Published online Feb 28, 2015. doi: 10.3748/wjg.v21.i8.2413
Peer-review started: October 17, 2014
First decision: November 14, 2014
Revised: December 18, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: February 28, 2015
Processing time: 134 Days and 6.8 Hours
AIM: To evaluate the feasibility of transjugular intrahepatic portosystemic shunt (TIPS) for severe jaundice secondary to acute Budd-Chiari syndrome (BCS).
METHODS: From February 2009 to March 2013, 37 patients with severe jaundice secondary to acute BCS were treated. Sixteen patients without hepatic venule, hepatic veins (HV) obstruction underwent percutaneous angioplasty of the inferior vena cava (IVC) and/or HVs. Twenty-one patients with HV occlusion underwent TIPS. Serum bilirubin, liver function, demographic data and operative data of the two groups of patients were analyzed.
RESULTS: Twenty-one patients underwent TIPS and the technical success rate was 100%, with no technical complications. Sixteen patients underwent recanalization of the IVC and/or HVs and the technical success rate was 100%. The mean procedure time for TIPS was 84.0 ± 12.11 min and angioplasty was 44.11 ± 5.12 min (P < 0.01). The mean portosystemic pressure in the TIPS group decreased significantly from 40.50 ± 4.32 to 16.05 ± 3.50 mmHg (P < 0.01). The mean portosystemic pressure gradient decreased significantly from 33.60 ± 2.62 to 7.30 ± 2.21 mmHg (P < 0.01). At 8 wk after the procedures, in the TIPS group, total bilirubin (TBIL) decreased significantly from 266.24 ± 122.03 before surgery to 40.11 ± 3.52 μmol/L (P < 0.01) and direct bilirubin (DBIL) decreased significantly from 194.22 ± 69.82 μmol/L to 29.82 ± 3.10 μmol/L (P < 0.01). In the angioplasty group, bilirubin returned to the normal range, with TBIL decreased significantly from 258.22 ± 72.71 μmol/L to 13.33 ± 3.54 μmol/L (P < 0.01) and DBIL from 175.08 ± 39.27 to 4.03 ± 1.74 μmol/L (P < 0.01). Liver function improved faster than TBIL. After 2 wk, in the TIPS group, alanine aminotransferase (ALT) decreased significantly from 50.33 ± 40.61 U/L to 28.67 ± 7.02 U/L (P < 0.01) and aspartate aminotransferase (AST) from 49.46 ± 34.33 U/L to 26.89 ± 8.68 U/L (P < 0.01). In the angioplasty group, ALT decreased significantly from 51.56 ± 27.90 to 14.22 ± 2.59 μmol/L (P < 0.01) and AST from 60.66 ± 39.89 μmol/L to 8.18 ± 1.89 μmol/L (P < 0.01). After mean follow-up of 12.6 mo, there was no recurrence of jaundice in either group.
CONCLUSION: Severe jaundice is not a contraindication for TIPS in patients with acute BCS and TIPS is appropriate for severe jaundice due to BCS.
Core tip: Jaundice with bilirubin > 51.3 μmol/L (3 mg/dL) is a contraindication for transjugular intrahepatic portosystemic shunt (TIPS) in patients with liver cirrhosis or end-stage liver disease. However, 21 patients in our single center underwent TIPS due to severe jaundice secondary to acute Budd-Chiari syndrome (BCS) and good clinical outcomes were achieved. We present a single center clinical experience and discuss the feasibility and effectiveness of treating severe jaundice secondary to acute BCS with TIPS.
