Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1675
Peer-review started: June 19, 2014
First decision: July 9, 2014
Revised: August 12, 2014
Accepted: September 19, 2014
Article in press: September 19, 2014
Published online: February 7, 2015
Processing time: 236 Days and 5.7 Hours
Castleman’s disease (CD) is a rare lymphoproliferative disorder of unknown etiology. Clinically, it occurs as a localized (unicentric) disease or as a systemic (multicentric) disease. Unicentric Castleman’s disease (UCD) presents as a solitary mass and primarily affects the mediastinal, retroperitoneal, and cervical lymph nodes. In contrast to multicentric CD, which involves peripheral lymphadenopathy and numerous systemic symptoms, UCD is not typically associated with generalized symptoms. Three main distinct histologic variants are recognized: hyaline-vascular type, plasma cell type, and mixed type. Extranodal CD is rare. Specifically, UCD exclusively in the spleen is extremely rare, with only 2 cases described in the literature to date. Here, we describe an asymptomatic 75-year-old man with a 5.7 cm × 4.5 cm sized heterogenous enhanced mass located in the spleen. He underwent surgical resection for diagnosis and treatment. A pathologic examination indicated the hyaline-vascular type of CD. In this patient, the preoperative diagnosis was difficult to determine, and therefore, invasive procedures were required.
Core tip: Unicentric Castleman’s disease (CD) of spleen is extremely rare, with only less than 5 cases described in the literature. We experienced a case of CD isolated spleen.
- Citation: Lee HJ, Jeon HJ, Park SG, Park CY. Castleman’s disease of the spleen. World J Gastroenterol 2015; 21(5): 1675-1679
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1675
Castleman’s disease (CD), also known as angiofollicular lymph node hyperplasia, is a rare lymphoproliferative disorder that was first described by Benjamin Castleman in 1954[1]. CD is categorized into two clinical types: unicentric and multicentric[2]. Pathologically, CD is subdivided into the hyaline-vascular (HV), plasma cell (PC), and mixed types[3]. Depending on the clinical and pathologic subtypes of CD, the clinical manifestations and management of the disease are distinct. Although CD can occur wherever lymphoid tissue is found, classically, it arises as a solitary mediastinal nodal mass[3]. The most common sites of UCD are the mediastinum, lung, neck, abdomen and retroperitoneum[3,4]. Less common sites include the axilla, groin, and pelvis[4]. Solid organ involvement is rare, and isolated splenic involvement is extremely rare. Thus far, only 2 cases have been reported in the literature: a case of mixed type UCD described by Kujat et al[5] and a case of hyaline-vascular type UCD described by Taura et al[6]. Here, we report a rare case of a patient with solitary splenic CD.
A 75-year-old man was referred to us with an isolated splenic mass that was detected by computed tomography of the abdomen during an evaluation for abdominal pain, diarrhea and fever. His symptoms began 3 d ago. His medical history included hypertension. His vital signs were as follows: body temperature 38.2 °C, blood pressure 110/70 mmHg, pulse 84 beats per minute, and respiratory rate of 20 breaths per minute. A physical examination revealed only non-specific findings with the exception of increased bowel sound. No lymphadenopathy was present.
Laboratory results were as follows: white blood cell count 13210/mm3 (normal range 4000-8000/mm3), hemoglobin 13.2 g/dL (normal range 14.0-18.0 g/dL), platelet count 143 × 10³/mm3 (normal range 150-400 × 10³/mm3), total protein 7.1 g/dL, albumin 3.6 g/dL, aspartate aminotransferase (AST) 57 IU/L, alkaline aminotransferase (ALT) 39 IU/L, alkaline phosphatase 45 IU/L, serum lactate dehydrogenase level 684 IU/L (normal range 200-450 IU/L), blood urea nitrogen 26.2 mg/dL, creatinine 1.19 mg/dL, serum sodium 133 mEq/L, serum potassium 4.0 mEq/L, chloride level 95 mEq/L, erythrocyte sedimentation rate 40 mm/h (normal range 10-20 mm/h), and C-reactive protein 11.0 mg/dL (normal range 0.0-0.3 mg/dL). Tests were negative for both HIV antigen and antibody.
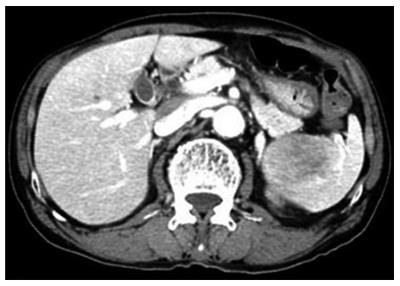
Computed tomography (CT) revealed a 5.7 cm × 4.5 cm sized single mass in the spleen (Figure 1). No enlarged abdominal lymph nodes were observed, and the rest of the abdominal organs appeared to be normal. No other masses or lymphadenopathy were noted on computed tomography of the chest.
A broad spectrum antibiotics (ceftriaxone) was prescribed to the patient due to fever, diarrhea, leukocytosis, elevated ESR, and elevated CRP. On day 6 of his hospital stay, the fever had subsided, and the patient’s symptoms and general condition had improved. On day 8 of the patient’s hospital stay, the underwent laparoscopic splenectomy for diagnosis and treatment because the lesion was limited to the spleen.
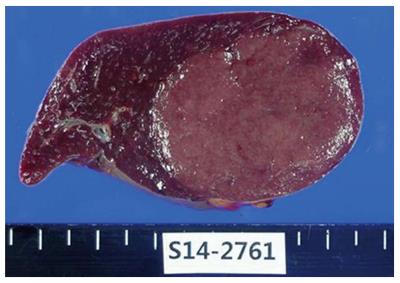
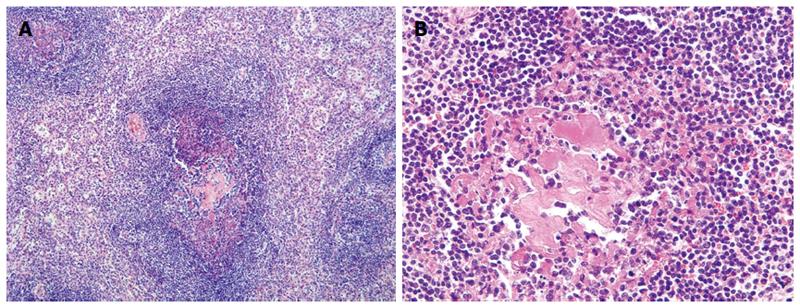
The lesion was a well circumscribed, rubbery tumor (Figure 2). A histopathologic examination revealed hyalinized germinal centers with expanded mantle zones of small lymphocytes that surrounded the germinal centers in addition to interfollicular hypervascularity without plasma cell sheets (Figure 3). Therefore, the hyaline vascular variant of CD was diagnosed. Test for human herpes virus (HHV)-8 was negative.
CD comprises a heterogeneous group of lymphoproliferative disorders with variable clinical presentations and prognoses.
The pathogenesis of CD is not clear, but it has been suggested that CD is associated with immunoregulatory defects in individuals infected with HHV-8 and human immunodeficiency virus (HIV)[7]. HHV-8 infection is universally found in cases of HIV-associated multicentric CD (MCD) and in 40%-50% of cases of HIV-negative MCD[8,9]. HHV-8 encodes viral interleukin (IL)-6, which can induce the secretion of endogenous human IL-6, and vascular endothelial growth factor and can also enhance angiogenesis[10,11]. Specifically, the role of HHV-8 and IL-6 in the pathogenesis of the PC type of MCD has long been recognized. During symptomatic episodes, the serum IL-6 level is elevated in patients with the PC type of CD[12].
Additionally, CD is associated with Kaposi’s sarcoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal M protein and skin lesions) and paraneoplastic pemphigus[7,13].
CD presents as three histological variants: hyaline-vascular (HVCD), plasma cell (PCCD) and mixed type[3]. A mixed subtype has been reported in a few patients. Clinically, CD presents as either a localized disease (UCD) or as a systemic disease (MCD)[2].
Approximately 80%-90% of cases of UCD are classified as HVCD, while only 10%-20% are classified as PCCD[3,4]. UCD is mostly asymptomatic and is diagnosed incidentally upon imaging. Symptoms may be seen in a few patients as a result of mass effects. While UCD can occur at any age, the median age of the patients was 35 years, with an equal male/female ratio[4]. The majority of cases of UCD originate in the mediastinum, lung, neck, pelvis, retroperitoneum and axilla[3,4]. Anemia, fever, fatigue, hypoalbuminemia, hypergammaglobulinemia, and elevation of erythrocyte sedimentation are typically observed in approximately 50% of patients who present with the plasma cell type[14].
Most patients with MCD have the plasma cell type, and most of them have B symptoms (e.g., fever, night sweats, weight loss). Fewer than 10% are asymptomatic. Peripheral lymphadenopathy is virtually always present in cases of MCD[15,16]. The majority of patients also present with hepatosplenomegaly[17,18]. While only approximately 10% of HIV-negative patients have mediastinal or abdominal lymphadenopathy at presentation, after disease progression or if patients are infected with HIV at diagnosis, approximately 50% show lymph node involvement at these sites[16]. A subset of patients experience skin rash, edema, body cavity effusion, and neurologic changes[19]. Laboratory abnormalities include anemia, hypoalbuminemia, hypergammaglobulinemia, increased IL-6, elevated erythrocyte sedimentation rate and elevated C-reactive protein[20]. HHV-8 is frequently associated with MCD, specifically in HIV-positive patients, and the disease prognosis is poor[8,9]. MCD typically presents in individuals between 50 and 65 years of age, although those who are infected with HIV tend to be younger[4,15,16,21]. Male sex is predominant (50%-65%)[4].
Generally, the workup for CD is the same that for any form of lymphadenopathy. Initial imaging is usually performed with CT, as the affected nodes or the localized mass in cases of CD demonstrate a dense homogeneous enhancement[22]. Clinical and radiologic findings are not pathognomonic for CD, and therefore, a definitive diagnosis is established only by histologic examination of the surgical specimen[23]. Tissue that is obtained by a fine needle aspiration or core needle biopsy is often nondiagnostic, therefore, an excisional biopsy is preferred. HVCD is characterized by distinctive follicles with atrophic hyalinized germinal centers and a broad mantle zone of lymphocytes that form concentric rings (so-called onion-skin arrangement). Regressed germinal centers are surrounded by an expanded mantle zone. Increased interfollicular vascularity with hyalinized vessels is another important feature[7,24]. A characteristic lollipop appearance is observed, that is the “onion-skin” of the mantle zone lymphocytes with a penetrating vessel to the germinal center[25]. PCCD has fewer distinctive histologic features. However, it is characterized by remained the lymph node architecture, variable hyperplastic germinal centers, interfollicular hypervascularity and marked plasma cell sheets cytosis[3,26].
The treatment of patients with CD depends on its presentation. Surgical resection is curative in UCD[4,27]. In the patients with UCD who have undergone an incomplete resection, radiotherapy has been shown to improve the outcome[28]. Medical therapy such as steroid and combination chemotherapy is considered a primary treatment option for patients with MCD[29].
This patient was diagnosed incidentally after imaging was performed. A CT scan revealed a solitary mass on the spleen without any lymphadenopathy or any other organ involvement. The patient underwent a splenectomy for diagnosis and curable treatment. The pathologic diagnosis was HVCD localized to the spleen. This patient is elderly relative to most other patients with UCD. He presented with fever, and blood tests revealed mild anemia, elevated ESR, and elevated CRP. These findings were characteristic of MCD rather than UCD, and of the plasma cell variant of UCD rather than hyaline-vascular variant. However, this patient presented with only a single mass on the spleen without any lymphadenopathy in the chest, abdomen and pelvis according to CT. These findings are adequate for the diagnosis of UCD. While the peak incidence of UCD occurs in the second to fourth decades of life, it can occur at any age. Fever had subsided and the elevated ESR and CRP improved after treatment with antibiotics that targeted colitis. These clinical symptoms and findings are associated with infection but not with CD. Furthermore, these findings are adequate for the diagnosis of the hyaline-vascular variant of CD in that the pathologic findings showed atrophic hyalinized germinal centers with expanded mantle zones of small lymphocytes that surround the germinal centers and interfollicular hypervascularity without plasma cell sheets. HHV-8 infection is universally found in HIV-associated MCD versus in HIV-negative MCD and is associated with PCCD rather than HVCD. Most cases of the hyaline-vascular variant of UCD are negative for HHV-8. Although, HHV-8 infection would support the diagnosis, a negative serologic or immunohistochemical assay for HHV-8 would not eliminate the diagnosis. Therefore, this patient was diagnosed with HVCD according to radiologic and histologic findings. We experienced and reported an extremely rare case of CD that originated in an extranodal organ, the spleen.
A 75-year-old man with an isolated splenic mass presented with abdominal pain, diarrhea and fever.
Findings were non-specific with the exception of increased bowel sound.
Splenic abscess, lymphoma, splenic hamartoma.
White blood cell 13210/mm3; Hb 13.2 g/dL; platelet count 143 × 10³/mm3; serum lactate dehydrogenase 684 IU/L; erythrocyte sedimentation rate 40 mm/h; C-reactive protein 11.0 mg/dL; metabolic panel and liver function test were within normal limits.
Computed tomography revealed a 5.7 cm × 4.5 cm sized single mass in the spleen.
A laparoscopic splenectomy revealed unicentric Castleman’s disease of the hyaline vascular type.
Laparoscopic splenectomy.
Unicentric Castleman’s disease (UCD) is mostly asymptomatic and is diagnosed incidentally upon imaging.
POEMS syndrome is a syndrome characterized by polyneuropathy, organomegaly, endocrinopathy, monoclonal M protein and skin lesions.
This case report describes the typical histologic findings of the hyaline-vascular variant of UCD, which are atrophic hyalinized germinal centers with expanded mantle zones of small lymphocytes that surround the germinal centers and interfollicular hypervascularity without plasma cell sheets.
This case is the third case reported in the literature as UCD localized to the spleen, and although it is atypical with regard to the age of onset, the pathologic findings are indicative of the typical hyaline-vascular variant of UCD.
P- Reviewer: Lhuaire M S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Gaba AR, Stein RS, Sweet DL, Variakojis D. Multicentric giant lymph node hyperplasia. Am J Clin Pathol. 1978;69:86-90. [PubMed] |
| 3. | Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29:670-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Kujat C, Müller-Leisse C, Lorbacher P, Seufert R, Falk S, Stutte HJ. [A unifocal manifestation of Castleman’s disease (angiofollicular lymphatic hyperplasia) in the spleen]. Rofo. 1990;152:615-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Taura T, Takashima S, Shakudo M, Kaminou T, Yamada R, Isoda K. Castleman’s disease of the spleen: CT, MR imaging and angiographic findings. Eur J Radiol. 2000;36:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276-1280. [PubMed] |
| 9. | Gessain A, Sudaka A, Brière J, Fouchard N, Nicola MA, Rio B, Arborio M, Troussard X, Audouin J, Diebold J. Kaposi sarcoma-associated herpes-like virus (human herpesvirus type 8) DNA sequences in multicentric Castleman’s disease: is there any relevant association in non-human immunodeficiency virus-infected patients? Blood. 1996;87:414-416. [PubMed] |
| 10. | Mori Y, Nishimoto N, Ohno M, Inagi R, Dhepakson P, Amou K, Yoshizaki K, Yamanishi K. Human herpesvirus 8-encoded interleukin-6 homologue (viral IL-6) induces endogenous human IL-6 secretion. J Med Virol. 2000;61:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034-4043. [PubMed] |
| 12. | Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, Nakahata T, Kawai H, Tagoh H, Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74:1360-1367. [PubMed] |
| 13. | Kop EN, MacKenzie MA. Clinical images: Castleman disease and paraneoplastic pemphigus. CMAJ. 2010;182:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Goldberg MA, Deluca SA. Castleman’s disease. Am Fam Physician. 1989;40:151-153. [PubMed] |
| 15. | Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Ann Intern Med. 1998;128:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 262] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Frizzera G, Peterson BA, Bayrd ED, Goldman A. A systemic lymphoproliferative disorder with morphologic features of Castleman’s disease: clinical findings and clinicopathologic correlations in 15 patients. J Clin Oncol. 1985;3:1202-1216. [PubMed] |
| 17. | Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116:4415-4421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Shin DY, Jeon YK, Hong YS, Kim TM, Lee SH, Kim DW, Kim I, Yoon SS, Heo DS, Park S. Clinical dissection of multicentric Castleman disease. Leuk Lymphoma. 2011;52:1517-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kawabata H, Kadowaki N, Nishikori M, Kitawaki T, Kondo T, Ishikawa T, Yoshifuji H, Yamakawa N, Imura Y, Mimori T. Clinical features and treatment of multicentric castleman’s disease : a retrospective study of 21 Japanese patients at a single institute. J Clin Exp Hematop. 2013;53:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Bower M, Powles T, Williams S, Davis TN, Atkins M, Montoto S, Orkin C, Webb A, Fisher M, Nelson M. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Bower M, Newsom-Davis T, Naresh K, Merchant S, Lee B, Gazzard B, Stebbing J, Nelson M. Clinical Features and Outcome in HIV-Associated Multicentric Castleman’s Disease. J Clin Oncol. 2011;29:2481-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Moon WK, Im JG, Kim JS, Choi CG, Kim HC, Yeon KM, Han MC. Mediastinal Castleman disease: CT findings. J Comput Assist Tomogr. 1994;18:43-46. [PubMed] |
| 23. | Tunru-Dinh VW, Ghani A, Tom YD. Rare case of Castleman disease involving the pancreas. Am Surg. 2007;73:1284-1287. [PubMed] |
| 24. | Nguyen DT, Diamond LW, Hansmann ML, Alavaikko MJ, Schröder H, Fellbaum C, Fischer R. Castleman’s disease. Differences in follicular dendritic network in the hyaline vascular and plasma cell variants. Histopathology. 1994;24:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Nagubandi R, Wang Y, Dutcher JP, Rao PM. Classic case of unicentric mixed-type Castleman’s disease. J Clin Oncol. 2013;31:e452-e455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Amin HM, Medeiros LJ, Manning JT, Jones D. Dissolution of the lymphoid follicle is a feature of the HHV8+ variant of plasma cell Castleman’s disease. Am J Surg Pathol. 2003;27:91-100. [PubMed] |
| 27. | Dispenzieri A, Armitage JO, Loe MJ, Geyer SM, Allred J, Camoriano JK, Menke DM, Weisenburger DD, Ristow K, Dogan A. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Bowne WB, Lewis JJ, Filippa DA, Niesvizky R, Brooks AD, Burt ME, Brennan MF. The management of unicentric and multicentric Castleman’s disease: a report of 16 cases and a review of the literature. Cancer. 1999;85:706-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |