Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2467
Peer-review started: August 17, 2014
First decision: September 15, 2014
Revised: September 29, 2014
Accepted: December 8, 2014
Article in press: December 8, 2014
Published online: February 28, 2015
Processing time: 196 Days and 18 Hours
AIM: To investigate the efficacy and safety of percutaneous needle decompression in the treatment of malignant small bowel obstruction (MSBO).
METHODS: A prospective analysis of the clinical data of 52 MSBO patients undergoing percutaneous needle decompression was performed.
RESULTS: Percutaneous needle decompression was successful in all 52 patients. Statistically significant differences were observed in symptoms such as vomiting, abdominal distension and abdominal pain before and after treatment (81.6% vs 26.5%, 100% vs 8.2%, and 85.7% vs 46.9%, respectively; all P < 0.05). The overall significantly improved rate was 19.2% (11/52) and the response rate was 94.2% (49/52) using decompression combined with nasal tube placement, local arterial infusion of chemotherapy and nutritional support. During the one-month follow-up period, puncture-related complications were acceptable.
CONCLUSION: Percutaneous needle intestinal decompression is a safe and effective palliative treatment for MSBO.
Core tip: Malignant small bowel obstruction (MSBO) is a common complication in patients with advanced cancer. In this study, we prospectively analyzed MSBO patients undergoing percutaneous needle decompression in our hospital over the past few years to identify the value of this technique in the palliative treatment of MSBO. We concluded that percutaneous needle intestinal decompression is a safe and effective palliative treatment for MSBO. When combined with local arterial infusion of chemotherapy and nasal intestinal decompression tube placement, percutaneous needle decompression can further improve the clinical remission.
- Citation: Jiang TH, Sun XJ, Chen Y, Cheng HQ, Fang SM, Jiang HS, Cao Y, Liu BY, Wu SQ, Mao AW. Percutaneous needle decompression in treatment of malignant small bowel obstruction. World J Gastroenterol 2015; 21(8): 2467-2474
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2467
Malignant small bowel obstruction (MSBO) is a common complication in patients with advanced cancer, with a reported incidence of approximately 3%-6% in advanced malignancies[1]. Statistics show that in some European countries, the hospitalization cost of patients with small bowel obstruction (SBO) exceeded that of patients with gastric cancer, and was comparable to that for colon cancer[2,3]. Unfortunately, the incidence and natural course of SBO has not reduced in recent years, nor has any improvement in the prevention and management been reported. The treatment of SBO should be based on the specific conditions of the patients and should be individualized and comprehensive. Surgery is still a good treatment choice for SBO. Other available options include minimally invasive treatment (such as laparoscopic surgery, endoscopic balloon dilatation, stent placement, and local perfusion of chemotherapy for tumors) and conservative treatment (such as nasal bowel decompression, water-soluble hyperosmolar contrast agents, and nutritional support)[4-9]. As MSBO is mainly found in patients with advanced cancer, surgery is not tolerable, and the presence of closed-loop obstruction or multiple sites in some patients with severe obstruction even precludes the possibility of nasal intestinal decompression tube placement and stent usage, or makes treatment futile. Significantly dilated bowels, abdominal distension, abdominal pain, vomiting, and other progressive obstructive symptoms seriously affect the quality of life and survival of such patients[10]. For patients with advanced diseases, instant relief from intestinal pressure may reduce the obstructive symptoms, and in turn improve the quality of life, or even provide the opportunity for further treatment. In the present study, we prospectively analyzed MSBO patients undergoing percutaneous needle decompression in our hospital over the past few years to identify the value of this technique in the palliative treatment of MSBO.
Fifty-two patients, 18 men and 34 women aged 27-86 years (median age 62 years), undergoing percutaneous needle decompression for SBO secondary to moderate-to-advanced malignancies in our hospital from June 2004 to August 2010 were enrolled.
The patients were unable to eat, had obvious abdominal distention, abdominal pain, and had ceased anal flatus and defecation. Some patients experienced nausea and vomiting. Abdominal distension was found, with drum sound in percussion. Abdominal computed tomography (CT) imaging showed significantly dilated intestines.
Criteria for study inclusion were: (1) patients with SBO confirmed by CT imaging and pathologically or clinically diagnosed as having malignancies; (2) patients with SBO unresponsive to conservative medical treatment and cannot tolerate surgery because of closed-loop or multiple-sites obstruction for which the placement of nasal intestinal decompression tubes or stents had failed, or had been unable to effectively relieve the obstructive symptoms; (3) patients with clinically significant abdominal distention, abdominal pain, and intestinal patterns with radiographic implication of serious dilation, inflation, or effusions of the intestines with a high risk of rupture; and (4) patients who were willing to participate in the study voluntarily with signed informed consent, were cooperative and compliant, and could be followed-up.
Criteria for exclusion from the study were: (1) patients with SBO caused by mesenteric artery embolization or mesenteric vein thrombosis; (2) patients with severe coagulopathy, platelets < 30 × 109/L, prothrombin time > 30 s, prothrombin activity < 40%, which were not improved by blood transfusion, hemostatic therapy, or other treatments; (3) patients with portal hypertension and severe esophageal and gastric varices with bleeding episodes; (4) patients with intestinal necrosis, or bowel obstruction complicated by intra-abdominal infections and abscesses; (5) patients with severe heart and lung failure; and (6) patients who failed to complete the treatment for subjective or objective reasons, making it difficult or impossible to evaluate the efficacy because of insufficient data.
All treatments were approved by the Ethics Committee of our hospital. Informed consent was obtained from all patients before the initiation of treatment, and all operations were conducted by at least two interventional radiologists. Enhanced CT scans were performed one week before the procedure to identify the location and number of obstruction sites.
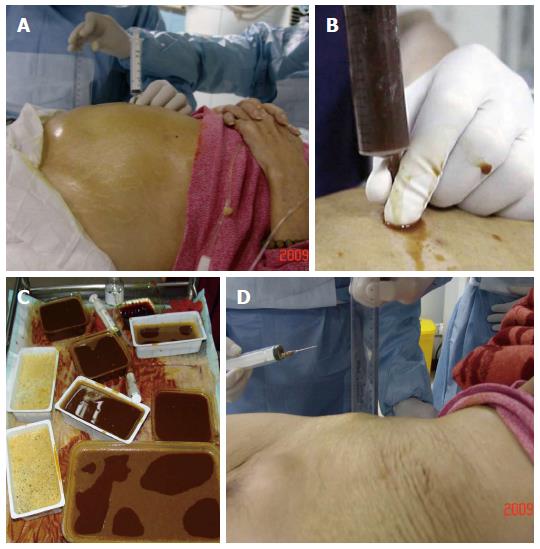
Patients were placed in the supine position. The procedure was performed under ultrasonic monitoring to avoid vital abdominal organs and large blood vessels. Lidocaine infiltration anesthesia was delivered layer by layer from the abdominal wall where bowel gas accumulation was identified from ultrasonography. A gauge-6 needle or pericardial puncture needle contained in a syringe was used to puncture the dilated intestinal cavity under ultrasonic guidance. After puncture, gas and liquid was continuously withdrawn from the intestines until a significant reduction in gas was observed and the intestines returned to normal widths under ultrasonography. Complete decompression of the intestines was determined by complete removal of gas and liquid. In the case of multiple closed-loop obstructions, aspiration was conducted section by section until all dilated intestines were relieved and the patient reported significant improvement of abdominal distention and pain.
After removal of the needle, patients were instructed to remain supine for two hours. Conventional antibiotic treatment was administered after the procedure. Somatostatin was injected to reduce intestinal fluid secretion. Multiple courses of intestinal needle decompression were performed if necessary. Nasal intestinal tube placement and local arterial infusion of chemotherapy treatments were delivered based on the remission status and physical conditions of the patient after decompression. All patients were administered subsequent nutritional support treatment.
Relevant medical history was recorded in all 52 patients before treatment who met the study criteria. The following parameters were observed: (1) tumor location, nature, stage, presence or absence of peritoneal metastases, previous anti-cancer treatment; (2) clinical signs and symptoms; (3) sites and nature of obstruction as shown on CT imaging one week before the procedure, and if ascites was present (amount, nature, and other aspects of ascites); (4) weight loss in the past 3 mo; and (5) serum albumin, hemoglobin, and potassium levels.
Responses following decompression were recorded on a daily basis, including: (1) obstruction remission; (2) improvement in abdominal distention, abdominal pain, nausea, vomiting, eating, flatus and defecation; (3) subsequent possibility of further treatment and implementation; and (4) occurrence of complications, including death, peritonitis, and bleeding.
The statistical analysis was carried out using SPSS 17.0 software (SPSS Inc., Chicago, IL, United States). Qualitative data were compared using the χ2 tests, and quantitative data were compared using Student’s t tests. A P value < 0.05 was considered statistically significant.
Baseline patient and tumor characteristics are presented in Table 1. Among the 52 patients, there were 27 cases of gastrointestinal cancer, 21 cases of gynecologic tumors, 1 case of lymphoma, and 3 cases of metastatic peritoneal cancer (of unknown sources). Thirty-seven patients had a weight loss > 5 kg in the past three months with dyscrasia; 11 patients had bloody ascites in diagnostic paracentesis. Thirty-nine patients had a previous history of chemotherapy; 20 had a previous history of radiotherapy (seven of which also had a previous history of intraperitoneal chemotherapy), 37 had previous abdominal surgery.
| Characteristic | Value |
| Age (yr), median (range) | 62 (27-86) |
| Gender (male/female) | 18 (34.6)/34 (65.4) |
| Weight loss > 5 kg in 90 d | 37 (71.2) |
| ECOG status, median ± SD | 2.84 ± 0.91 |
| Albumin (g/L), median ± SD | 30.24 ± 4.96 |
| Hemoglobin (g/L), median ± SD | 90.01 ± 22.99 |
| Bloody ascites | 11 (21.1) |
| Alimentary system malignancy (n = 27) | |
| Gastric | 13 |
| Colorectal | 9 |
| Pancreas | 2 |
| Small bowel carcinoma | 1 |
| Hepatic | 2 |
| Gynecologic malignancy (n = 21) | |
| Ovarian | 14 |
| Uterine and cervical | 7 |
| Lymphoma | 1 |
| Peritoneal metastatic carcinoma of unknown primary origin | 3 |
| Prior therapy | |
| Chemotherapy | 39 (75.0) |
| Radiotherapy | 20 (38.5) |
| Surgery | 37 (71.2) |
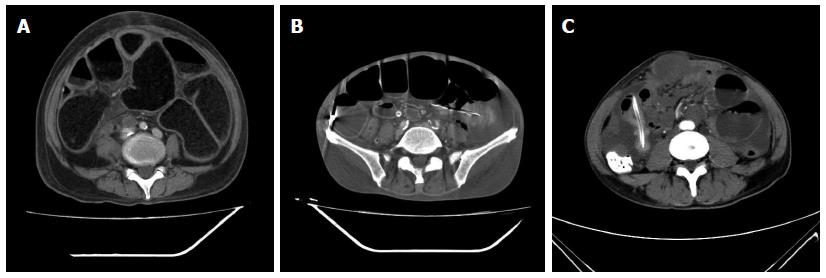
All 52 patients underwent contrast-enhanced CT scans one week before decompression. CT imaging suggested the presence of dilated intestines in all patients with varying degrees of small intestinal fluid levels (Table 2). Some had effusions as the primary findings, and others had inflation. Fifteen patients showed bowel wall thickening, suggesting the presence of primary or metastatic tumors, 28 patients had peritoneal nodules, suggesting peritoneal metastases, and 6 had bowel and abdominal wall adhesions (large masses) (Figure 1 and Table 2).
| Findings | n (%) |
| Dilation of the small intestine | 52 (100.0) |
| Small intestinal fluid level | 52 (100.0) |
| Wall thickening | 15 (28.8) |
| Mesenteric edema and congestion | 36 (69.2) |
| Ascites | 19 (36.5) |
| Closed loop | 28 (53.8) |
| Extensive adhesions | 14 (26.9) |
| Large masses | 6 (11.5) |
| Peritoneal metastasis | 28 (53.8) |
| Intestinal fecal signs | 7 (13.5) |
| Multiple small bowel obstructions | 39 (75.0) |
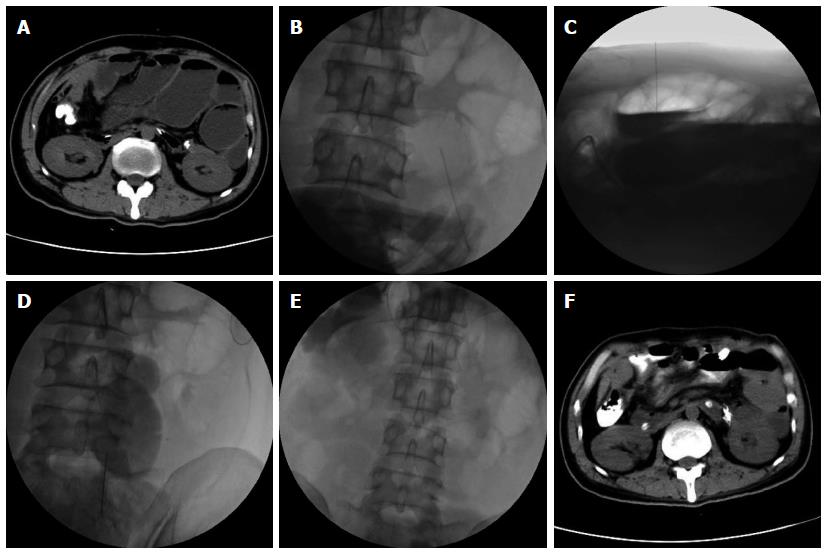
In terms of symptoms, typical cases had obvious abdominal pain, abdominal distension and skin tension before decompression, which returned to normal following extraction of large amounts of gas and liquid (Figure 2). Radiologic findings confirmed that a large amount of gas and a large number of effusions in the intestines were removed (Figure 3).
To evaluate the efficacy of this treatment, the patients were followed-up for one month. Remission of obstructive symptoms was considered effective treatment, and resumption of eating was considered complete remission. In this study, percutaneous needle decompression was successful in all 52 patients. No failures such as unintended penetration were observed, as it was not difficult to puncture the highly dilated intestines under imaging guidance. The operations were mainly completed in the anterior abdominal cavity based on previous CT findings and intraoperative ultrasonic guidance. Most abdominal blood vessels and nerves were located close to the posterior abdominal wall. The average number of punctures per patient was 3.5 (range: 1-12), with a median of 4.6 d (range: 2-15 d) between punctures.
Vomiting, distention, and abdominal pain improved significantly after decompression compared with baseline symptoms (all P < 0.05 by χ2 test) (Table 3). The average time to symptom improvement after needle decompression alone was 3.7 d. Four patients (4/52; 7.7%) had complete remission and began eating after decompression, and 8/52 (15.4%) patients had partial remission and successful nasal intestinal decompression tube placement, although one patient (1.9%) did not show significant relief of obstructive symptoms after tube placement. This patient underwent multiple decompression procedures, and the intestinal obstruction symptoms resolved. Partial remission after needle decompression was achieved following arterial infusion of chemotherapy in 5/52 (9.6%) patients, the obstructions resolved and the patients resumed eating; 6/52 (11.5%) patients were regarded as having a partial remission after decompression followed by local arterial chemotherapy, and underwent successful placement of nasal intestinal decompression tubes. Obstructions resolved in 2/52 (2.8%) patients after local arterial chemotherapy following decompression with nasal intestinal tube placement, and the patients resumed eating. An overall significantly improved rate of 19.2% (11/52) and a response rate of 94.2% (49/52) were observed following decompression combined with nasal tube placement, local arterial infusion of chemotherapy, and subsequent nutritional support treatment.
Of the 52 patients included in the study, 13.4% (7/52) experienced puncture-related complications during the observation period, the main complication was intestinal contents leak and sepsis, but if decompression is thorough and successful, the intestinal pinholes will heal naturally and avoid fistulas. Other complications including abdominal infections due to spontaneous peritonitis, and puncture site metastases due to extensive abdominal metastasis. Other complications such as abdominal pain and ascites extravasation through puncture sites were all resolved by symptomatic treatment. One patient died five days after surgery due to tumor progression (Table 4).
| Patient No. | Primary malignancy | Complication | Postoperative time to complication (d) | Outcome |
| 1 | Pancreas | Death | 5 | Died of tumor progression, no autopsy was performed |
| 2 | Gastric | Peritonitis | 3 | Intravenous systemic antibiotics therapy, ascites culture found growing Klebsiella pneumoniae; discharged 3 wk after surgery, palliative care was continued |
| 3 | Ovarian | Abdominal pain, intestinal contents leak | Intraoperative | Anti-analgesic treatment, complete extraction of the intestinal contents, abdominal flush with metronidazole; after 14 d, patient was voluntarily discharged |
| 4 | Gastric | Puncture site metastases | 17 | No obvious symptoms at metastatic sites, postoperative local arterial infusion of chemotherapy was delivered; discharged after 4 wk for palliative care |
| 5 | Ovarian | Intestinal contents leak, sepsis | < 1 | Persistent abdominal flush with metronidazole and abdominal percutaneous catheter drainage, systemic antibiotics, nasal intestinal drainage tube was placed postoperatively; discharged after 1 wk for rehabilitation |
| 6 | Ovarian | Ascites extravasation | < 1 | Closed drainage with abdominal decompression, ascites smears showed tumor cells; discharged after 28 d following peritoneal local chemotherapy |
| 7 | Cervical | Exudates at puncture sites | < 1 | Exudates disappeared following local pressure dressing, intravenous albumin and intensified nutrition therapy for 2 d; discharged 10 d after surgery |
MSBO is a common complication in patients with moderate-to-advanced malignancies, with an incidence up to 20%-50% in ovarian cancer, and 10%-29% in colon cancer[11,12]. Long-term SBO may cause retention of the proximal intestine contents, leading to intestinal swelling and increased pressure, and in turn intestinal mucosal edema, blood circulation disorders, ischemia, and hypoxia, which will eventually lead to intestinal necrosis and perforation[13]. For the diagnosis of SBO, abdominal X-ray examination is only effective in identifying 46-80% of SBOs, and even less useful in determining the cause and presence of strangulation. In contrast, CT scans can additionally reveal the situation outside the intestinal walls, mesentery, and peritoneal clearance, which has significant advantages in identifying the site of obstruction, cause, and presence of strangulation[14]. Therefore, in this study, all patients underwent abdominal and pelvic enhanced CT examinations before the decompression procedures to identify the obstructions. Medical treatment of SBO can relieve nausea, vomiting, and abdominal pain in some patients. Due to mechanical obstruction and persistent adhesions, however, drugs alone may often be ineffective in some patients. Bypass surgery is currently the standard treatment for these patients, however, palliative surgery is not possible in advanced patients with multiple obstructions and poor physical conditions[15]. As a result, for those who cannot tolerate surgery, the key to successful treatment is to reduce the intestinal pressure and effectively drain the contents to restore intestinal blood circulation and further improve the quality of life. Nasal intestinal decompression tube placement and self-expanding metal stents can effectively relieve SBO symptoms. However, in cases with serious narrowing due to obstruction, it is extremely difficult or even impossible to introduce the enteroscope or guidewire. In addition, they are not sufficiently effective in patients with multiple obstructions, and have limited success rates due to their technical difficulties[12,16]. As there is still no effective treatment for such patients in clinical settings, their quality of life is severely affected by the condition.
Intestinal puncture is mostly used for percutaneous intestinal ostomy, which is mainly conducted under ultrasonic guidance to provide a long-term enteral nutrition supply to patients who cannot eat[17-19]. Direct intestinal puncture has also been reported[20]. Clinical studies have shown that these patients often have tumors extensively seeded in the peritoneum with varying degrees of adhesion, thus the small intestine is relatively fixed and difficult to move, and the obstructions are obviously dilated. In contrast to creating a nutrient supply passage, percutaneous intestinal stoma is a technically easier option for treating SBO. Investigators have used 12-Fr or 14-Fr drainage tubes to successfully treat 21 patients, and their obstruction symptoms were effectively alleviated and clinical complications are acceptable[21]. However, this method requires a long-term indwelling drainage tube in the intestinal cavity, increasing the risk of intestinal perforation, necrosis or infection, and tube movement. In this study, a one-time application of percutaneous puncture decompression was used to treat MSBOs. The procedure was successful in all subjects, resulting in obvious improvement in clinical symptoms, including complete remission in four patients undergoing intestinal needle decompression alone. The puncture-related complications are acceptable.
Percutaneous needle intestinal decompression only resolves the symptoms but cannot control the tumors. But patients with advanced tumors can rarely tolerate the toxicity or side effects of traditional radio- and/or chemotherapy. In fact, the sensitivity toward traditional radio- and chemotherapy in the majority of patients with a solid tumor of the digestive tract is rather poor. As chemotherapeutics have killing and injurious effects on most tumor cells, the difference in therapeutic effect is determined mainly by whether or not the drug in the target organ can reach an effective antitumor blood concentration. The general effect of traditional chemotherapy makes it difficult to reach an effective drug concentration in the target organ of the gastrointestinal tract at a safe dosage. Interventional chemotherapy by drug infusion of the supplying artery can make the drug concentration in the vascular network of the tumor area reach an effective antitumor level at a relatively safe dosage, thus decreasing toxic and side effects and increasing the therapeutic efficacy. This method may play a role in the treatment of primary or secondary tumors that cause malignant digestive tract obstruction[22-25]. Thus, following intestinal decompression, physical conditions were improved in some patients, and low-dose intra-arterial chemotherapy and nasal intestinal decompression tube placement were performed in accordance with patients’ physical conditions. Decompression combined with nasal tube placement, local arterial infusion of chemotherapy resulted in an overall significantly improved rate of 19.2% and a response rate of 94.2%.
In this study, we found that complete extraction of the intestinal gas and fluids and thorough decompression of the intestinal cavity were the key to successful treatment. Multiple punctures are needed when there is a large amount of viscous content that prevents this goal. Patients with gas as the primary radiologic manifestation are more likely to achieve complete decompression, as observed in four patients in this study. If decompression is thorough and successful, the intestinal pinholes will heal naturally. Otherwise, the intestinal contents will continuously emerge through the holes to contaminate the abdominal cavity, causing peritonitis.
In conclusion, percutaneous needle intestinal decompression is a safe and effective palliative treatment for unresectable MSBO. In combination with local arterial infusion of chemotherapy and nasal intestinal decompression tube placement, it can relieve clinical symptoms, improve quality of life, and prevent mortality and morbidity of intestinal obstructions. In addition, this technique can provide opportunities for tumor treatment to further improve the remission and clinical cure rates.
Malignant small bowel obstruction (MSBO) is a common complication in patients with advanced cancer, with a reported incidence of approximately 3%-6% in advanced malignancies. Statistics show that in some European countries, the hospitalization cost of patients with small bowel obstruction (SBO) exceeded that of patients with gastric cancer, and was comparable to that for colon cancer. Unfortunately, the incidence and natural course of SBO has not reduced in recent years, nor has any improvement in the prevention and management been reported.
Significantly dilated bowels, abdominal distension, abdominal pain, vomiting, and other progressive obstructive symptoms seriously affect the quality of life and survival of patients with MSBO. Long-term SBO may cause retention of the proximal intestine contents, leading to intestinal swelling and increased pressure, and in turn intestinal mucosal edema, blood circulation disorders, ischemia, and hypoxia, which will eventually lead to intestinal necrosis and perforation. For patients with advanced diseases, instant relief from intestinal pressure may reduce the obstructive symptoms, and in turn improve the quality of life, or even provide the opportunity for further treatment.
Percutaneous needle decompression was successful in all patients in this study. Statistically significant differences were observed in symptoms such as vomiting, abdominal distension, and abdominal pain before and after treatment. The overall significantly improved rate was 19.2% (11/52) and the response rate was 94.2% (49/52) using decompression combined with nasal tube placement, local arterial infusion of chemotherapy, and nutritional support. During the one-month follow-up period, puncture-related complications were acceptable.
Percutaneous needle intestinal decompression is a safe and effective palliative treatment for MSBO. When combined with local arterial infusion of chemotherapy and nasal intestinal decompression tube placement, percutaneous needle decompression can further improve the clinical remission rates.
MSBO is a common complication in patients with moderate-to-advanced malignancies; clinical manifestations include dilated bowels, abdominal distension, abdominal pain, and vomiting.
This is a very interesting study about the safety and efficacy of percutaneous needle decompression in treatment of malignant small bowel obstruction. In this study, the authors made a prospective analysis of the clinical data of 52 MSBO patients undergoing percutaneous needle decompression. They concluded that the percutaneous needle intestinal decompression is a safe and effective palliative treatment for malignant small bowel obstruction.
P- Reviewer: Nicole G, Casper S S- Editor: Yu J L- Editor: AmEditor E- Editor: Wang CH
| 1. | Klein C, Stiel S, Bükki J, Ostgathe C. [Pharmacological treatment of malignant bowel obstruction in severely ill and dying patients: a systematic literature review]. Schmerz. 2012;26:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Tingstedt B, Johansson J, Nehez L, Andersson R. Late abdominal complaints after appendectomy--readmissions during long-term follow-up. Dig Surg. 2004;21:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Kössi J, Salminen P, Rantala A, Laato M. Population-based study of the surgical workload and economic impact of bowel obstruction caused by postoperative adhesions. Br J Surg. 2003;90:1441-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Dayton MT, Dempsey DT, Larson GM, Posner AR. New paradigms in the treatment of small bowel obstruction. Curr Probl Surg. 2012;49:642-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Popa D, Ramesh J, Peter S, Wilcox CM, Mönkemüller K. Small Bowel Stent-in-Stent Placement for Malignant Small Bowel Obstruction Using a Balloon-Assisted Overtube Technique. Clin Endosc. 2014;47:108-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Laval G, Rousselot H, Toussaint-Martel S, Mayer F, Terrebonne E, François E, Brixi H, Nguyen T, Bourdeix I, Bisot-Locard S. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer. 2012;99:E1-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Kitani K, Yukawa M, Fujiwara Y, Tsujie M, Hara J, Ikeda M, Sato K, Isono S, Kawai K, Miura K. [Palliative surgery for malignant bowel obstruction in patients with advanced and recurrent gastroenterological cancer]. Gan To Kagaku Ryoho. 2013;40:1699-1701. [PubMed] |
| 8. | Carmelo B. [Stents in the digestive tract: state of the art]. Rev Gastroenterol Peru. 2013;33:43-51. [PubMed] |
| 9. | Baron TH. Interventional palliative strategies for malignant bowel obstruction. Curr Oncol Rep. 2009;11:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Dalal KM, Gollub MJ, Miner TJ, Wong WD, Gerdes H, Schattner MA, Jaques DP, Temple LK. Management of patients with malignant bowel obstruction and stage IV colorectal cancer. J Palliat Med. 2011;14:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44:1105-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep. 2009;11:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Frager D. Intestinal obstruction role of CT. Gastroenterol Clin North Am. 2002;31:777-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Dolan EA. Malignant bowel obstruction: a review of current treatment strategies. Am J Hosp Palliat Care. 2011;28:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Lee H, Park JC, Shin SK, Lee SK, Lee YC. Preliminary study of enteroscopy-guided, self-expandable metal stent placement for malignant small bowel obstruction. J Gastroenterol Hepatol. 2012;27:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Hu HT, Shin JH, Song HY, Kim JH, Yoon HK, Gwon DI, Ko GY, Sung KB. Fluoroscopically guided percutaneous jejunostomy with use of a 21-gauge needle: a prospective study in 51 patients. J Vasc Interv Radiol. 2009;20:1583-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Yang ZQ, Shin JH, Song HY, Kwon JH, Kim JW, Kim KR, Kim JH. Fluoroscopically guided percutaneous jejunostomy: outcomes in 25 consecutive patients. Clin Radiol. 2007;62:1061-105; discussion 1061-105;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Rondelli F, Balzarotti R, Bugiantella W, Mariani L, Pugliese R, Mariani E. Temporary percutaneous ileostomy versus conventional loop ileostomy in mechanical extraperitoneal colorectal anastomosis: a retrospective study. Eur J Surg Oncol. 2012;38:1065-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Sparrow P, David E, Pugash R. Direct percutaneous jejunostomy--an underutilized interventional technique? Cardiovasc Intervent Radiol. 2008;31:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Kim YJ, Yoon CJ, Seong NJ, Kang SG, An SW, Woo YN. Safety and efficacy of radiological percutaneous jejunostomy for decompression of malignant small bowel obstruction. Eur Radiol. 2013;23:2747-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Wang Z, Chen K, Gong J, Zheng Y, Wang T. Combined arterial infusion and stent implantation compared with metal stent alone in treatment of malignant gastroduodenal obstruction. Cardiovasc Intervent Radiol. 2009;32:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Zhang CW, Zou SC, Shi D, Zhao DJ. Clinical significance of preoperative regional intra-arterial infusion chemotherapy for advanced gastric cancer. World J Gastroenterol. 2004;10:3070-3072. [PubMed] |
| 24. | Iida T, Hirata N, Hirakawa M, Noguchi T. Preoperative intraarterial infusion chemotherapy for advanced gastric cancer--a retrospective review of four cases. Radiat Med. 2003;21:172-177. [PubMed] |
| 25. | Mao AW, Gao ZD, Xu JY, Yang RJ, Xiao XS, Jiang TH, Jiang WJ. Treatment of malignant digestive tract obstruction by combined intraluminal stent installation and intra-arterial drug infusion. World J Gastroenterol. 2001;7:587-592. [PubMed] |