loss, stool consistency, and perianal bleeding (Table 1).
| Score | Parameter | ||
| Weight loss | Stool consistency | Perianal bleeding | |
| 0 | None | Normal | None |
| 1 | 1%-5% | ||
| 2 | 5%-10% | Pasty stools | Occult bleeding |
| 3 | 10%-20% | ||
| 4 | > 20% | Diarrhea | Gross bleeding |
Rats in the CRC group (n = 15) were subjected to three cycles of alternating administration of distilled water containing DSS for 7 d followed by distilled water for 14 d after intraperitoneal pretreatment with 20 mg/kg 1,2-DMH (Sigma Aldrich). Rats in the UC group (n = 15) were subjected to three cycles of alternating administration of distilled water containing DSS for 7 d followed by distilled water for 14 d after intraperitoneal pretreatment with 0.5 mL saline. Rats in the control group received distilled water only after intraperitoneal pretreatment with 0.5 mL saline.
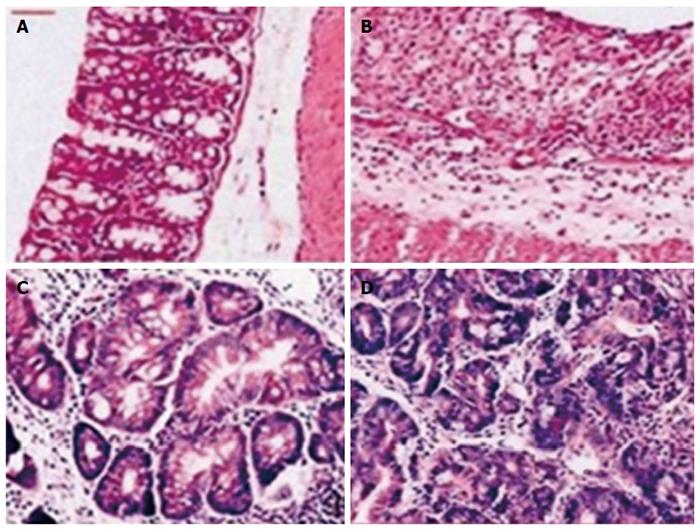
Rats were anesthetized with diethyl ether and perfused through the aorta with physiologic saline, followed by periodate-lysine-paraformaldehyde. The distal part of the rectum were removed and immersed in the same fixative liquid and stored overnight at 4 °C, then placed in 30% sucrose in 0.01 mol/L PBS for 6 h at 4 °C. The specimens were embedded in paraffin and then frozen in -80 °C until sectioning. Serial tissue sections (5 μm) of paraffin-embedded specimens were cut perpendicular to the mucosal surface and stained with hematoxylin-eosin to assess the morphology of the tissue. The severity of damage in the colonic tissue was graded according to the histopathologic standard scoring system as follows: 0, normal; 1, focal inflammatory cell infiltration (including polymorphonuclear leukocytes); 2, inflammatory cell infiltration, gland dropout, and crypt abscess; 3, mucosal ulceration; and 4, two or more areas of mucosal ulceration.
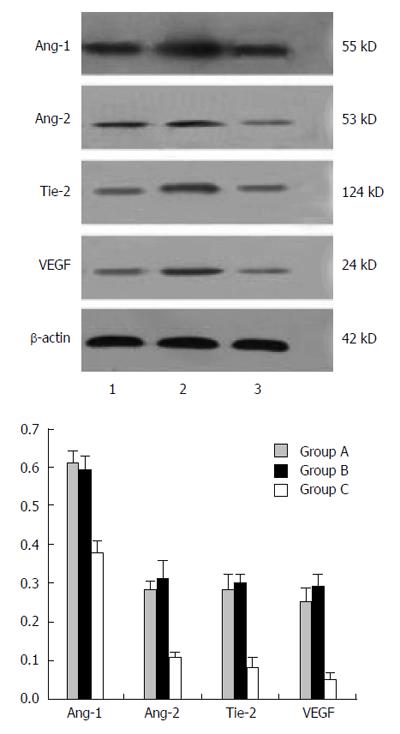
Colorectal tissue samples were homogenized at 4 °C in 5 vol buffer containing 320 mmol/L sucrose, 1 mmol/L, DL-dithiothreitol, 10 μg/mL leupeptin, 10 μg/mL soybean trypsin inhibitor, 2 μg/mL aprotinin, and 50 nmol/L Tris (pH 7.0) and centrifuged at 12000 ×g for 20 min. The supernatants were diluted and heated at 95 °C for 5 min. After loading (20 μg protein), proteins were separated in 12 or 14% SDS-PAGE (90 mV). Proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, United States) and incubated with polyclonal rabbit anti-rat Ang-1 (1:1000), Ang-2 (1:1000), Tie-2 (1:500), and VEGF (1:1000) antibodies (all from Santa Cruz Biotechnology, Dallas, TX, United States). After incubating with peroxidase secondary antibody, proteins were visualized on X-ray film by chemiluminescence according to the manufacturer’s instructions (Amersham of GE Healthcare, Little Chalfont, United Kingdom). Autoradiographs were quantified by densitometry software (Total Lab Dynamics Ltd, Newcastle, United Kingdom), and several time exposures were analyzed to ensure the linearity of the band intensities.
All experiments were performed with triplicate or greater samples, and data shown are representative of five or more independent experiments. The differences among groups in disease activity index, colon length, pathology score, and protein expression were evaluated by one-way analyses of variance followed by least significant difference tests. Data are expressed as mean ± SD. All statistical analyses were conducted using SPSS 16.0 software (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered as statistically significant.
Compared with control rats, rats in the CRC and UC groups developed symptoms of acute colitis with diarrhea, rectal bleeding, wasting, and loss of body weight. This was paralleled by a dramatic rise in the disease activity index starting at day 3 after DSS induction in the CRC group and day 4 in the UC group. In addition, the mean length of colorectum of CRC and UC rats was significantly shorter than that of control rats (P < 0.05) (Table 2).
In the control group, colonic membrane structure was intact, without epithelial sloughing, trimmed glands, and with only a few red blood cells appearing in the mucosa. In the CRC and UC groups, slight damage to the epithelium was observed, with trimmed glands, a few glands detaching, as well as some red blood cells and inflammatory cells appearing in the mucosa (Figure 1). Histopathologic scores in the CRC and UC groups were significantly higher than in the control group (Ps < 0.05) (Table 2). However, there was no significant difference in the histopathologic scores between the CRC and the UC groups.
Rats in the CRC group developed multiple tumors in the colorectal region. Eleven of fifteen rats had at least one dysplasia and/or cancer lesion; gross lesions were noted in nine rats. These lesions were dome shaped and ranged from 1.5 to 3.5 mm in size, which mainly appeared in the distal portion of the large intestine, then in the middle portion, and none in the proximal part. Most tumor tissues were tubular lesions with atypical severe cellular and structural high-grade dysplasia. A small number of lesions revealed relatively moderate dysplasia. Eight samples of dysplasia were categorized as low-grade dysplasia, and three samples were categorized as high-grade dysplasia. Among the rats with dysplasia and/or cancer, the incidence of dysplasia and/or cancer was 18.18, 27.27 and 54.55% in the proximal, middle and distal colon segments, respectively. Furthermore, 63.64% rats with dysplasia and/or cancer had lesions limited to only one colon segment, 27.27% of rats had lesions in two different segments, and 9.09% of rats had lesions in all three segments. No sign of tumor development was detected in UC or control groups.
As shown in Figure 2, the protein levels of Ang-1, Ang-2, Tie-2, and VEGF were markedly increased in the CRC and UC groups as compared to controls. However, the differences did not reach statistical significance.
The administration of DMH and/or DSS in rats induces CRC and UC with histopathology very similar to that seen in humans[22,23]. The study of animal models allows us to better understand the cause and mechanisms of UC and CRC. Clinical study of large patient populations and the introduction of specialized instrumentation such as colonoscopy have helped to answer questions regarding epidemiology and anatomic changes, but these are limited methods for investigating the molecular mechanisms underlying the disease in humans. CRC in patients with UC develops mainly on the left side of the large intestine and transverse colon. In our experiment, DSS and DMH induced colitis-related tumors that were predominantly in the distal part of colon. In patients with UC, dysplasia was found in two or more segments in 42%-75% of the cases, and the incidence of multiple synchronous cancers varied from 22% to 50%[24-26]. Our results show that dysplasia was present in two or more segments in 36.36% of the rats, and the incidence of synchronous cancers was 27.27%.
Although Ang has been suggested to play a role in the initiation of angiogenesis and progression from inflammation to cancer, the role of Ang-1, Ang-2, Tie-2, and VEGF in UC and CRC, particularly the relationship between Ang and Tie systems, has not been fully clarified. Our study shows that Ang-1, Ang-2, Tie-2, and VEGF were expressed in normal epithelium, but there were differential expression patterns in epithelial/tumor and endothelial cells depending on the stage of disease. It has also been suggested that a decrease in Ang-1 and increase in Ang-2 would result in destabilization of the vessels, thereby allowing the vessels to be acted upon by other angiogenesis factors, such as VEGF. The current data show that although vascular Ang-1 remained constant at the earliest stages of hyperplasia, there was a decrease in the epithelial expression of Ang-1, suggesting less available ligand for binding to Tie-2 on the endothelial cell surface. Simultaneously, there was an increase in both epithelial and endothelial Ang-2, which may compete with Ang-1 for Tie-2 binding, resulting in vascular destabilization. As some studies have reported a significant increase in epithelial VEGF expression at this early stage in colon cancer development, our data suggest that the Ang and Tie-2 pathway may act in conjunction with VEGF to stimulate the angiogenic switch at the onset of hyperplasia. However, it must be acknowledged that the difficulties in drawing clear boundaries among usual and atypical hyperplasia, low-grade DCIS, and the small size of the lesions limit the accuracy of quantitative immunohistochemistry, and mask the difference between UC and CRC.
In conclusion, our findings suggest that Ang-2 expression may negatively regulate angiogenesis and decrease vascular permeability by stabilizing epithelial cells and increasing periendothelial support. Ang-2 is an important mediator of neoplastic and nonneoplastic angiogenesis, but its precise role in this process remains to be elucidated. Sequential expression of Ang-1, Ang-2, Tie-2, and VEGF has been shown to be crucial for successful angiogenesis. Therefore, any interruption or disturbance in this balanced expression will likely significantly affect the angiogenic process. Such a disturbance could occur at the level of continuous Tie-2 activation (by Ang-1) or by Tie-2 interruption (soluble Tie-2, Tie-2 receptor antagonists). Future studies are required to provide further insight into this complex process.
Colorectal cancer (CRC) is the most severe complication in ulcerative colitis (UC). Angiopoietin (Ang)-1, Ang-2, tyrosine kinase with immunoglobulin-like and EGF-like domains (Tie)-2 and vascular endothelial growth factor (VEGF) may play a key role in forming vasculature that is involved in carcinogenesis.
The role of Ang-1, Ang-2, Tie-2, and VEGF in UC and CRC, particularly the relationship between Ang and Tie systems, needs to be clarified.
The results indicate that Ang-2 and Tie-2 may be involved in the early induction of proinflammatory factors, which may contribute to the development of colon cancer. Furthermore, the expression of Ang-2, Tie-2, and VEGF in UC-derived CRC might lead to chronic colitis and pathologic angiogenesis in rat.
Further research concerning Ang-1, Ang-2, Tie-2, and VEGF in UC and CRC might unravel the underlying pathogenesis of UC and CRC.
Angiogenesis is necessary for the initiation and progression of malignant tumors and thus is considered as a hallmark of cancer.
In the present study, the authors investigated the protein expression of Ang-1, Ang-2, Tie-2, and VEGF in colons of rats with UC and CRC to elucidate the potential role of these factors in the progression from inflammation to malignant transformation. The study is well designed, and results are interesting.
P- Reviewer: Cignarelli M, Okamoto T S- Editor: Yu J L- Editor: AmEditor E- Editor: Wang CH
| 1. | Binefa G, Rodríguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786-6808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (3)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Seidelin JB, Coskun M, Nielsen OH. Mucosal healing in ulcerative colitis: pathophysiology and pharmacology. Adv Clin Chem. 2013;59:101-123. [PubMed] |
| 4. | Magro F, Rodrigues A, Vieira AI, Portela F, Cremers I, Cotter J, Correia L, Duarte MA, Tavares ML, Lago P. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Delcò F, Sonnenberg A. A decision analysis of surveillance for colorectal cancer in ulcerative colitis. Gut. 2000;46:500-506. [PubMed] |
| 6. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 659] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 7. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] |
| 8. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [PubMed] |
| 9. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47059] [Article Influence: 3361.4] [Reference Citation Analysis (5)] |
| 10. | Mihalache A, Rogoveanu I. Angiogenesis factors involved in the pathogenesis of colorectal cancer. Curr Health Sci J. 2014;40:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 11. | Luvuno FM, Mtshali Z, Baker LW. Vascular occlusion in the pathogenesis of complicated amoebic colitis: evidence for an hypothesis. Br J Surg. 1985;72:123-127. [PubMed] |
| 12. | Wang HL, Deng CS, Lin J, Pan DY, Zou ZY, Zhou XY. Expression of angiopoietin-2 is correlated with vascularization and tumor size in human colorectal adenocarcinoma. Tohoku J Exp Med. 2007;213:33-40. [PubMed] |
| 13. | Yoshizaki A, Nakayama T, Naito S, Sekine I. Expression patterns of angiopoietin-1, -2, and tie-2 receptor in ulcerative colitis support involvement of the angiopoietin/tie pathway in the progression of ulcerative colitis. Dig Dis Sci. 2009;54:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 494] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 15. | Ahmad SA, Liu W, Jung YD, Fan F, Reinmuth N, Bucana CD, Ellis LM. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92:1138-1143. [PubMed] |
| 16. | Reiss Y. Angiopoietins. Recent Results Cancer Res. 2010;180:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bach F, Uddin FJ, Burke D. Angiopoietins in malignancy. Eur J Surg Oncol. 2007;33:7-15. [PubMed] |
| 18. | Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1-10. [PubMed] |
| 19. | Metheny-Barlow LJ, Li LY. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003;13:309-317. [PubMed] |
| 20. | Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557-1570. [PubMed] |
| 21. | Jackson PE, O’Connor PJ, Cooper DP, Margison GP, Povey AC. Associations between tissue-specific DNA alkylation, DNA repair and cell proliferation in the colon and colon tumour yield in mice treated with 1,2-dimethylhydrazine. Carcinogenesis. 2003;24:527-533. [PubMed] |
| 22. | Hashimoto M, Ayada T, Kinjyo I, Hiwatashi K, Yoshida H, Okada Y, Kobayashi T, Yoshimura A. Silencing of SOCS1 in macrophages suppresses tumor development by enhancing antitumor inflammation. Cancer Sci. 2009;100:730-736. [PubMed] |
| 23. | Onose J, Imai T, Hasumura M, Ueda M, Hirose M. Rapid induction of colorectal tumors in rats initiated with 1,2-dimethylhydrazine followed by dextran sodium sulfate treatment. Cancer Lett. 2003;198:145-152. [PubMed] |
| 24. | Taylor BA, Pemberton JH, Carpenter HA, Levin KE, Schroeder KW, Welling DR, Spencer MP, Zinsmeister AR. Dysplasia in chronic ulcerative colitis: implications for colonoscopic surveillance. Dis Colon Rectum. 1992;35:950-956. [PubMed] |
| 25. | Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934-944. [PubMed] |