- Citation: He FL, Wang L, Zhao HW, Fan ZH, Zhao MF, Dai S, Yue ZD, Liu FQ. Transjugular intrahepatic portosystemic shunt for severe jaundice in patients with acute Budd-Chiari syndrome. World J Gastroenterol 2015; 21(8): 2413-2418
- URL: https://www.wjgnet.com/1007-9327/full/v21/i8/2413.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i8.2413
Budd-Chiari syndrome (BCS) is a group of disorders resulting from hepatic venous obstruction at the level of the hepatic vein (HV), the inferior vena cava (IVC) or the hepatic venules. The clinical features include jaundice, digestive discomfort, abdominal distention, abdominal pain, refractory ascites, hepatosplenomegaly, portal hypertension, gastrointestinal bleeding, liver cirrhosis and even liver failure. Patients with acute BCS rarely benefit from conservative treatment. The major interventional treatment for BCS is percutaneous angioplasty of outflow vessels, including the IVC and HV; however, when all the hepatic venules are occluded, recanalization of the IVC and HVs did not relieve the symptoms. Transjugular intrahepatic portosystemic shunt (TIPS) has been performed since 1992 and good outcomes have been achieved. Jaundice of acute BCS is caused by HV occlusion. In the past, jaundice with total bilirubin (TBIL) > 51.3 μmol/L (3 mg/dL) was a contraindication for TIPS in patients with liver cirrhosis or end-stage liver diseases[1]. However, from February 2009 to March 2013, 21 patients in our single center underwent TIPS for severe jaundice secondary to acute BCS and good clinical outcomes were achieved.
Here, we retrospectively review these patients and compare them with patients with jaundice secondary to acute BCS that was relieved by percutaneous angioplasty of the HV and/or IVC during the same period. We present a single center clinical experience and discuss the feasibility and effectiveness of treating severe jaundice secondary to acute BCS with TIPS.
Between February 2009 and March 2013, 37 patients (22 female, 15 male; all from China) were referred to the Department of Interventional Therapy of Beijing Shijitan Hospital due to jaundice symptoms, including severe yellow sclera and film, pruritus, abdominal discomfort and other features. Three patients also had massive ascites. All the patients were diagnosed with acute BCS in the local hospital. The mean age was 39.67 ± 2.74 years. Duration of symptoms ranged from 5 to 42 d (mean 14.5 ± 7.89 d).
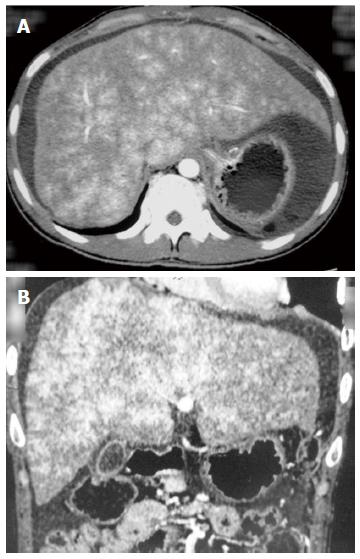
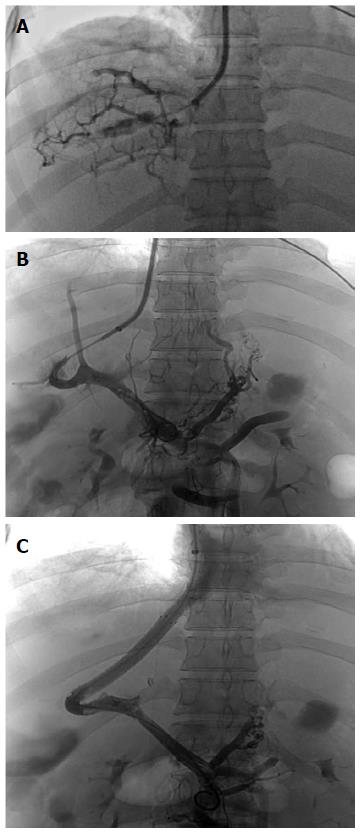
After administration, abdominal computed tomography (CT), a magnetic resonance imaging of the portal vein (Figure 1) and vena cava angiography were performed on each patient. Occlusion of the IVC or HVs was confirmed for each patient. Percutaneous angioplasty of the IVC and/or HVs was performed on 16 (10 female, 6 male) patients without hepatic venule obstruction. TIPS was performed on the other 21 (12 female, 9 male) patients (2 also had massive ascites) with occluded hepatic venules (Figure 2). Laboratory tests, including TBIL, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and blood ammonia, were recorded for each patient before the procedures. The two groups of patients were compared with regard to operative and postoperative data. Anti-coagulation treatment with warfarin was administered to both groups of patients after the procedures and low-molecular-weight heparin was given before the international normalized ratio reached 2-3.
The TIPS procedures were performed under local anesthesia in the Interventional Radiology Suite of Shijitan Hospital of Capital Medical University. The Rösch-Uchida Transjugular Liver Access Set (Cook) was used for each patient. After right jugular venous access was gained with a 10 F sheath, a 5 F multipurpose catheter was inserted into the hepatic vein and angiography was performed. As already stated, 21 patients had occlusion of the hepatic venules. TIPS was then performed. A TIPS needle was advanced into the portal vein through the liver parenchyma from the vena cava and the guide wire was placed into the portal vein through the sheath. Subsequently, the shunt was dilated with an angioplasty balloon of 8 mm diameter and a covered stent with a diameter of 8 mm was deployed. If the stent was not long enough, one or two additional stents were utilized to extend the length. Portal vein angiography and pressure measurements were repeated.
All the patients were under close monitoring during the perioperative period. Reduction of jaundice was recorded and laboratory tests were repeated. Complications, including abdominal cavity hemorrhage, acute hepatic failure and hepatic encephalopathy (HE), were observed during the perioperative period.
Monthly follow-up was planned during the first 6 mo after the procedure and then at 6 mo intervals thereafter. Clinical observations included reduction of jaundice, hepatic failure and HE. Abdominal CT was repeated at each point. Laboratory investigations, including TBIL, ALT, AST and ammonia, were also checked at each follow-up point.
SPSS for Windows version 17.0 was utilized for data processing. Statistical analysis with paired sample t test was used for measurement data. Numerical data were summarized as frequencies and continuous variables as ± standard deviation. P < 0.05 was considered to be statistically significant.
In the TIPS group, the procedures were successful in all 21 patients, with no technical complications. In the percutaneous angioplasty group, recanalization of the IVC and/or HVs was successful in all 16 patients. In the TIPS group, 42 covered stents were deployed in the patients and four bare stents were used in four patients to extend the shunt in the portal vein. The mean operation time of the TIPS group was 84.0 ± 12.11 min and the mean procedure time of the angioplasty group was 44.11 ± 5.12 min (P < 0.01). Portal vein hypertension was found in all patients and was improved after TIPS. The mean portosystemic pressure prior to TIPS placement was 40.50 ± 4.32 mmHg, which decreased significantly to 16.05 ± 3.50 mmHg after the shunt was established (P < 0.01). The mean PSG prior to TIPS was 33.60 ± 2.62 mmHg and decreased significantly to 7.30 ± 2.21 mmHg after the procedure (P < 0.01). Mild-to-moderate gastroesophageal varices were found in two patients in TIPS group and were embolized with coils after the TIPS shunt was formed.
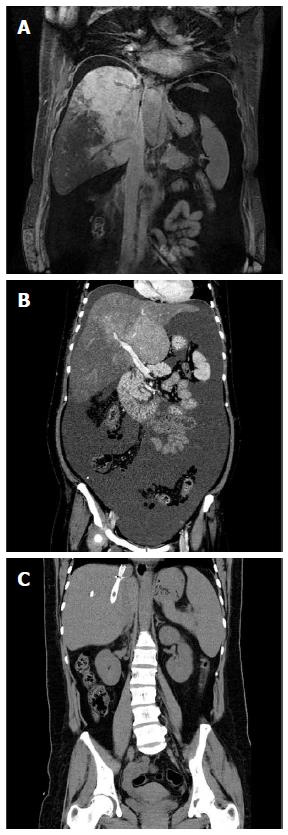
Clinical improvement was seen in both groups of patients. In the TIPS group, jaundice symptoms were relieved within 2-8 wk and in the angioplasty group, the symptoms were relieved within 1-4 wk. Ascites decreased obviously in both groups of patients within 1-2 wk after the procedure (Figure 3).
At 8 wk after TIPS, TBIL decreased significantly from 266.24 ± 122.03 μmol/L before surgery to 40.11 ± 3.52 μmol/L (P < 0.01) and direct bilirubin (DBIL) dropped significantly from 194.22 ± 69.82 μmol/L to 29.82 ± 3.10 μmol/L (P < 0.01). At 8 wk after angioplasty, bilirubin level returned to normal, with TBIL decreasing significantly from 258.22 ± 72.71 μmol/L to 13.33 ± 3.54 μmol/L (P < 0.01) and DBIL from 175.08 ± 39.27 μmol/L to 4.03 ± 1.74 μmol/L (P < 0.01) (Table 1).
| TIPS group | Angioplasty group | Pvalue1 | |||||
| Before (mean ± SD) | After (mean ± SD) | Pvalue | Before (mean ± SD) | After (mean ± SD) | Pvalue | ||
| TBIL (μmol/L) | 266.24 ± 122.03 | 40.11 ± 3.52 | < 0.01 | 258.22 ± 72.71 | 13.33 ± 3.54 | < 0.01 | < 0.01 |
| DBIL (μmol/L) | 194.22 ± 69.82 | 29.82 ± 3.10 | < 0.01 | 175.08 ± 39.27 | 4.03 ± 1.74 | < 0.01 | < 0.01 |
| ALT (U/L) | 50.33 ± 40.61 | 28.67 ± 7.02 | < 0.01 | 51.56 ± 27.90 | 14.22 ± 2.59 | < 0.01 | < 0.01 |
| AST (U/L) | 49.46 ± 34.33 | 26.89 ± 8.68 | < 0.01 | 60.66 ± 39.89 | 8.18 ± 1.89 | < 0.01 | < 0.01 |
| Ammonia (μmol/L) | 50.21 ± 21.17 | 39.09 ± 9.90 | < 0.01 | 56.78 ± 18.90 | 26.38 ± 5.19 | < 0.01 | < 0.01 |
Before the procedures, liver function was abnormal in 28 patients (18 in the TIPS group and 10 in the angioplasty group). After the procedures, liver function improved faster than TBIL. ALT and AST improved significantly within 1 wk after the procedures and returned to normal within 2 wk in both groups. At 2 wk after TIPS, ALT decreased significantly from 50.33 ± 40.61 U/L to 28.67 ± 7.02 U/L (P < 0.01) and AST from 49.46 ± 34.33 U/L to 26.89 ± 8.68 U/L (P < 0.01). Two weeks after angioplasty, ALT decreased significantly from 51.56 ± 27.90 μmol/L to 14.22 ± 2.59 μmol/L (P < 0.01) and AST from 60.66 ± 39.89 to 8.18 ± 1.89 μmol/L (P < 0.01).
Although ammonia level was abnormal in 17 patients (10 in the TIPS group and 7 in the angioplasty group), no patient had HE after TIPS or percutaneous angioplasty of the IVC and/or HV. Mean ammonia level decreased significantly from 50.21 ± 21.17 μmol/L before the procedures to 39.09 ± 9.90 μmol/L 2 wk after TIPS (P < 0.01) and from 56.78 ± 18.90 μmol/L to 26.38 ± 5.19 μmol/L in the angioplasty group (P < 0.01).
The patients underwent a mean follow-up of 12.6 mo without loss. No recurrence of jaundice was observed during follow-up and no stenosis of the recanalization or TIPS shunt was found. No patients had HE during follow-up. In the TIPS group, TBIL, ALT, AST and ammonia were 37.23 ± 3.24 μmol/L, 25.66 ± 4.51 U/L, 22.87 ± 5.15 U/L and 41.56 ± 2.42 μmol/L at 6 mo follow-up, respectively. In the angioplasty group, TBIL, ALT, AST and ammonia were 14.55 ± 3.21 μmol/L, 12.64 ± 1.12 U/L, 6.62 ± 2.73 U/L and 18.52 ± 4.91 μmol/L at 6 mo follow-up, respectively. Before surgery, abdominal CT showed hepatomegaly but the liver returned to its normal size at 6 mo follow-up.
BCS is a group of disorders caused by occlusion of hepatic venules, HVs and/or conterminous IVC affecting younger to middle-aged patients (median age 40 years). The average age of the 21 patients in our study was 42.64 ± 2.13 years, which was similar to a previously reported series. The symptoms of BCS include abdominal pain, jaundice, hepatosplenomegaly, HE and ascites or gastrointestinal bleeding caused by portal hypertension, and hepatic failure and cirrhosis are often also observed. The natural course of BCS with symptoms is progressive, while congestion caused by total occlusion of HVs leads to fulminant jaundice, ascites, hepatic failure and cirrhosis without proper treatment. In our study, the symptoms of jaundice progressed rapidly within a few weeks.
Randomized clinical trials of treatment for BCS are still lacking. Anti-coagulation therapy has been proved to be sufficient in controlling the liver disease in cases of mild BCS and is recommended by British and American guidelines at diagnosis[2,3]. In the present study, both groups of patients received anti-coagulation therapy after the procedures and no internal bleeding was seen in the perioperative or follow-up periods. Acute BCS treated with catheter-directed thrombolysis might be effective for hyperacute thrombosis within 3 d. Once thrombolytic therapy is given, no interventional measures should be performed until the drug is metabolized completely.
Interventional management of patients with BCS focuses on the relief of hepatic venous outflow tract obstruction. About 30% of BCS patients present with stenosis or occlusion of IVC and/or HVs. The efficacy of percutaneous angioplasty for these patients has been confirmed. In a retrospective study of one single center in China that involved 177 patients[4], the survival rates at 1, 5 and 10 years after the procedures were 96%, 83% and 73%, respectively. Active searching for such stenosis or occlusion following angioplasty is recommended in patients with symptomatic BCS. Patients with occlusion of both IVC and HVs require recanalization of both segments. Stents may be placed when necessary. Transjugular and transfemoral routes are used in these patients and in some cases a combined jugular and femoral approach is utilized. Percutaneous angioplasty of IVC and/or HVs is frequently practiced in our institute and the prognosis is favorable. In the present study, the jaundice decreased significantly in the angioplasty group and liver function recovered to normal within a few weeks after the procedures. Good clinical outcomes have also been achieved in patients with other symptoms, including abdominal pain, ascites and hepatosplenomegaly, after percutaneous angioplasty of IVC and/or HVs. In patients with asymptomatic BCS, aggressive management of IVC and/or HVs is still debated[1].
When BCS symptoms cannot be controlled by angioplasty of the IVC and/or HVs, the next step should be TIPS. With a relatively good midterm prognosis, TIPS is now the preferred treatment for BCS. A 90% of 5-year and 80% of 10-year survival can now be achieved after TIPS[5]. The transcaval approach performed by an experienced doctor is technically and clinically successful in most patients. In our single center, > 200 TIPS procedures have been performed annually since 2009 and the success rate is > 95%. Meanwhile, with covered stent grafts routinely applied in TIPS procedures, the patency rates have clearly increased and in the present study, no stenosis of the TIPS shunt was found during follow-up.
When dealing with patients with end-stage liver disease or advanced cirrhosis, severe jaundice with bilirubin > 3 mg/dL is a contraindication for TIPS. Furthermore, pre-TIPS bilirubin level is an independent predictor of 30-d mortality of TIPS and a 1-mg/dL increase above 3.0 mg/dL leads to a 40% increased risk of death[6]. Patients with MELD (Model for End-Stage Liver Disease) score > 18 have a significantly higher mortality than patients with scores of ≤ 18[7,8] and MELD score is better in predicting 3-mo survival of patients undergoing TIPS than Child-Pugh score.
However, patients in the present study had acute or sub-acute BCS, with mildly abnormal liver function. Portal hypertension was observed in all patients, with mild-to-moderate gastroesophageal varices in two patients, and no advanced cirrhosis was observed. Although the mean bilirubin of the TIPS group was > 3 mg/dL, a good clinical outcome was achieved. In other words, severe jaundice with bilirubin > 3 mg/dL is not a contraindication for TIPS in patients with acute or sub-acute BCS. In the present study, there was a significant difference in postprocedural data between the TIPS and angioplasty groups. However, the data were close to normal and improved greatly compared with before TIPS. Thus, we suggest that severe jaundice due to BCS is an indication for TIPS after unsuccessful angioplasty of IVC and/or HVs.
The utilization of covered stent grafts has greatly increased the patency rates after TIPS in patients with cirrhosis, yet the risk of HE has increased. The incidence of HE is between 33% and 48% in patients with liver cirrhosis at 6 mo after TIPS[9]. Some risk factors for HE after TIPS have already been mentioned: age > 60 years, female sex, previous history of HE, hypoproteinemia, final pressure gradient, basal disease severity and shunt diameter[10]. In our previous study on parallel TIPS shunting in patients with hepatitis B virus cirrhosis, HE was the major complication after the second shunt was established[11]. However, according to the literature, the risk of HE is relatively low after TIPS for BCS, with an incidence of 0-25%[12]. In the present study, no patient had HE after TIPS. Nevertheless, we still suggest that treatment for HE should be given before and after TIPS.
In conclusion, severe jaundice due to acute BCS can be relieved by TIPS when hepatic venules are occluded, leading to a satisfactory clinical outcome. Severe jaundice with bilirubin > 3 mg/dL is not a contraindication for TIPS in patients with acute BCS. On the contrary, TIPS is an appropriate option for severe jaundice due to BCS.
In the past, jaundice with total bilirubin of over 51.3 μmol/L (3 mg/dL) was a contraindication for transjugular intrahepatic portosystemic shunt (TIPS) in patients with liver cirrhosis or end-stage liver diseases and pre-TIPS bilirubin level was an independent predictor of 30-d mortality of TIPS.
TIPS has been applied for patients with Budd-Chiari syndrome (BCS) since 1992 and good outcomes have been achieved.
With a relatively good mid-term prognosis, TIPS is now the preferred treatment for BCS. A 90% of 5-year and 80% of 10-year survival can now be achieved after TIPS. There has been no research about treatment of severe jaundice due to acute BCS.
The authors present a single center clinical experience in treating severe jaundice secondary to acute BCS with TIPS and believe that TIPS is an appropriate option for severe jaundice due to BCS.
TIPS is a shunt (tube) placed between the portal vein which carries blood from the intestines and intraabdominal organs to the liver and the hepatic vein which carries blood from the liver back to the vena cava and the heart.
This is a study about TIPS for severe jaundice in patients with acute BCS. The authors believe that severe jaundice with bilirubin above 3 mg/dL is not a contraindication for TIPS in patients with acute BCS and TIPS is an appropriate option for severe jaundice due to BCS. The idea is very interesting and the article is of great value.
Biostatistics: The statistical methods of this study were reviewed by Professor Li-Ping Mei form Beijing Boai Hospital, Capital Medical University.
P- Reviewer: Lundahl J, Stebbing J S- Editor: Yu J L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 2. | DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 3. | Tait C, Baglin T, Watson H, Laffan M, Makris M, Perry D, Keeling D. Guidelines on the investigation and management of venous thrombosis at unusual sites. Br J Haematol. 2012;159:28-38. [PubMed] |
| 4. | Han G, Qi X, Zhang W, He C, Yin Z, Wang J, Xia J, Xu K, Guo W, Niu J. Percutaneous recanalization for Budd-Chiari syndrome: an 11-year retrospective study on patency and survival in 177 Chinese patients from a single center. Radiology. 2013;266:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Garcia-Pagán JC, Heydtmann M, Raffa S, Plessier A, Murad S, Fabris F, Vizzini G, Gonzales Abraldes J, Olliff S, Nicolini A. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Rajan DK, Haskal ZJ, Clark TW. Serum bilirubin and early mortality after transjugular intrahepatic portosystemic shunts: results of a multivariate analysis. J Vasc Interv Radiol. 2002;13:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Schepke M, Roth F, Fimmers R, Brensing KA, Sudhop T, Schild HH, Sauerbruch T. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Boone MD, Celi LA, Ho BG, Pencina M, Curry MP, Lior Y, Talmor D, Novack V. Model for End-Stage Liver Disease score predicts mortality in critically ill cirrhotic patients. J Crit Care. 2014;29:881.e7-881.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Hausegger KA, Karnel F, Georgieva B, Tauss J, Portugaller H, Deutschmann H, Berghold A. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | He FL, Wang L, Yue ZD, Zhao HW, Liu FQ. Parallel transjugular intrahepatic portosystemic shunt for controlling portal hypertension complications in cirrhotic patients. World J Gastroenterol. 2014;20:11835-11839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Qi X, Yang M, Fan D, Han G. Transjugular intrahepatic portosystemic shunt in the treatment of Budd-Chiari syndrome: a critical review of literatures. Scand J Gastroenterol. 2013;48:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |