Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1749
Peer-review started: August 8, 2014
First decision: September 15, 2014
Revised: September 27, 2014
Accepted: November 18, 2014
Article in press: November 19, 2014
Published online: February 14, 2015
Processing time: 188 Days and 18 Hours
AIM: To determine the expression of neurokinin-1 receptor (NK-1R), phosphorylated epidermal growth factor receptor (pEGFR), cyclooxygenase-2 (Cox-2), and vitamin D receptor (VDR) in normal, inflammatory bowel disease (IBD), and colorectal neoplasia tissues from Puerto Ricans.
METHODS: Tissues from patients with IBD, colitis-associated colorectal cancer (CAC), sporadic dysplasia, and sporadic colorectal cancer (CRC), as well as normal controls, were identified at several centers in Puerto Rico. Archival formalin-fixed, paraffin-embedded tissues were de-identified and processed by immunohistochemistry for NK-1R, pEGFR, Cox-2, and VDR. Pictures of representative areas of each tissues diagnosis were taken and scored by three observers using a 4-point scale that assessed intensity of staining. Tissues with CAC were further analyzed by photographing representative areas of IBD and the different grades of dysplasia, in addition to the areas of cancer, within each tissue. Differences in the average age between the five patient groups were assessed with one-way analysis of variance and Tukey-Kramer multiple comparisons test. The mean scores for normal tissues and tissues with IBD, dysplasia, CRC, and CAC were calculated and statistically compared using one-way analysis of variance and Dunnett’s multiple comparisons test. Correlations between protein expression patterns were analyzed with the Pearson’s product-moment correlation coefficient. Data are presented as mean ± SE.
RESULTS: On average, patients with IBD were younger (34.60 ± 5.81) than normal (63.20 ± 6.13, P < 0.01), sporadic dysplasia (68.80 ± 4.42, P < 0.01), sporadic cancer (74.80 ± 4.91, P < 0.001), and CAC (57.50 ± 5.11, P < 0.05) patients. NK-1R in cancer tissue (sporadic CRC, 1.73 ± 0.34; CAC, 1.57 ± 0.53) and sporadic dysplasia (2.00 ± 0.45) were higher than in normal tissues (0.73 ± 0.19). pEGFR was significantly increased in sporadic CRC (1.53 ± 0.43) and CAC (2.25 ± 0.47) when compared to normal tissue (0.07 ± 0.25, P < 0.05, P < 0.001, respectively). Cox-2 was significantly increased in sporadic colorectal cancer (2.20 ± 0.23 vs 0.80 ± 0.37 for normal tissues, P < 0.05). In comparison to normal (2.80 ± 0.13) and CAC (2.50 ± 0.33) tissues, VDR was significantly decreased in sporadic dysplasia (0.00 ± 0.00, P < 0.001 vs normal, P < 0.001 vs CAC) and sporadic CRC (0.47 ± 0.23, P < 0.001 vs normal, P < 0.001 vs CAC). VDR levels negatively correlated with NK-1R (r = -0.48) and pEGFR (r = -0.56) in normal, IBD, sporadic dysplasia and sporadic CRC tissue, but not in CAC.
CONCLUSION: Immunohistochemical NK-1R and pEGFR positivity with VDR negativity can be used to identify areas of sporadic colorectal neoplasia. VDR immunoreactivity can distinguish CAC from sporadic cancer.
Core tip: This study compares for the first time the expression of neurokinin-1 receptor, phosphorylated epidermal growth factor receptor, cyclooxygenase-2, and vitamin D receptor in samples from patients with sporadic colorectal neoplasia, colitis, and colitis-associated colorectal neoplasia and obtained from a Puerto Rican population. We believe that this knowledge could be of great use for the further identification of reliable markers of cancer risk and of potential therapeutic targets in a population where colorectal cancer is the deadliest cancer, and that our findings could be used in the day-to-day examination of colonic biopsies for establishing a diagnosis and distinguishing dysplasia from reactive inflammatory changes.
- Citation: Isidro RA, Cruz ML, Isidro AA, Baez A, Arroyo A, González-Marqués WA, González-Keelan C, Torres EA, Appleyard CB. Immunohistochemical expression of SP-NK-1R-EGFR pathway and VDR in colonic inflammation and neoplasia. World J Gastroenterol 2015; 21(6): 1749-1758
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1749.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1749
Not only is colorectal cancer (CRC) the second most commonly occurring cancer among Puerto Rican men and women combined but it is also the deadliest cancer in the Puerto Rican population[1]. This situation contrasts with that of both the general United States population - where CRC is less frequent than prostate, breast, and lung cancer and less deadly than lung cancer - and in United States Hispanics - where CRC incidence is surpassed by that of prostate and breast cancer and more people die from lung cancer than from CRC[2,3]. Although the exact cause remains unknown, this disparity is likely due to a complex interplay between several factors, such as differences in genetic admixture and environment. Facilitating an accurate diagnosis of CRC and its precursor lesions in Puerto Ricans could help mitigate the disparate incidence and mortality of CRC.
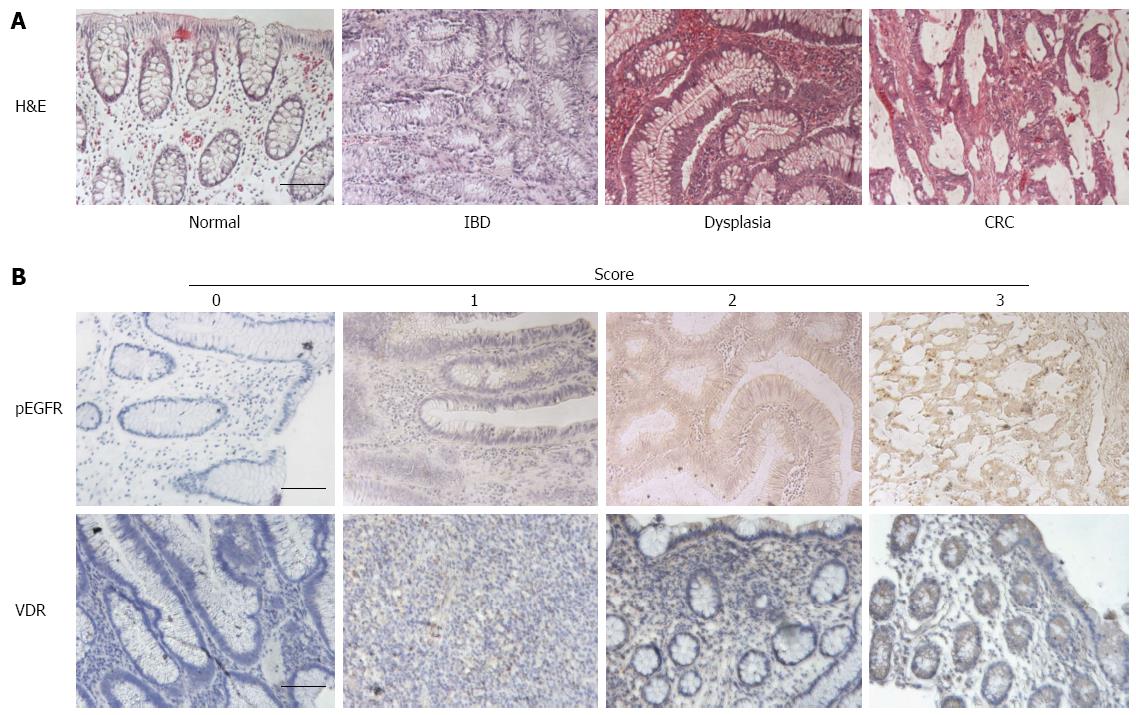
CRC encompasses several different modalities of cancer, ranging from familial syndromes to colitis-associated malignancy to sporadic cancer. The latter two serve as an example of how the same pathology can arise through different pathways: sporadic CRC arises from adenomatous polyps that progress to carcinoma, also known as the adenoma-carcinoma sequence. In contrast, colitis-associated colorectal cancer (CAC) begins with the chronic inflammation characteristic of inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn’s disease, and progresses to dysplasia and subsequently carcinoma, also known as the colitis-dysplasia-carcinoma sequence (Figure 1A)[4]. Dysplasia is a definite neoplastic change of the intestinal epithelium that does not penetrate the basement membrane[5-7]. The architectural changes that define colitis-associated dysplasia are analogous to those seen in adenomatous polyps[5]. Although dysplasia is currently the best marker of CRC risk in IBD, the wide range of variability between observers has led to a rising need for more reliable criteria and markers of cancer risk[8]. Interestingly, the prevalence of IBD in Puerto Rico has recently been estimated to be one of the highest among Hispanic groups[9], and Hispanics display phenotypic differences in the manifestations of IBD when compared to non-hispanic whites[10]. These two points emphasize the existence of differences not only between Hispanics and non-Hispanics but also between different Hispanic populations, thus indicating a need for studies that investigate disease processes in specific populations.
Much attention has been paid to signaling pathways and their components because of their involvement in carcinogenesis and their potential as therapeutic targets[11]. The substance P-neurokinin-1 receptor-epidermal growth factor receptor (SP-NK-1R-EGFR) pathway is of particular interest, since previous studies from our laboratory have shown that signaling components involved in these pathways are up-regulated in rat models of chronic inflammation and colitis-associated neoplasia[12-15]. SP and its endogenous receptor, NK-1R, are known to play a pivotal role in the pathophysiology of intestinal inflammation and are also involved in many processes related to oncogenesis[16,17]. Binding of SP and NK-1R causes transactivation of the EGFR, with subsequent activation of other pathways, such as Raf/MEK/ERK and PI3K/PDK/Akt, which can lead to increased inflammation and proliferation, along with decreased apoptosis and differentiation[18,19]. A downstream product of several of these pathways is cyclooxygenase-2 (Cox-2), an isozyme involved in the synthesis of prostaglandins. The expression of Cox-2 is normally minimal under basal conditions but is increased when it is stimulated by inflammatory cytokines, growth factors, and endotoxins[20].
There has been increasing awareness regarding the possible importance of vitamin D levels in various cancers, including CRC[21]. Interestingly, binding of calcitriol (1,2-dihydroxycholecalciferol, the active form of vitamin D) to the vitamin D receptor (VDR) has been shown to block the EGFR and to inhibit several of its pathways (particularly, Raf/MEK/ERK), resulting in an increase in apoptosis and a decrease in proliferation and angiogenesis[22].
Previous studies have assessed the expression of several of the aforementioned signaling components in chronic colitis and in CRC. Levels of NK-1R, EGFR, and Cox-2 have been shown to be elevated both in patients with colitis and in patients with CRC[17,23-27]. VDR levels have been shown to be decreased in patients with colitis but either minimally expressed or over-expressed in CRC[21,28]. Importantly, little is known regarding the expression of these proteins during dysplasia. Furthermore, no study to date has investigated the expression pattern of these proteins in either Puerto Ricans or United States Hispanics. A unique expression pattern for these proteins in dysplasia could further elucidate their potentially critical roles in the development of cancer. Therefore, the aim of this study was to determine and compare the expression of NK-1R, pEGFR, Cox-2, and VDR in patients with sporadic and colitis-associated colorectal neoplasia and how it compares to that found in normal tissues and in patients with IBD, in samples obtained from a Puerto Rican population. This knowledge could be of great use for the further identification of reliable markers of cancer risk and of potential therapeutic targets in a population where CRC is the deadliest cancer.
This study was carried out in compliance with all NIH regulations concerning the Protection of Human Subjects and human tissue specimens were approved for use by Institutional Review Boards (IRB) as follows. The Ponce School of Medicine and Health Sciences Institutional Review Board approved this study under IRB protocol 080506-CA and the University of Puerto Rico Medical Sciences Campus Institutional Review Board approved this study under IRB protocol MSC IRB 1250112. Both institutional review boards waived the need for informed consent given that the samples were existing archived pathological specimens which were de-identified, and thus did not contain any identifiable information that could be linked to the subjects.
Archived pathology laboratory samples, embedded in paraffin and devoid of personal information, were obtained from pathology laboratories of local hospitals. Samples (paraffin blocks) of colonic tissue from 26 different patients were randomly selected by laboratory staff for each of the following diagnoses: normal (n = 5), IBD (n = 5), dysplasia (n = 5), cancer (n = 5) and CAC (n = 6; Table 1). Patients with a histopathologic diagnosis of CAC in biopsy or surgical specimens were identified in the UPR Center for Inflammatory Bowel Diseases database (Table 2). Corresponding archived paraffin-embedded tissue blocks were retrieved from UPR School of Medicine Pathology Laboratory and de-identified by two pathologists (WGM and CGK), with the identification list saved for re-identification. All blocks were transferred to the Gastrointestinal Research Laboratory at Ponce School of Medicine and Health Sciences. Tissue sections from each of the selected samples were stained with hematoxylin and eosin in order for an independent pathologist (AAI) to confirm the diagnosis.
| Sample ID | IBD diagnosis | Duration of disease (yr) | Sex | Age (yr) | Graph symbol |
| 1 | UC | 9 | M | 61 | ● |
| 2 | UC | 7 | M | 61 | ■ |
| 3 | CD | 20 | F | 49 | ▲ |
| 4 | UC | 27 | F | 40 | ▼ |
| 5 | UC | 35 | M | 57 | ◆ |
| 6 | UC | 57 | F | 77 | ★ |
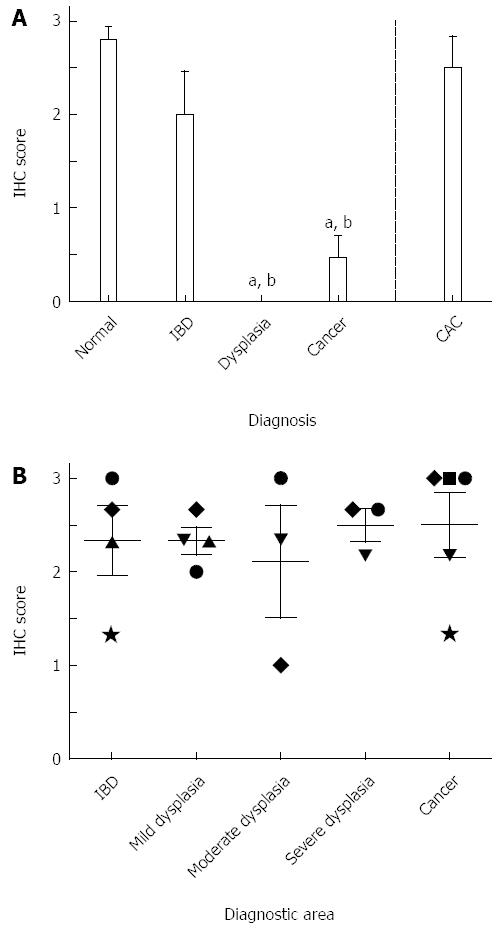
Tissue sections were deparaffinized in two 15-min Hemo-De xylene-substitute baths. Graded ethanol dilutions and distilled water were used for tissue rehydration. Hydrogen peroxide (3%, aqueous) was used to block endogenous peroxidase activity. After washing with phosphate-buffered saline, antigen retrieval was achieved by placing sections in citrate-EDTA buffer (10 mmol/L; 2 mmol/L EDTA, 0.05% Tween 20, pH 6.2) at 95 °C-99 °C for 40 min and at room temperature for 20 min. Sections were then washed with distilled water for 2 min (twice), phosphate-buffered saline for 5 min, and normal serum (Biogenex, San Ramon, CA) for 15 min before overnight incubation with the primary antibody. Antibodies used included NK-1R (sc-15323; Santa Cruz Biotechnology, dilution 1:100), pEGFR (#2236; Cell Signaling Technology, dilution 1:400), Cox-2 (#160106; Cayman Chemical Co., dilution 1:200), and VDR (Ab3508; Abcam Inc, dilution 1:2000). Secondary antibody was added to the sections for 20 min using the Super Sensitive Link-Label IHC Detection System (Biogenex, San Ramon, CA); sections were incubated with the biotinylated secondary antibody for 20 min and then with a streptavidin-peroxidase conjugate for 20 min. 3,3-Diaminobenzidine chromogen solution (Biogenex, San Ramon, CA) was used to achieve color development. Tissue sections were counterstained with hematoxylin for 20 s, dehydrated with graded ethanol dilutions, cleared with xylene, and mounted with a xylene-based mounting medium. Pictures were taken of representative areas containing the diagnosis of interest for each sample and blindly scored by three observers. A four-point scale ranging from 0 to 3 was used to assign scores according to staining intensity. In this scale, a score of 0 corresponds to the lightest, or absence of, staining, whereas 3 corresponds to the strongest staining for a particular antibody (Figure 1B). Additionally, areas diagnostic of IBD, dysplasia (mild, moderate, and severe), and cancer in tissue from CAC patients were photographed and scored.
Graphpad Prism V6.0a (Graphpad Software, San Diego, CA) was used to perform all statistical analyses. One-way analysis of variance and post hoc multiple comparisons tests were used to determine the statistical significance of the measured variables. A Tukey-Kramer multiple comparisons test was used to analyze differences between age distributions in the five patient groups. Dunnett’s multiple comparisons tests were used to analyze differences in protein expression. Pearson’s product-moment correlation coefficient was used to analyze the associations between expression of the four proteins measured. A difference was considered significant if P < 0.05. Data presented as mean ± SE.
There were no significant differences in gender between groups. As might be expected, IBD patients were significantly younger than normal, sporadic dysplasia, sporadic cancer, and CAC patients (P < 0.01, P < 0.01, P < 0.001, and P < 0.05 respectively; Table 1). There were no significant differences in age between any of the other groups.
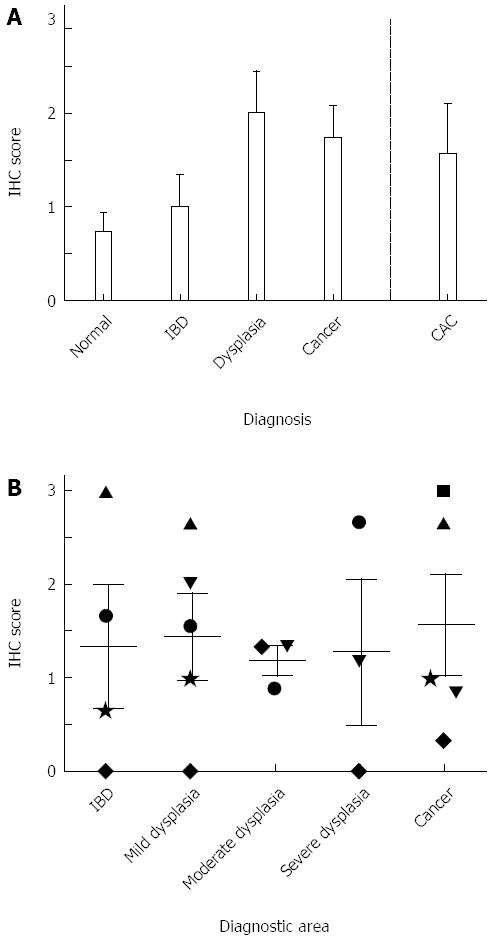
All diagnoses showed higher NK-1R expression when compared to normal tissues, although this failed to reach statistical significance (Figure 2A). NK-1R levels doubled in sporadic cancer and CAC when compared to normal. In normal tissues, staining was found in the glands and surface epithelium. In IBD, staining was found predominantly in the columnar cells of the glands, with some additional staining in the surface epithelium (in 40% of the samples). Sporadic dysplasia stained predominantly in the cytoplasm of columnar cells of the glands, whereas in sporadic cancer the staining was mostly found in the cytoplasm of poorly differentiated cancer cells. In CAC, staining was mainly observed in the cytoplasm of transformed glandular epithelial cells, and did not vary significantly between different diagnostic areas (Figure 2B).
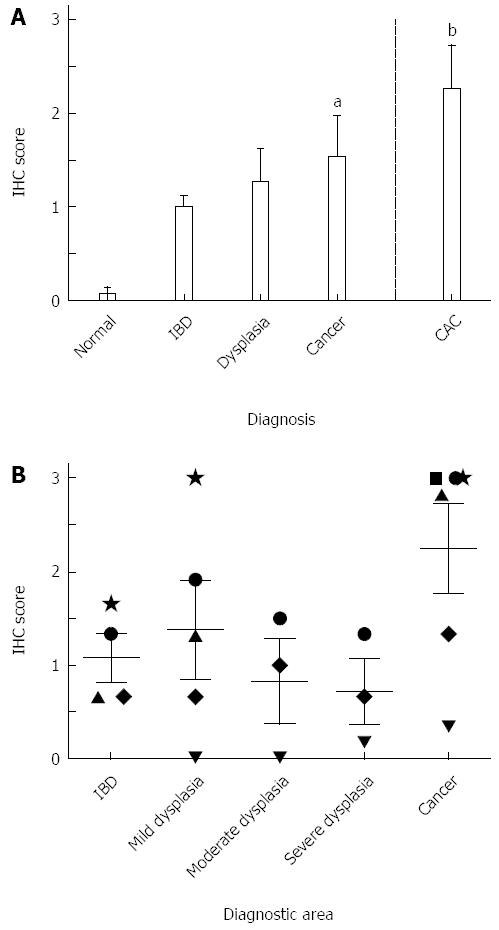
For pEGFR, a 15-fold increase and a 19-fold increase in expression were observed in IBD (1.00 ± 0.10) and sporadic dysplasia (1.27 ± 0.34), respectively, when compared to normal patients (0.07 ± 0.07; Figure 3A). Patients with sporadic cancer or CAC had significantly higher expression levels than those found in normal tissues (P < 0.05 and P < 0.001, respectively). In normal tissues, minimal to no staining was found, with only one sample showing nonspecific staining in the surface epithelium. Staining in IBD, sporadic dysplasia, sporadic cancer, and CAC tissues was observed in the columnar cells of the glands, mainly in the cytoplasm. Areas of cancer within CAC tissue had higher levels of pEGFR than other diagnostic areas present, but these differences were not statistically significant (Figure 3B). Figure 1B (top row) shows an example of pEGFR staining.
Cox-2 expression had an increasing trend similar to that of pEGFR, with higher expression occurring with worsening diagnosis. In patients with sporadic cancer, levels more than doubled (2.20 ± 0.23) and were significant when compared with normal tissues (P < 0.05; Figure 4A). Cox-2 levels in CAC tissue were not as elevated as in tissues with sporadic cancer. No significant differences were found between the other groups. In normal tissues, staining was observed in the glands and surface epithelium, when present. Tissues from patients with IBD, sporadic dysplasia, sporadic cancer, and CAC showed staining in the columnar cells of the glands, mainly in the cytoplasm. In CAC tissues, Cox-2 expression did not vary significantly between different diagnostic areas (Figure 4B) and negatively correlated with increased disease duration (r = -0.87, P < 0.05).
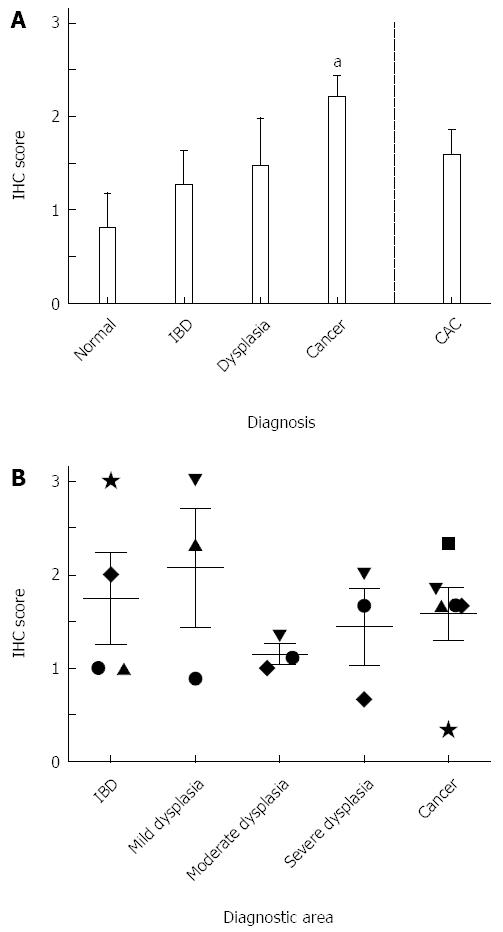
The highest levels of VDR expression were found in normal tissues. In patients who had been diagnosed with sporadic dysplasia, no expression was found (P < 0.001 vs normal), with minimal VDR expression occurring in sporadic CRC (P < 0.001 vs normal; Figure 5A). VDR levels in CAC tissue were significantly higher than those of sporadic dysplasia and cancer (P < 0.001) and more similar to those found in IBD and normal tissue. Normal tissues stained in the cytoplasm of the columnar cells of the glands. In IBD tissues, staining was found in the cytoplasm of the columnar cells of the glands and the acute inflammatory cell infiltrate. In sporadic cancer, 4 out of 5 tissues had no staining in the glands; 3 of these had light staining limited to inflammatory infiltrate within the areas of necrosis. Only 1 of the samples had slight staining in the columnar cells of the glands. In CAC, staining was mostly in the cytoplasm of glandular epithelial cells and inflammatory lamina propria cells and was consistent across the different diagnostic areas (Figure 5B). Figure 1B (bottom row) shows an example of VDR staining.
Statistical tests showed that VDR expression had a significant negative correlation with NK-1R (r = -0.48) and pEGFR (r = -0.56) expression (P < 0.05) when CAC tissue was excluded. These associations were lost when analysis included results from CAC tissue.
Our laboratory has previously studied the role of NK-1R, EGFR, Cox-2, and VDR in a rat model of colitis-associated dysplasia and cancer[12]. We demonstrated that antagonizing either the NK-1R or the EGFR delayed progression to dysplasia, decreased Cox-2 levels, and reduced inflammation[13,14]. Also, we demonstrated that delaying the transition to dysplasia by administering probiotics was associated with an increase in VDR expression[29]. In the present study, we report the comparative expression patterns of NK-1R, EGFR, Cox-2, and VDR in colonic tissue from Puerto Rican patients with IBD, sporadic colorectal neoplasia, and colitis-associated colorectal neoplasia.
Prior reports have demonstrated that there is an alteration in the expression of NK-1R, pEGFR, Cox-2, and VDR both in chronic colitis and in neoplasm of the colon. However, the expression of these proteins in Hispanic patients has never been studied. Furthermore, NK-1R has been found to be increased in tissue from patients with IBD and colon cancer[30,31]. Here, we showed that NK-1R levels were increased in colonic inflammation and neoplasia samples from Puerto Rican patients, although these results were not significantly different, most likely due to the small sample size. Finding tissue from Puerto Rican patients with colitis-associated dysplasia or cancer was extremely difficult. After 2 years of actively seeking said tissue, we were able to find only those tissues that were used in this study. Curiously, NK-1R levels in sporadic dysplasia did reach statistical significance, but only if the CAC group was excluded. Nevertheless, our results suggest that NK-1R is a relevant player in colonic inflammation and neoplasia in Puerto Rican patients. Gillespie et al[32] demonstrated that a truncated form of NK-1R, rather than its full-length counterpart, is increased in colectomy tissue from patients with CAC who were treated at the Boston University Medical Center. Our present study, however, seems to indicate that levels of the full-length NK-1R were increased in both sporadic and colitis-associated colorectal neoplasia tissue samples from Puerto Rican patients.
The role of EGFR in inflammatory and malignant pathologies of the colon has long been known: Sottili et al[24] demonstrated that EGFR is upregulated in experimental colitis, and Malecka-Panas et al[25] demonstrated that EGFR is increased in the colonic mucosa of patients with ulcerative colitis, adenomatous polyps, and CRC, The relevance of EGFR in CRC is such that anti-EGFR antibodies are currently being used as adjuvant therapy in metastatic CRC. Our current findings in samples from Puerto Rican patients are consistent with the aforementioned studies: pEGFR is increased in colonic inflammation and neoplasia, with significantly elevated levels found in tissue from both sporadic CRC and CAC. It is interesting to note that the increased pEGFR expression does not exactly match the increase in NK-1R levels. This suggests that a factor other than NK-1R may account for the marked increase seen in CAC. One possibility is that, in Puerto Rican patients, EGFR might become an oncogene in the colitis-dysplasia-carcinoma sequence of CAC through a mechanism different from that which takes place in the adenoma-carcinoma sequence of sporadic CRC. Our present findings also suggest that therapies directed against EGFR might be of benefit to Puerto Rican CAC patients, thus warranting further investigation. Our findings also underscore the necessity to include different ethnic groups in clinical trials, since these may respond differently to the same treatment.
In ulcerative colitis, Cox-2 expression is up-regulated in the inflamed mucosa, and there is evidence showing an overexpression of Cox-2 in cancer tissues, suggesting an important role in tumorigenesis[26,27,33,34]. Several epidemiological studies have found that these levels are reduced in individuals with CRC who are taking non-steroidal anti-inflammatory drugs[35]. Our findings show that, although expression is increased in tissue from IBD and CAC patients, Cox-2 levels are highest in sporadic CRC. Intriguingly, Cox-2 levels in CAC patients negatively correlated with disease duration, leading us to speculate that medications used to ameliorate the inflammation of IBD could alter the expression of Cox-2. Unfortunately, we were unable to confirm this suspicion due to the limited patient information available to us.
VDR is expressed in early-stage neoplasias but is repressed in high-grade and metastatic cancers[28,36]. Wada et al[21] (2009) demonstrated that VDR expression decreases in ulcerative colitis, dysplasia, and cancer. We have also shown that, in Puerto Rican patients, there is a significant decrease in VDR expression in sporadic dysplasia and in CRC, but not in CAC, when compared to normal tissue. In sporadic CRC, it is highly likely that without VDR, calcitriol cannot regulate the EGFR-related pathways, resulting in uncontrolled proliferation in the absence of differentiation and apoptosis[22]. Although the exact cause of the reduced VDR expression is yet to be elucidated, one possibility is that cellular signaling events may culminate in the nuclear translocation of certain factors that inhibit the transcription of the VDR gene. A possible candidate is SNAIL, a transcription factor that represses VDR expression and whose upregulation in colonic tumors has been associated with VDR downregulation[37]. Intriguingly VDR levels in CAC were not significantly lower than those seen in normal or IBD tissues. Although it has been reported that VDR can negatively regulate EGFR-related pathways[22], this does not seem to be the case in CAC, since both VDR and pEGFR are overexpressed. Perhaps another factor is affecting VDR expression in CAC. Although vitamin D deficiency is common among patients with IBD[38], the effect of this deficiency on colonic VDR expression is not clear. A possible explanation may lie in the different molecular mechanisms responsible for the development of CAC and sporadic CRC. Cancer-associated mutant p53 can affect VDR-mediated transcriptional activation and repression[39]. Both CAC and sporadic CRC tend to have p53 mutations; however, these mutations tend to occur at later stages in sporadic CRC and at very early stages in CAC[4]. Therefore, the long-term effects of mutant p53 could be responsible for the increased VDR levels seen in Puerto Rican patients with CAC.
To our knowledge, this study is the first to address NK-1R, pEGFR, Cox-2, and VDR expression in Puerto Ricans or any other Hispanic group. It is also one of the few studies to examine and compare changes in expression for these proteins in sporadic and colitis-associated neoplasia. Doing so allowed us to determine the expression pattern for these proteins in IBD, sporadic dysplasia, sporadic CRC, and CAC. Of particular interest is the differential expression of pEGFR and VDR in sporadic CRC. Negative or weak immunohistochemical staining for VDR and strong immunohistochemical staining for pEGFR in colonic gland cytoplasm in conjunction with architectural changes can be very useful in distinguishing areas of sporadic colonic dysplasia from cancer and from non-neoplastic changes. These stains can further help the pathologist pinpoint the regions in which the presence of atypical mitosis and/or invasion will render a diagnosis of malignancy, and absence of these findings will establish the diagnosis of dysplasia. This should greatly aid in reducing inter-observer variability in establishing these crucial diagnoses, and, by doing so, may help mitigate the current disparity in CRC incidence and mortality between Puerto Ricans and the general United States population. Unfortunately, these changes are not as useful in colitis-associated neoplasia. Whether these findings are limited to Puerto Rican patients or generalizable to other Hispanic groups or the general population remains to be determined. Finally, although inflammation is involved in sporadic neoplasia[40] as well as in colitis-associated colorectal neoplasia, our findings further emphasize the different molecular events underlying the pathogenesis of sporadic CRC and CAC.
Preliminary data from this study were previously communicated at the Annual Meeting of the American Gastroenterological Association (New Orleans, 2010), Experimental Biology 2011 (Washington DC, 2011) and Experimental Biology 2013 (Boston, 2013). The authors would like to thank Alcira Benitez Barros, Victor Rodríguez Rapale, and Antonio Ramírez for their technical assistance and Rasa G. Hamilton (Moffitt Cancer Center) for her editorial assistance.
Colorectal cancer (CRC) is the deadliest cancer in the Puerto Rican population and the second deadliest cancer in both the general United States population and in United States Hispanics. Signaling components of the substance P (SP)-neurokinin-1 receptor (NK-1R)-epidermal growth factor receptor (EGFR) pathway are involved in development of intestinal inflammation and in processes related to oncogenesis. Calcitriol and the vitamin D receptor (VDR) can regulate EGFR and its pathways and may antagonize the neoplastic process.
Expression of these signaling components has not been examined in Puerto Ricans or United States Hispanics, populations where colorectal cancer is one of the deadliest cancers, in colon tissues with cancer and/or with the different histopathological changes that lead up to cancer. This knowledge might be important for CRC prevention and treatment.
This is the first study to address the expression of NK-1R, pEGFR, Cox-2, and VDR in colonic tissue from Puerto Rican patients. In these patients, pEGFR was significantly increased in sporadic and colitis-associated colorectal cancer (CAC), Cox-2 was significantly increased in sporadic CRC, and VDR was significantly decreased in sporadic dysplasia and sporadic CRC. Overall, VDR levels negatively correlated with NK-1R and pEGFR in tissues without CAC.
These findings suggest that, in these patients, NK-1R and pEGFR immunohistochemical positivity together with VDR immunohistochemical negativity could be used to identify areas of neoplastic change in sporadic colorectal neoplasia, with changes in VDR immunoreactivity distinguishing CAC from sporadic cancer.
Sporadic CRC is the type of cancer of the colon and rectum that tends to develop in the general population after the age of 50. In contrast, CAC is a type of colorectal cancer that arises in patients with inflammatory bowel disease (IBD). The risk of developing CAC increases with the duration, extent, and severity of colonic inflammation. Dysplasia of the colon and rectum is defined as a neoplastic change of the epithelium that is not invasive, and therefore non-cancerous or benign. Nonetheless, dysplastic epithelium may eventually become cancerous, and therefore malignant and invasive. Neoplasia includes both benign changes, such as dysplasia, and malignant changes, such as CRC.
In this article, the authors compared for the expression of NK-1R, pEGFR, Cox-2, and VDR with sporadic colorectal neoplasia, colitis, and colitis-associated colorectal neoplasia from a Puerto Rican population. The findings suggest that NK-1R and pEGFR immunohistochemical positivity together with VDR immunohistochemical negativity could be used to identify areas of neoplastic change in sporadic colorectal neoplasia, with changes in VDR immunoreactivity distinguishing CAC from sporadic cancer. This is a well-written paper containing interesting results which merit publication.
P- Reviewer: Esmat S, Fei BY, Luo HS, Ozen H S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Figueroa-Vallés NR, Ortiz-Ortiz KJ, Villanueva-Rosa E, Traverso-Ortiz M, Torres-Cintrón CR, Suárez-Ramos T. Cancer in Puerto Rico, 2004-2009. San Juan, PR. 2012;. |
| 3. | American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2012-2014. Atlanta. 2012;3-8. |
| 4. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 5. | Harpaz N, Polydorides AD. Colorectal dysplasia in chronic inflammatory bowel disease: pathology, clinical implications, and pathogenesis. Arch Pathol Lab Med. 2010;134:876-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Harpaz N, Ward SC, Mescoli C, Itzkowitz SH, Polydorides AD. Precancerous lesions in inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2013;27:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1213] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-774, 774.e1-e4; quiz e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Vendrell R, Venegas HL, Pérez CM, Morell C, Roman RV, Torres EA. Differences in prevalence of inflammatory bowel disease in Puerto Rico between commercial and government-sponsored managed health care insured individuals. Bol Asoc Med P R. 2013;105:15-19. [PubMed] |
| 10. | Damas OM, Jahann DA, Reznik R, McCauley JL, Tamariz L, Deshpande AR, Abreu MT, Sussman DA. Phenotypic manifestations of inflammatory bowel disease differ between Hispanics and non-Hispanic whites: results of a large cohort study. Am J Gastroenterol. 2013;108:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Santiago C, Pagán B, Isidro AA, Appleyard CB. Prolonged chronic inflammation progresses to dysplasia in a novel rat model of colitis-associated colon cancer. Cancer Res. 2007;67:10766-10773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Pagán B, Isidro AA, Coppola D, Chen Z, Ren Y, Wu J, Appleyard CB. Effect of a neurokinin-1 receptor antagonist in a rat model of colitis-associated colon cancer. Anticancer Res. 2010;30:3345-3353. [PubMed] |
| 14. | Pagán B, Isidro AA, Cruz ML, Ren Y, Coppola D, Wu J, Appleyard CB. Erlotinib inhibits progression to dysplasia in a colitis-associated colon cancer model. World J Gastroenterol. 2011;17:4858-4866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Appleyard CB, Morales M, Percy WH. Regional variations in neurokinin receptor subtype contributions to muscularis mucosae and epithelial function in rat colon. Dig Dis Sci. 2006;51:506-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem. 2004;279:45519-45527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Esteban F, Muñoz M, González-Moles MA, Rosso M. A role for substance P in cancer promotion and progression: a mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer Metastasis Rev. 2006;25:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Castagliuolo I, Valenick L, Liu J, Pothoulakis C. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J Biol Chem. 2000;275:26545-26550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda). 2010;25:85-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 20. | Habib A, Créminon C, Frobert Y, Grassi J, Pradelles P, Maclouf J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J Biol Chem. 1993;268:23448-23454. [PubMed] |
| 21. | Wada K, Tanaka H, Maeda K, Inoue T, Noda E, Amano R, Kubo N, Muguruma K, Yamada N, Yashiro M. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1015] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 23. | Goode T, O’Connell J, Anton P, Wong H, Reeve J, O’Sullivan GC, Collins JK, Shanahan F. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut. 2000;47:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Sottili M, Sternini C, Reinshagen M, Brecha NC, Nast CC, Walsh JH, Eysselein VE. Up-regulation of transforming growth factor alpha binding sites in experimental rabbit colitis. Gastroenterology. 1995;109:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Malecka-Panas E, Kordek R, Biernat W, Tureaud J, Liberski PP, Majumdar AP. Differential activation of total and EGF receptor (EGF-R) tyrosine kinase (tyr-k) in the rectal mucosa in patients with adenomatous polyps, ulcerative colitis and colon cancer. Hepatogastroenterology. 1997;44:435-440. [PubMed] |
| 26. | O’Sullivan MG, Chilton FH, Huggins EM, McCall CE. Lipopolysaccharide priming of alveolar macrophages for enhanced synthesis of prostanoids involves induction of a novel prostaglandin H synthase. J Biol Chem. 1992;267:14547-14550. [PubMed] |
| 27. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 28. | Kure S, Nosho K, Baba Y, Irahara N, Shima K, Ng K, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004-G1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | ter Beek WP, Biemond I, Muller ES, van den Berg M, Lamers CB. Substance P receptor expression in patients with inflammatory bowel disease. Determination by three different techniques, i.e., storage phosphor autoradiography, RT-PCR and immunohistochemistry. Neuropeptides. 2007;41:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Rosso M, Robles-Frías MJ, Coveñas R, Salinas-Martín MV, Muñoz M. The NK-1 receptor is expressed in human primary gastric and colon adenocarcinomas and is involved in the antitumor action of L-733,060 and the mitogenic action of substance P on human gastrointestinal cancer cell lines. Tumour Biol. 2008;29:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Gillespie E, Leeman SE, Watts LA, Coukos JA, O’Brien MJ, Cerda SR, Farraye FA, Stucchi AF, Becker JM. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc Natl Acad Sci USA. 2011;108:17420-17425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 657] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 35. | Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14:2370-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Larriba MJ, Muñoz A. SNAIL vs vitamin D receptor expression in colon cancer: therapeutics implications. Br J Cancer. 2005;92:985-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Stambolsky P, Tabach Y, Fontemaggi G, Weisz L, Maor-Aloni R, Siegfried Z, Shiff I, Kogan I, Shay M, Kalo E. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010;17:273-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1041] [Article Influence: 80.1] [Reference Citation Analysis (0)] |