Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1606
Peer-review started: July 31, 2014
First decision: August 15, 2014
Revised: August 22, 2014
Accepted: October 15, 2014
Article in press: October 15, 2014
Published online: February 7, 2015
Processing time: 194 Days and 2.6 Hours
AIM: To describe the learning curves of hand-assisted laparoscopic D2 radical gastrectomy (HALG) for the treatment of gastric cancer.
METHODS: The HALG surgical procedure consists of three stages: surgery under direct vision via the port for hand assistance, hand-assisted laparoscopic surgery, and gastrointestinal tract reconstruction. According to the order of the date of surgery, patients were divided into 6 groups (A-F) with 20 cases in each group. All surgeries were performed by the same group of surgeons. We performed a comprehensive and in-depth retrospective comparative analysis of the clinical data of all patients, with the clinical data including general patient information and intraoperative and postoperative observation indicators.
RESULTS: There were no differences in the basic information among the patient groups (P > 0.05). The operative time of the hand-assisted surgery stage in group A was 8-10 min longer than the other groups, with the difference being statistically significant (P = 0.01). There were no differences in total operative time between the groups (P = 0.30). Postoperative intestinal function recovery time in group A was longer than that of other groups (P = 0.02). Lengths of hospital stay and surgical quality indicators (such as intraoperative blood loss, numbers of detected lymph nodes, intraoperative side injury, postoperative complications, reoperation rate, and readmission rate 30 d after surgery) were not significantly different among the groups.
CONCLUSION: HALG is a surgical procedure that can be easily mastered, with a learning curve closely related to the operative time of the hand-assisted laparoscopic surgery stage.
Core tip: In order to explore the learning curves and impact factors of hand-assisted laparoscopic D2 radical gastrectomy (HALG) for the treatment of advanced gastric cancer, plenty of pre-, intra-, and post-operative data was involved in this study. We found that the HALG learning curve was closely related to the operative time of the hand-assisted laparoscopic surgery stage and was not related to surgical quality indicators. The HALG learning curve indicates that it is a surgical procedure that can be easily mastered.
- Citation: Gong JQ, Cao YK, Wang YH, Zhang GH, Wang PH, Luo GD. Learning curve for hand-assisted laparoscopic D2 radical gastrectomy. World J Gastroenterol 2015; 21(5): 1606-1613
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1606.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1606
Laparoscopic-assisted D2 radical gastrectomy (LAG) is capable of the same degrees of thoroughness and safety as laparotomy for the treatment of advanced gastric cancer, which is a view that has been accepted by the majority of surgeons[1,2]. Compared to laparotomic radical gastrectomy, laparoscopic techniques have obvious advantages, such as reduced invasiveness, cosmetic incisions, reduced pain in patients, and shorter postoperative hospital stays[3,4]. However, the laparoscopic surgical procedure is difficult, with long learning curves[5]. To determine a starting point between the two procedures that not only maintains their individual advantages, but also avoids their shortcomings, we have created our own surgical route, hand-assisted laparoscopic D2 radical gastrectomy (HALG), on the basis of the characteristics of hand-assisted laparoscopic radical colectomy[6,7]. A few HALG cases have been reported in recent years, but there have been no systematic reports on the topic[8-13]. From July 2008 to June 2013, we conducted 120 HALG procedures and have generated a fixed, convenient, and safe surgical protocol. This procedure has been widely reported in China and introduced in multiple keynote speeches in national meetings, which has inspired widespread acclaim[12,13]. For LAG, D2 lymph node dissection is the principal factor that prevents its comprehensive application and is also the point of difficulty and bottleneck for this technique[14,15]. In our preliminary work, we performed an in-depth prospective study focusing on HALG and LAG, and we believe that the former effectively breaks the latter’s point of difficulty and bottleneck while maintaining the same degrees of thoroughness and safety as laparotomy[16]. To promote this procedure, we retrospectively analyzed the learning curve of HALG performed by the same surgical team in our center. We hope to provide a reference for surgeons who have already mastered the technique of laparotomic radical gastrectomy to then successfully perform HALG and thereby surpass the learning curve in a smooth, safe, and fast manner.
From July 2008 to June 2013, our center conducted 120 HALG procedures. All patients received preoperative histopathological examination under gastroscopy to confirm the diagnosis. The patients underwent preoperative upper gastrointestinal imaging, chest X-ray, and abdominal computed tomography scans to exclude distant metastases, such as pulmonary and liver metastases, and to confirm that the tumors did not show signs of invasion of adjacent organs and were resectable. Based on the order of the date of the surgery, patients were divided into 6 groups, A-F, with 20 cases in each group. General patient information included age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification[17], history of abdominal surgery, tumor size, tumor TNM stage, and the rate of switching to laparotomy during surgery. All surgeries were performed by the same group of surgeons that had mastered the technique of laparotomic D2 radical gastrectomy and had amassed a wealth of clinical experience. All patients were attended to under uniform treatment principles and discharge standards.
Tumor staging was performed via abdominal exploration, with D2 radical surgery implemented and perigastric lymph node dissection performed according to Japan’s “Statute of gastric cancer treatment”. The HALG surgical method can be divided into three phases using total gastrectomy as an example: (1) Surgery under direct vision via the port for hand-assistance: in this stage, an incision approximately 7 cm under the xiphoid in the middle of the upper abdomen was made, and the omentum, 5th, 6th, 14th, and portions of 8a groups of lymph nodes were dissected; (2) Hand-assisted laparoscopic surgery: in this stage, after placing the LapDisc hand-assisted device, the 1st, 2nd, 3rd, 4th, 7th, 9th, 10th, 11th, and remaining 8a groups of lymph nodes were dissected; and (3) Gastrointestinal tract reconstruction phase. Specific surgical procedures and our preliminary work have been published in Surgical Endoscopy[16].
The observation and recording of all indicators were completed by dedicated personnel in our center, followed by statistical analysis. For the 6 groups of patients, intraoperative observation of the following indicators was conducted: surgical approach (total gastrectomy, proximal gastrectomy, and distal gastrectomy), operative time, blood loss, number of detected lymph nodes, and intraoperative side injury. The postoperative observation indicators were: intestinal function recovery time, postoperative hospital stay, postoperative complications (pulmonary infection, cardiac arrhythmias, gastrointestinal fistula, gastrointestinal disorders, bile reflux, abdominal infection, and wound infection), reoperation rate, readmission rate after 30 d, and mortality. Perigastric lymph nodes were removed one by one from the resected specimens by a pathologist, and were classified based on pathological examination. To facilitate statistical analysis, the intraoperative and postoperative indicators of cases switched to laparotomy were not included in the statistics. To facilitate a more intuitive evaluation of the learning curve for time, we divided operative times into two parts: total operative time (from opening the incision to completion of incision suturing) and hand-assisted laparoscopic surgery time (from the insertion of the LAP DISC to the removal of the trocars).
We used SPSS 16.0 software for the statistical analysis. Measurement data are expressed as mean ± SD and analyzed using one-way analysis of variance. Pairwise comparisons were conducted using the Tamhane and least significant difference tests. Count data were analyzed using the χ2 test. Significance was set at P < 0.05.
As shown in Table 1, the patient groups were not statistically significantly different in terms of age (P = 0.82), gender (P = 0.95), BMI (P = 0.64), ASA classification (P = 0.70), history of abdominal surgery (P = 0.89), tumor size (P = 0.89), and tumor TNM stage (P = 0.99). In terms of the rate of switching to laparotomy in surgery, there were two cases that were switched to laparotomy (P = 0.54); group A had one case with tumors that invaded the splenic hilum and group C had one case with tumors that had invaded the celiac trunk. Those patients who were switched to laparotomy in surgery were not included in the intraoperative and postoperative statistics. Therefore, the intraoperative and postoperative statistics had 19 cases each in groups A and C, and 20 cases each in the remaining groups.
| Cohort | A (n = 20) | B (n = 20) | C (n = 20) | D (n = 20) | E (n = 20) | F (n = 20) | P value |
| Age (yr), mean ± SD | 58.45 ± 7.80 | 56.45 ± 9.67 | 57.30 ± 9.89 | 59.05 ± 11.66 | 59.65 ± 7.32 | 59.95 ± 8.51 | 0.82 |
| Median (range) | 60.0 (34-71) | 57.0 (40-75) | 56.5 (40-72) | 63.0 (36-73) | 59.5 (41-75) | 60.5 (45-75) | |
| Sex (male:female) | 12:8 | 13:7 | 12:8 | 11:9 | 14:6 | 13:7 | 0.95 |
| BMI; mean ± SD | 24.24 ± 3.15 | 24.04 ± 2.94 | 25.47 ± 3.31 | 25.28 ± 3.59 | 24.93 ± 3.26 | 25.30 ± 3.31 | 0.64 |
| median (range) | 24.7 (19.6-32.0) | 23.9 (19.0-31.7) | 25. 6 (19.9-31.8) | 26.2 (19.0-31.5) | 25.1 (19.3-31.9) | 25.0 (19.5-31.7) | |
| ASA | 0.70 | ||||||
| I | 1 | 3 | 2 | 1 | 2 | 3 | |
| II | 14 | 9 | 14 | 16 | 14 | 12 | |
| III | 5 | 8 | 4 | 3 | 4 | 5 | |
| Pre-abdominal operation, n (%) | 1 (5) | 2 (10) | 2 (10) | 3 (15) | 2 (10) | 1 (5) | 0.89 |
| Size of tumor (cm), mean ± SD | 4.57 ± 1.54 | 4.64 ± 1.49 | 4.53 ± 1.46 | 4.66 ± 1.29 | 4.96 ± 1.41 | 4.94 ± 1.54 | 0.89 |
| median (range) | 4.6 (2.2-6.8) | 5.3 (2.3-6.2) | 4.5 (2.4-6.8) | 4.4 (2.2-6.7) | 5.2 (2.3-6.7) | 5.1 (2.2-6.8) | |
| TNM stage (n) | 0.99 | ||||||
| I | 3 | 2 | 3 | 3 | 2 | 2 | |
| II | 3 | 4 | 4 | 3 | 2 | 3 | |
| IIIA | 3 | 4 | 3 | 4 | 3 | 5 | |
| IIIB | 6 | 4 | 3 | 4 | 5 | 2 | |
| IV | 5 | 6 | 7 | 6 | 8 | 8 | |
| Open conversion | 1 | 0 | 1 | 0 | 0 | 0 | 0.54 |
The intraoperative indicators included a total of five items: surgical approach (total gastrectomy, proximal gastrectomy, and distal gastrectomy), operative time, blood loss, number of detected lymph nodes, and unexpected injuries. As shown in Table 2, the various groups were not statistically significantly different in terms of surgical approach (P = 0.99). Group A had a total operative time of 166.26 ± 12.15 min, 4-8 min longer than the rest of the groups, but the difference was not statistically significant (P = 0.30). For groups A-F, the operative time of hand-assisted surgery stage was 43.21 ± 11.20, 35.00 ± 7.3, 34.11 ± 8.07, 35.20 ± 7.63, 33.65 ± 6.68, and 35.55 ± 9.92 min, respectively, with group A requiring 8-10 min longer than the other groups, which was a statistically significant difference (P = 0.01); however, there were no statistically significant differences among the remaining groups (P > 0.05). Intraoperative blood loss was 112-343 mL, with no statistically significant differences among the groups (P = 0.96). The number of detected lymph nodes was 10-23, with no statistically significant differences among the groups (P = 0.78). In all groups, there were no unexpected operation-induced injuries.
| Cohort | A (n = 19) | B (n = 20) | C (n = 19) | D (n = 20) | E (n = 20) | F (n = 20) | P value |
| Type of operation (n) | 0.99 | ||||||
| Total gastrectomy | 8 | 7 | 8 | 7 | 8 | 8 | |
| Distal gastrectomy | 10 | 11 | 9 | 12 | 10 | 9 | |
| Proximal gastrectomy | 1 | 2 | 2 | 1 | 2 | 3 | |
| Operative time (min) | |||||||
| Total, mean ± SD | 166.26 ± 12.15 | 159.70 ± 12.36 | 161.68 ± 12.01 | 158.30 ± 11.97 | 162.95 ± 11.27 | 158.80 ± 11.85 | 0.30 |
| median (range) | 169 (137-185) | 158 (139-184) | 164 (136-183) | 157.5 (136-184) | 164.5 (140-182) | 157.5 (138-180) | |
| Lap, mean ± SD | 43.21 ± 11.20 | 35.00 ± 7.31 | 34.11 ± 8.07 | 35.20 ± 7.63 | 33.65 ± 6.68 | 35.55 ± 9.92 | 0.01 |
| median (range) | 43 (25-65) | 33 (24-50) | 33 (23-54) | 36 (22-47) | 34 (21-48) | 35.5 (18-51) | |
| Blood loss (mL) | |||||||
| mean ± SD | 237.89 ± 71.77 | 231.90 ± 68.42 | 242.42 ± 71.96 | 245.55 ± 70.70 | 225.75 ± 68.65 | 235.55 ± 64.59 | 0.96 |
| median (range) | 267 (120-340) | 248 (125-343) | 265 (112-335) | 260.5 (120-342) | 223 (117-341) | 226 (116-335) | |
| Lymph nodes harvested | |||||||
| mean ± SD | 17.74 ± 4.56 | 18.25 ± 3.78 | 19.26 ± 2.26 | 18.47 ± 3.58 | 18.37 ± 3.55 | 19.16 ± 3.39 | 0.78 |
| median (range) | 17 (10-23) | 18.5 (11-23) | 19 (12-23) | 19 (12-23) | 19 (12-23) | 19 (13-23) | |
| Unexpected injury | 0 | 0 | 0 | 0 | 0 | 0 |
The postoperative indicators included a total of 5 items: intestinal function recovery time, postoperative hospital stay, postoperative complications (pulmonary infection, cardiac arrhythmias, gastrointestinal fistula, gastrointestinal disorders, bile reflux, abdominal infection, and wound infection), reoperation rate, and readmission rate after 30 d. As shown in Table 3, group A had a postoperative intestinal function recovery time of 65.58 ± 10.53 h, 8-11 h longer than the rest of the groups, which was statistically significantly different (P = 0.02); however, there were no statistically significant differences among groups B-F (P > 0.05). Group A had a hospital stay of 9.79 ± 1.78 d, which was longer than the other groups, but not statistically significantly different (P = 0.11). Groups A-F had 3, 3, 3, 1, 2, and 2 respective cases of postoperative complications that were all cured, with no statistically significant differences (P = 0.89), of which group A had 1 case of pan-peritonitis caused by duodenal stump leakage that was cured by re-surgery and drainage; the other groups had no gastrointestinal leak. There were 5 cases that were re-admitted within 30 d of surgery, including 1 case in group A that was hospitalized due to poor appetite, 1 case in group B that was re-admitted due to constipation, 2 and 1 cases in groups C and F, respectively, that were hospitalized due to intestinal adhesions. The above patients exhibited symptom relief after 2-5 d of treatment and were discharged; there were no statistically significant differences among the groups (P = 0.59). There were no postoperative deaths.
| Cohort | A (n = 19) | B (n = 20) | C (n = 19) | D (n = 20) | E (n = 20) | F (n = 20) | P value |
| Functional recovered bowel (h) | |||||||
| mean ± SD | 65.58 ± 10.53 | 55.65 ± 12.13 | 57.42 ± 10.92 | 54.65 ± 11.33 | 55.35 ± 9.81 | 56.25 ± 9.95 | 0.02 |
| Median (range) | 63 (44-93) | 52.5 (41-83) | 56 (37-82) | 54 (38-75) | 54 (38-77) | 55 (41-80) | |
| Length of stay (d) | |||||||
| mean ± SD | 9.79 ± 1.78 | 8.75 ± 1.77 | 8.53 ± 1.74 | 8.55 ± 1.28 | 8.60 ± 1.67 | 8.50 ± 1.40 | 0.11 |
| median (range) | 10 (7-13) | 8.5 (6-13) | 8 (6-12) | 8 (7-12) | 8.5 (6-13) | 8 (6-12) | |
| Complication (total) | 3 | 3 | 3 | 1 | 2 | 2 | 0.89 |
| Pulmonary infection | 1 | 1 | |||||
| Arrhythmia | 1 | ||||||
| Anastomotic leak | 1 | ||||||
| Gastrointestinal dysfunction | 1 | 1 | 1 | ||||
| Bile back flow | 1 | 1 | |||||
| Abdominal cavity infection | 1 | ||||||
| Wound infect | 1 | 1 | 1 | 1 | |||
| Reoperation | 1 | 0.39 | |||||
| Readmission | 1 | 1 | 2 | 1 | 0.59 |
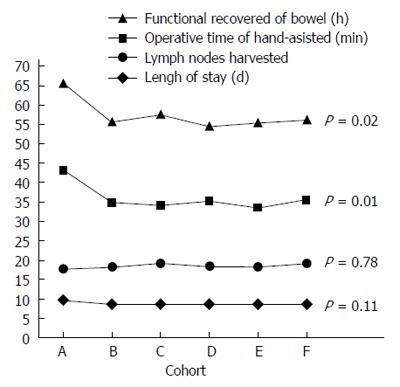
As shown in Figure 1, group A had an operative time in the hand-assisted laparoscopic surgery stage of 43.21 ± 11.20 min, significantly longer than the other groups (P = 0.01), whereas there were no statistically significant differences among groups B-F (P > 0.05); the learning curve had a significant downward trend from point A to point B, and showed a flat trend from point B to point F. When comparing postoperative intestinal function recovery time, recovery time in group A was 65.58 ± 10.53 h, significantly longer than the other groups (P = 0.02), whereas there were no significant differences among groups B-F (P > 0.05); therefore, the learning curve had a significant downward trend from point A to point B, and showed a flat trend from point B to point F. When comparing the lengths of hospital stay, group A had a hospital stay of 9.79 ± 1.78 d, which was longer than the rest of groups; however, the differences were not statistically significant (P = 0.11). The learning curve showed a downward trend from point A to point B, but the trend was not significant, while the curve was flat from point B to point F. The most important quality indicator for D2 radical gastrectomy is the number of obtained lymph nodes. Group A obtained 17.74 ± 4.56 lymph nodes, with no statistically significant differences (P = 0.78) when compared with the other groups; therefore, the curve was flat. No group had a statistically significant difference in terms of total operative time (P = 0.30); therefore, the learning curve trend was not obvious. There were no significant differences among the groups (P > 0.05) for the other related quality indicators, such as blood loss, intraoperative side injury, rate of postoperative complications, reoperation rate, and readmission rate 30 d after surgery. Therefore, these indicators did not show learning curve trends.
After searching the relevant literature, we determined that this study is the first retrospective analysis and investigation of the learning curve for HALG. We compared general patient information (age, gender, BMI, ASA classification, history of abdominal surgery, tumor size, TNM stage, and rate of switching to laparotomy) for each group of patients and found that there were no significant differences (P > 0.05) among the groups. We performed an in-depth analysis and investigation of the intraoperative and postoperative indicators of various groups and established our own HALG learning curve to provide a reference for surgeons who have mastered the skill of laparotomic D2 radical gastrectomy to smoothly utilize HALG.
When starting to master a surgical procedure, beginners inevitably go through a process of imitation, exploration, mastering, and stereotyping; this process is considered to be a learning curve. Usually, the learning curve is measured not only by operative time, but, more importantly, by recent quality of surgeries[18]. In this study, we applied the following quality indicators: blood loss, number of lymph nodes obtained, unexpected injuries, rate of switching to laparotomy, postoperative intestinal function recovery time, postoperative complications, length of stay, and readmission rate after 30 d. Analyzing the HALG learning curve statistics, we believe that the HALG learning curve was only closely related to the operative time of the hand-assisted laparoscopic surgery stage and was not related to any indicators of surgical quality.
In HALG surgery, we took full advantage of the port for hand-assistance to complete most of the lymph node dissections and related tissue dissociations required by the D2 radical treatment principles under direct vision. Only portions of the lymph node and tissue dissociations were completed in the hand-assisted stage, which greatly reduced the laparoscopic workload. Analysis of the total operative time showed that group A took 4-8 min longer than the other groups, but the difference was not statistically significant (P = 0.30). We then analyzed the operative time of the hand-assisted stage and found that the time for group A was 8-10 min longer than other groups, which was statistically significant (P = 0.01); however, there were no significant differences among groups B-F (P > 0.05). These data suggest that hand-assisted laparoscopic surgery stage is key to the HALG learning curve, with the operative time learning curve of this stage determining the total operative time learning curve of HALG.
We also analyzed the learning curves of indicators related to surgical quality. The postoperative intestinal function recovery time of group A was 8-11 h longer than the rest of the groups, which was a statistically significant difference (P = 0.02); there were no statistically significant differences among groups B-F (P > 0.05). The length of hospital stay of group A was longer than the other groups, but this difference was not statistically significant (P = 0.11). The above data did not suggest that the HALG learning curve was directly related to surgery quality indicators, instead being only directly related to the operative time of the hand-assisted laparoscopic surgery stage. Hand-assisted and laparoscopic-assisted techniques are minimally invasive surgical techniques that have been developed in recent years. Studies have shown that minimal invasiveness is closely related to the stability of the internal environment. A shorter operative time is associated with less trauma to the body; lower levels of serum inflammatory cytokines, such as interleukin-6 (IL-6), IL-10, C-reactive protein, and tumor necrosis factor-α; and a more stable internal body environment[19,20]. Analysis of the HALG time learning curve revealed that group A had a significantly longer hand-assisted surgical time than the other groups. This group experienced the largest interference to the internal environment, thereby leading to longer intestinal function recovery and hospital stay times than the other groups.
When comparing other elements of quality indicators, group A did not show statistically significant differences in terms of blood loss, number of lymph nodes obtained, intraoperative side injury, rate of switching to laparotomy, postoperative complications, reoperation rate, or readmission rate after 30 d compared with the other groups (P > 0.05). Therefore, the learning curve trends were not obvious. Our analysis indicated some reasons for these findings: (1) The surgical team had mastered the laparotomic D2 radical gastrectomy technique. Most lymph node dissection and tissue separation work was completed under direct vision via the port for hand assistance, and the surgical techniques were fully consistent with those of laparotomy; and (2) The HALG hand-assisted laparoscopic stage accounted for a relatively low proportion of the total operative time, with only group A having a significantly longer operative time in the hand-assisted stage than the other groups (P = 0.01).
The greatest point of difficulty and bottleneck for the promotion of LAG in clinical practices is laparoscopic D2 lymph node dissection[14,21]. To overcome this difficulty and break through this bottleneck, we have successfully performed HALG and formed a fixed, safe, and convenient surgical protocol that features minimal invasiveness of LAG and the safety and lymphadenectomy thoroughness of laparotomy[12,14,16]. In this study, the HALG learning curve included 20 cases, which is a significantly reduced number compared to the LAG learning curves reported by others[22,23]. Our analysis indicated some possible reasons for this difference: (1) D2 lymph node dissection is the key step and point of difficulty for radical treatment. HALG divides the lymph node dissection into two stages, with the first taking full advantage of direct vision via the port for hand assistance to complete most of the lymph node dissections and the subsequent hand-assisted stage only completing the remaining lymph node dissections, thereby greatly reducing the laparoscopic operation time; (2) Completion of LAG requires the close coordination of three people: the surgeon, the assistant, and the camera assistant. The coordination of these three people is achieved through rigorous training and the accumulation of experience over a long period of time. HALG only requires cooperation between the surgeon and the camera assistant, greatly reducing the difficulty of cooperation and shortening training time; and (3) In HALG, the surgeon’s left hand works closely with the ultrasonic scalpel, the thumb and forefinger of the assisting hand stretch out in reverse directions to separate the tissue from the parts that are being exposed, and the remaining three fingers cooperate with these two fingers to form a triangle of support. HALG can fully reveal the operative field, which has an important advantage in the dissection of tissues around the splenic hilum, the lesser curvature of the stomach, and the cardia; thus, the complexity of the surgical procedure is significantly reduced when compared with LAG.
Analysis of the HALG learning curve revealed that it was influenced by simple impact factors: (1) A reasonable surgical route is one of the important factors affecting the HALG learning curve and is also important in ensuring smooth surgical performance. In the beginner stage, the surgical route is not finalized, and skill stability is still lacking, which are factors that seriously affect the surgical process. When we first launched HALG, the hand-assist port was close to the upper edge of the umbilicus. The biggest drawback of this type of port was that it was difficult to complete lymph node dissection for the upper edge of the lesser curvature of the stomach under direct vision. Inappropriate selection of the trocar location for the ultrasonic scalpel could also greatly affect the operation of the assisting hand in the limited space in the upper abdomen. Inappropriate selection of the observation hole could also seriously affect the surgeon’s viewing angle and could sometimes lead to visual dislocation; and (2) In the surgical procedure, the surgeons need to utilize hand dexterity as much as possible to fully expose the operative field, protect important blood vessels and tissue, and stop bleeding using the thumb and forefinger in a timely manner so that the cutting, slicing, separating, and sectioning functions of the ultrasonic scalpel can be utilized to the extreme, thus greatly reducing the operative time.
Previous literature has shown that many authors regard hand-assisted laparoscopic surgery as a bridge from laparotomy to full laparoscopic surgery[11,24,25]. However, we have a different view. As early as 2004, Hunter[26] described very optimistic predictions on the prospects of HALG. We believe that regardless of the procedure type, a prerequisite should be to ensure the personal safety of the patient, and the goals should be to achieve tumor-free and tumor-cure states. The short-term efficacy of HALG does not differ significantly from those of laparoscopic surgery and laparotomy. The HALG learning curve is far less complex than that of LAG[22,23]. In the present study, we found that there were only learning curve trends for operative time and intestinal function recovery time. Other indicators, such as quality of surgery, did not show learning curve trends. Therefore, for surgeons who have mastered the technique of open radical D2 gastrectomy, the time taken to master the technique of HALG was significantly shorter than that of LAG; thus, surgeons only required short-term training to master the key points of the procedure.
In the preliminary work, the authors believed that hand-assisted laparoscopic D2 radical gastrectomy (HALG) effectively removes the laparoscopic-assisted D2 radical gastrectomy (LAG) point of difficulty and bottleneck while maintaining the same degrees of thoroughness and safety as laparotomy. However, the learning curves and impact factors of HALG for the treatment of advanced gastric cancer have not been reported. In order to provide a reference for surgeons who have already mastered the technique of laparotomic radical gastrectomy to then successfully perform HALG and surpass the learning curve in a smooth, safe, and fast manner, the authors retrospectively analyzed the learning curve of HALG performed by the same surgical team in their center.
LAG is capable of the same degrees of thoroughness and safety as laparotomy for the treatment of advanced gastric cancer. However, the laparoscopic surgical procedure is difficult, with long learning curves. In order to determine a starting point between the two procedures that not only maintains the advantages of the two procedures but also avoids their shortcomings, a few HALG cases have been reported in recent years; however, there have been no systematic reports on HALG.
The present study indicates that HALG broke through the bottleneck of the LAG learning curve, with a learning curve that was closely related to the operative time of the hand-assisted laparoscopic surgery stage, but not related to surgical quality indicators.
This study provides a reference for surgeons who have already mastered the technique of laparotomic radical gastrectomy to then successfully perform HALG and surpass the learning curve in a smooth, safe, and fast manner.
This research is the first retrospective analysis and investigation of the learning curve for HALG, and which is an interesting presentation on an openly debated topic, and also presents a possible solution for reducing the long learning curve for gastric cancer surgery. This article is therefore innovative and worth publishing.
P- Reviewer: Ji JF, Morgagni P, Pan WS, Wang ZW, Yoshikawa T S- Editor: Gou SX L- Editor: Rutherford A E- Editor: Ma S
| 1. | Wang W, Zhang X, Shen C, Zhi X, Wang B, Xu Z. Laparoscopic versus open total gastrectomy for gastric cancer: an updated meta-analysis. PLoS One. 2014;9:e88753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Saeki H, Oki E, Tsuda Y, Ando K, Hiyoshi Y, Itoh S, Morita M, Ikeda T, Sugimachi K, Yamashita Y. Relevance of totally laparoscopic gastrectomy for patients with advanced gastric cancer. Fukuoka Igaku Zasshi. 2013;104:405-412. [PubMed] |
| 3. | Xiong JJ, Nunes QM, Huang W, Tan CL, Ke NW, Xie SM, Ran X, Zhang H, Chen YH, Liu XB. Laparoscopic vs open total gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:8114-8132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Cui M, Xing JD, Yang W, Ma YY, Yao ZD, Zhang N, Su XQ. D2 dissection in laparoscopic and open gastrectomy for gastric cancer. World J Gastroenterol. 2012;18:833-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Kim HG, Park JH, Jeong SH, Lee YJ, Ha WS, Choi SK, Hong SC, Jung EJ, Ju YT, Jeong CY. Totally laparoscopic distal gastrectomy after learning curve completion: comparison with laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2013;13:26-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Nam SE, Jung EJ, Ryu CG, Paik JH, Hwang DY. Feasibility of hand-assisted laparoscopic surgery as compared to open surgery for sigmoid colon cancer: a case-controlled study. Ann Coloproctol. 2013;29:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Meshikhes AW. Controversy of hand-assisted laparoscopic colorectal surgery. World J Gastroenterol. 2010;16:5662-5668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Zhang GT, Liang D, Zhang XD. Comparison of hand-assisted laparoscopic and open radical distal gastrectomy for obese patients. Am Surg. 2013;79:1273-1278. [PubMed] |
| 9. | Zhang GT, Zhang XD. A hand-assisted laparoscopic distal gastrectomy can be an effective way in obese patients. Surg Laparosc Endosc Percutan Tech. 2013;23:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Wong SK, Tsui DK, Li MK. Laparoscopic distal gastrectomy for gastric cancer: initial experience on hand-assisted technique and totally laparoscopic technique. Surg Laparosc Endosc Percutan Tech. 2009;19:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Kim YW, Bae JM, Lee JH, Ryu KW, Choi IJ, Kim CG, Lee JS, Rho JY. The role of hand-assisted laparoscopic distal gastrectomy for distal gastric cancer. Surg Endosc. 2005;19:29-33. [PubMed] |
| 12. | Cao YK, Liu LY, Gong JQ, Wang YH, Luo GD, Zhou J, Gan W, Huang L. [Analysis of lymph node dissection patterns in D2 radical gastrectomy by hand-assisted laparoscopic technique]. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:970-973. [PubMed] |
| 13. | Cao YK, Liu LY, Zhou J, Luo GD, Wang YH, Zhang GH, Wang PH, Gong JQ, Zhang L. [Hand-assisted laparoscopic radical gastrectomy: comparison between laparoscopic and open approach]. Zhonghua Weichang Waike Zazhi. 2012;15:740-742. [PubMed] |
| 14. | Lee SS, Kim IH. Are there any disbenefits to patients in choosing laparoscopic gastrectomy by an expert in open gastrectomy? Aspects of surgical outcome and radicality of lymphadenectomy. Chin Med J (Engl). 2013;126:4247-4253. [PubMed] |
| 15. | Mou TY, Hu YF, Yu J, Liu H, Wang YN, Li GX. Laparoscopic splenic hilum lymph node dissection for advanced proximal gastric cancer: a modified approach for pancreas- and spleen-preserving total gastrectomy. World J Gastroenterol. 2013;19:4992-4999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gong J, Cao Y, Li Y, Zhang G, Wang P, Luo G. Hand-assisted laparoscopic versus laparoscopy-assisted D2 radical gastrectomy: a prospective study. Surg Endosc. 2014;28:2998-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Tan WP, Talbott VA, Leong QQ, Isenberg GA, Goldstein SD. American Society of Anesthesiologists class and Charlson’s comorbidity index as predictors of postoperative colorectal anastomotic leak: a single-institution experience. J Surg Res. 2013;184:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Vickers AJ. What are the implications of the surgical learning curve? Eur Urol. 2014;65:532-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Bobocea AC, Trandafir B, Bolca C, Cordoş I. Minimally invasive surgery in cancer. Immunological response. Chirurgia (Bucur). 2012;107:154-157. [PubMed] |
| 20. | Misawa T, Shiba H, Usuba T, Nojiri T, Kitajima K, Uwagawa T, Toyama Y, Ishida Y, Ishii Y, Yanagisawa A. Systemic inflammatory response syndrome after hand-assisted laparoscopic distal pancreatectomy. Surg Endosc. 2007;21:1446-1449. [PubMed] |
| 21. | Wang JB, Huang CM, Zheng CH, Li P, Xie JW, Lin JX, Lu J. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol. 2014;20:4797-4805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Yoo CH, Kim HO, Hwang SI, Son BH, Shin JH, Kim H. Short-term outcomes of laparoscopic-assisted distal gastrectomy for gastric cancer during a surgeon’s learning curve period. Surg Endosc. 2009;23:2250-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc. 2009;23:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Meshikhes AW, El Tair M, Al Ghazal T. Hand-assisted laparoscopic colorectal surgery: initial experience of a single surgeon. Saudi J Gastroenterol. 2011;17:16-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Sheng QS, Lin JJ, Chen WB, Liu FL, Xu XM, Lin CZ, Wang JH, Li YD. Hand-assisted laparoscopic versus open right hemicolectomy: short-term outcomes in a single institution from China. Surg Laparosc Endosc Percutan Tech. 2012;22:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Hunter JG. Hand-assisted laparoscopic gastrectomy for cancer: the next last frontier. J Am Coll Surg. 2004;199:436. [PubMed] |