Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1468
Peer-review started: June 6, 2014
First decision: July 9, 2014
Revised: July 31, 2014
Accepted: September 19, 2014
Article in press: September 19, 2014
Published online: February 7, 2015
Processing time: 248 Days and 19.9 Hours
AIM: To investigate whether electroacupuncture ST36 activates enteric glial cells, and alleviates gut inflammation and barrier dysfunction following hemorrhagic shock.
METHODS: Sprague-Dawley rats were subjected to approximately 45% total blood loss and randomly divided into seven groups: (1) sham: cannulation, but no hemorrhage; (2) subjected to hemorrhagic shock (HS); (3) electroacupuncture (EA) ST36 after hemorrhage; (4) vagotomy (VGX)/EA: VGX before hemorrhage, then EA ST36; (5) VGX: VGX before hemorrhage; (6) α-bungarotoxin (BGT)/EA: intraperitoneal injection of α-BGT before hemorrhage, then EA ST36; and (7) α-BGT group: α-BGT injection before hemorrhage. Morphological changes in enteric glial cells (EGCs) were observed by immunofluorescence, and glial fibrillary acidic protein (GFAP; a protein marker of enteric glial activation) was evaluated using reverse transcriptase polymerase chain reaction and western blot analysis. Intestinal cytokine levels, gut permeability to 4-kDa fluorescein isothiocyanate (FITC)-dextran, and the expression and distribution of tight junction protein zona occludens (ZO)-1 were also determined.
RESULTS: EGCs were distorted following hemorrhage and showed morphological abnormalities. EA ST36 attenuated the morphological changes in EGCs at 6 h, as compared with the VGX, α-BGT and HS groups. EA ST36 increased GFAP expression to a greater degree than in the other groups. EA ST36 decreased intestinal permeability to FITC-dextran (760.5 ± 96.43 ng/mL vs 2466.7 ± 131.60 ng/mL, P < 0.05) and preserved ZO-1 protein expression and localization at 6 h after hemorrhage compared with the HS group. However, abdominal VGX and α-BGT treatment weakened or eliminated the effects of EA ST36. EA ST36 reduced tumor necrosis factor-α levels in intestinal homogenates after blood loss, while vagotomy or intraperitoneal injection of α-BGT before EA ST36 abolished its anti-inflammatory effects.
CONCLUSION: EA ST36 attenuates hemorrhage-induced intestinal inflammatory insult, and protects the intestinal barrier integrity, partly via activation of EGCs.
Core tip: The most important findings from this study were that enteric glial cells (EGCs) were distorted following hemorrhage and showed morphological abnormalities. Electroacupuncture (EA) ST36 attenuated the morphological changes in EGCs and intestinal inflammation, and decreased intestinal permeability, which is considered to be the possible mechanism of EA’s regulation of the intestinal barrier function after hemorrhage shock.
- Citation: Hu S, Zhao ZK, Liu R, Wang HB, Gu CY, Luo HM, Wang H, Du MH, Lv Y, Shi X. Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J Gastroenterol 2015; 21(5): 1468-1478
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1468.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1468
Gut barrier dysfunction is a common complication following hemorrhagic shock (HS) and severe burn, and plays an important role in the development of sepsis and multiple organ dysfunction[1,2]. The gut becomes a source of proinflammatory mediators resulting from an impaired intestinal mucosal barrier, which may amplify systemic inflammation response syndrome, develop a systemic inflammatory response state and distant organ failure, and lead to multiple organ dysfunction syndrome, or even death[3-5]. Although several studies have investigated gut barrier function, the mechanisms responsible for injury-induced gut barrier dysfunction and repair following hemorrhagic injury are ambiguous. In particular, the role played by the enteric nervous system has been poorly studied.
The enteric glial cells (EGCs) play an important role in maintaining gut barrier function. Recently, increased attention has been paid to EGCs. EGCs are known to secrete many factors, such as s-nitrosoglutathione (GSNO)[6], transforming growth factor (TGF)-β[7], and pro-epidermal growth factor (EGF)[8], which improve the expression and localization of intestinal tight junction proteins, nourish intestinal epithelial cells, and regulate gut barrier function. Loss of EGCs was fatal in an animal model of hemorrhagic necrosis of the small intestine[9]. Clinically, loss of EGCs has been implicated in the pathogenesis of inflammatory bowel disease. Recent studies have shown that electrical stimulation of the vagus nerve can protect the intestinal barrier and alleviate inflammatory intestinal injury in animals following burn injury[10]. However, the effect of stimulating the vagus nerve and its mechanism of action on EGCs remains unknown. It is still difficult to apply electrical stimulation to the vagus nerve in clinical practice because of the complicated manipulation and untoward side effects, including serious tissue injury.
Acupuncture is one of the therapeutic techniques in traditional Chinese medicine and has been applied clinically for thousands of years. It has a bidirectional neuron-endocrine-immune system regulatory effect, and it antagonizes systemic inflammatory responses, with few side effects. We demonstrated previously that electroacupuncture (EA) at ST36 has a significant positive effect on enteric tissue[11]. EA ST36 also protects the intestinal barrier function and prevents remote organ injury after prolonged HS in rats with delayed fluid replacement, through activating the cholinergic anti-inflammatory dependent mechanism[12]. However, the effect and mechanism of EA on EGCs remain unknown and require further study. In the present study, we investigated the effect of EA on EGCs and hypothesized that EA ST36 can activate EGCs through the cholinergic anti-inflammatory pathway to protect the gut barrier function against prolonged HS.
Male Sprague-Dawley rats (aged 8-10 wk, weighing 250-270 g) were purchased from Experimental Animal Center of Military Medical Sciences of the Chinese PLA. Rats were acclimatized in mesh cages in a temperature-controlled room with a 12-h light-dark cycle, and fasted overnight, but allowed free access to water until 4 h before surgery. The Committee of Scientific Research of the First Hospital Affiliated to General Hospital of PLA, China approved all the research protocols. The experiment was conducted in compliance with the Guide for Care and Use of Laboratory Animals of National Research Council, China.
Rats were anesthetized with 3% isoflurane inhalation (Yeeran Technology Limited, Beijing, China). Ketamine 10 mg/kg was hypodermically injected for local anesthesia. Isoflurane (0.7%) was used to maintain anesthesia during the experiments. Animals were allowed to breathe spontaneously under a nose cone scavenging system, using a veterinary anesthesia delivery system (Kent Scientific TOPO, Torrington, CT, United States). Using aseptic technique, polyethylene (PE50) catheters were placed in the right carotid artery for continuous artery blood pressure monitoring, and in the left femoral artery for blood withdrawal. A 2-cm upper-midline laparotomy incision was performed to identify the gastroesophageal junction and expose the dorsal and ventral vagus nerve on the distal esophagus, using a Phoenix XLT165-LB stereomicroscope (Phoenix Optical Instrument Group Company, Jiangxi Province, China). The rectal temperature was maintained at 37 °C with a heating pad and a heating lamp.
The estimated blood volume of each animal was calculated using the formula[13]: total blood volume (TBV) (mL) = body weight (g) × 0.06 (mL/g) + 0.77. HS was induced by withdrawing 45% of the calculated TBV within 20 min (30% TBV withdrawal over the first 3 min, suspension for 7 min, then 15% TBV withdrawal over a further 10 min), using an infusion or a withdrawal pump (Kelifeng Apparatus, Beijing, China). Mean arterial pressure (MAP) and heart rate (HR) were continuously monitored using a PICCO-PLUS cardiopulmonary volume monitor (Pulsion, Germany), from initiation of exsanguination until 6 h after termination. The MAP following exsanguination dropped promptly from around 110 to 30 mmHg and gradually improved, and was maintained at 35-45 mmHg by further blood withdrawal or reinfusion as required. At 6 h after hemorrhage, animals that were still alive were euthanized, and samples of arterial blood and jejunal tissue were collected.
All the animals underwent the same surgical procedure and HS protocol, and then the experimental rats were randomly assigned to seven groups: (1) sham: cannulation, but no hemorrhage; (2) HS: HS alone; (3) EA: EA ST36 (located at the posterior and lateral side of the knee joint, 5 mm below the capitulum fibulae[14]) immediately after HS. EA ST36 (LH202H; HANS, China) was performed as described previously[11]. Both hind limbs were shaved and the skin was disinfected. The ST36 acupuncture point was punctured to a depth of 7 mm, and the needle was connected to the EA apparatus. Electric current at 2 mA, 2-100 Hz was continued for approximately 1.5 h immediately after hemorrhage; (4) vagotomy (VGX)/EA: VGX of the dorsal and ventral vagus nerve on the distal esophagus prior to EA at ST36. (5) VGX group: VGX without EA at ST36; (6) α-bungarotoxin (α-BGT)/EA group: intraperitoneal injection of α-BGT (1 μg/kg) prior to hemorrhage and followed by EA ST36 similar to the EA group. EA parameters were the same as the EA group. α-BGT is an antagonist of the α7 subunit of the cholinergic nicotinic receptor, which inhibits the α7 subunit of acetylcholine receptors by blocking a pivotal communication pathway between the efferent vagus and intestinal cells[15,16]; and (7) α-BGT group: α-BGT intraperitoneal injection prior to hemorrhage without EA at ST36.
Rats were anesthetized with 3% isoflurane inhalation. The animals were sacrificed for distal small intestine harvest at 6 h after blood loss. Segments of distal small intestine were harvested and immediately homogenized on ice with 1 mL denaturing lysis buffer or nondenaturing lysis buffer for western blotting or enzyme-linked immunosorbent assay (ELISA). The homogenate was centrifuged at 10000 ×g for 10 min at 4 °C. Aliquots of the supernatants of tissue were stored at -80 °C until use. Segments of intestine were harvested and fixed in 4% paraformaldehyde for histological evaluation and immunofluorescence. Segments of intestine were also harvested and immediately store in liquid nitrogen for reverse transcriptase polymerase chain reaction (RT-PCR).
We used modified Mandhan’s method[17] to prepare the myenteric plexuses. Rats were anesthetized with 3% isoflurane inhalation. The animals were sacrificed for distal small intestine harvest. The ileum was washed to remove any content. One side of the intestinal segment was tied, and the other side was tied after expanding the intestine with 4% paraformaldehyde. The segment was fixed in a solution of 4% paraformaldehyde and nifedipine for 2-4 h at 4 °C. We selected a few segments and removed the mesentery and fat tissue before dissection and opening cutting along the mesenteric border. The tissue was cleared of paraformaldehyde by washing in PBS. The tissue was laid on a slide glass. Under magnification on a contrasting background, we slightly stretched the gut layer, then marked the proximal and distal ends with oblique and transverse cuts, respectively, and the mucosa was separated in single layer from the muscularis mucosa. The circular muscle fibers were gently peeled off using high-power magnification, leaving behind the myenteric plexuses attached to the longitudinal muscle layer. The mucosa, submucosa and circular muscle were removed from the fixed tissue and whole-mount preparations consisting of the myenteric plexus adhering to the longitudinal muscle were prepared. The preparations were attached to an amino slide.
After deparaffinization, the intestine sections were rehydrated. The tissue sections or EGC preparations were incubated in citrate buffer (Zhongshan Jinqiao Biotechnology Co. Ltd., Beijing, China) for heat-induced antigen retrieval. After three washes with PBS, tissue sections were incubated with 3% BSA (Zhongshan Jinqiao Biotechnology) for 30 min to block nonspecific binding sites. The sections were incubated with an anti-zona occludens (ZO)-1 antibody (1:100; Life Technologies, Gaithersburg, MD, United States) or antibodies recognizing glial fibrillary acidic protein (GFAP; 1:500; Abcam Hong Kong Ltd., New Territories, Hong Kong) at 4 °C overnight. The following day, after washing with PBS three times, the tissue sections were treated with Alexa Fluor 488 secondary goat anti-rabbit antibody in 1% BSA for 1 h at room temperature. Prolong Fade (Antifade Mounting Medium, Beyotime Institute of Biotechnology) was added after placement of coverslips. Images were viewed using an Olympus fluorescence microscope (BX51-DP71) with exposure-matched settings.
The harvested gut tissues were placed in 1 mL lysis buffer (50 mmol/L Tris-HCl, pH 7.4; 150 mmol/L NaCl; 1% NP-40; 0.1% SDS), then homogenized and centrifuged at 12000 ×g for 10 min. Following centrifugation, the supernatant was collected and analyzed for protein concentration, using a protein assay kit (Applygen Technologies, Beijing, China). Total protein (100 μg) was loaded onto SDS-PAGE and run at 120 V for 2 h. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane (Applygen Technologies) and blocked for 2 h in TBST (50 mmol/L Tris; 150 mmol/L NaCl; 0.05% Tween 20) containing 5% milk (Applygen Technologies). The membrane was then incubated with the primary antibodies against GAPDH (1:1000; Zhongshan Jinqiao Biotechnology), and ZO-1 (1:500; Life Technologies) or GFAP (1:1000; Abcam, Hong Kong) at 4 °C overnight. After three washes in TBST, the membrane was incubated with corresponding secondary antibodies conjugated to horseradish peroxidase at room temperature for 40 min and chemiluminescence detection was performed using Super ECL Plus (Applygen Technologies). Films were developed using a standard photographic procedure. Densitometer scanning (Image J) carried out the quantitative analysis of the detected bands.
For determination of GFAP mRNA level, rats were sacrificed under anesthesia. The distal ileum was preserved in liquid nitrogen. The tissues were subsequently homogenized in TRIzol reagent to extract total RNA, according to the manufacturer’s standard procedure (Beijing Solarbio science and Technology Co., Ltd, Beijing, China). Reverse transcription of total RNA was performed after DNase I digestion with the M-MLV reverse transcriptase protocol for cDNA synthesis. Two micrograms of RNA was added to 20 μL RT-PCR reaction system and first-strand cDNA was synthesized by OligodT primer (Beijing DingGuo ChangSheng Biotechnology Co., Ltd, Beijing, China). The amplification reactions were performed in Perkin-Elmer thermal cycle 480 (Massachusetts, United States), with 1 μL 10 μmol/L primer, and 1 μL cDNA in a final volume of 20 μL. The reaction mixtures were incubated for 5 min at 95 °C to denature the template, and then 38 cycles (30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C) and a final cycle of 5 min at 72 °C. The primers for RT-PCR were as follows: GFAP forward 5’-TGGAGAGGAAGGTTGAGTCG -3’, reverse 5’-TTGGCGGCGATAGTCATTAG-3’; GAPDH forward: 5’-ATTCAACGGCACAGTCAAGG-3’, reverse: 5’-GCAGAAGGGGCGGAGATGA-3’. PCR products were run on 1.5% agarose gels stained with ethidium bromide. The results were observed and photographed with a UVP gel imaging system, and analyzed semiquantitatively by measuring the density of the specific bands.
An in vivo intestinal permeability assay was performed to assess gut barrier function, as described by Kao et al[18]. Thirty minutes before sacrifice, animals were anesthetized with inhaled isoflurane. A midline laparotomy incision was performed and a 10-cm segment of distal ileum was isolated between silk ties. A solution of 1.0 mL PBS (pH 7.2) containing 25 mg 4-kDa FITC-dextran (Sigma-Aldrich, St. Louis, MO, United States) was injected into the lumen of the isolated intestinal segment. The bowel was returned to the abdominal cavity and the abdomen was closed. Animals were maintained under general anesthesia for 30 min, at which time systemic blood was drawn by left femoral artery puncture and placed in heparinized Eppendorf tubes on ice. Plasma was obtained by centrifuging the blood at 10000 ×g for 10 min at -4 °C. Plasma fluorescence was measured in a fluorescence spectrophotometer (Synergy2; BioTek Multi-Detection Microplate Reader, Vermont, United States) and compared with a standard curve of known concentrations of FITC-dextran diluted in rat plasma.
Tumor necrosis factor (TNF)-α levels in the intestine were assessed using a commercially available ELISA kit in accordance with the manufacturer’s protocol (Nanjing Jiancheng Corp., Nanjing, China).
Data were analyzed using SPSS Statistics version 17.0. Continuous variables were expressed as mean ± SE. Statistical significance of differences between groups was determined using one-way analysis of variance followed by Dunnett’s test and SNK-q for multiple comparisons. If some variables were non-normally distributed, the Kruskal-Wallis H test was used. Significance was defined as P < 0.05.
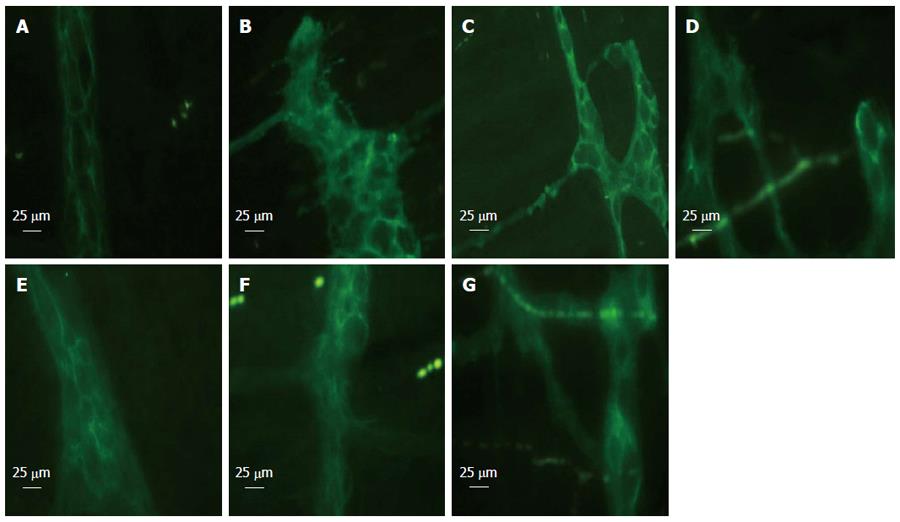
EGCs express the intermediate filament GFAP, and the calcium-binding protein S100. We identified the EGCs via immunofluorescence of GFAP. Normal GFAP immunoreactivity revealed thin processes of EGCs and a thin rim of immunoreactivity around the nucleus (Figure 1A). EGCs were distorted following hemorrhage and showed morphological abnormalities compared with those in the sham group (Figure 1B-F). We observed distorted GFAP processes. After HS, the processes of EGCs were distorted and strongly immunoreactive (Figure 1B). EA ST36 attenuated the morphological change in EGCs (Figure 1C) at 6 h, as compared with the VGX, α-BGT and HS groups.
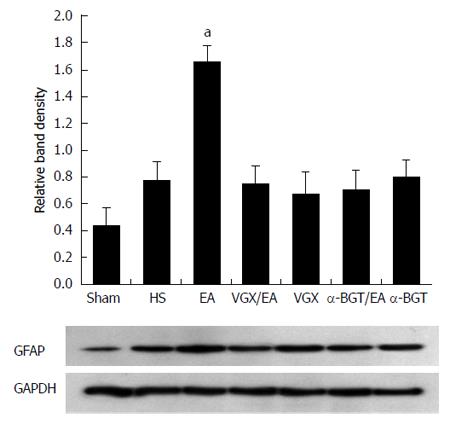
These results were confirmed by western blotting for GFAP in intestinal tissue lysates (Figure 2). The GFAP levels in the sham group were lower than in the HS group. When compared with the average relative band density of rats in the HS group, animals treated with EA ST36 had significantly higher GFAP expressions (P < 0.05). In contrast, intestinal GFAP levels were significantly decreased in rats that underwent abdominal VGX or α-BGT injection before EA ST36. GFAP levels in the VGX or α-BGT groups also were not significantly different from those in the HS group.
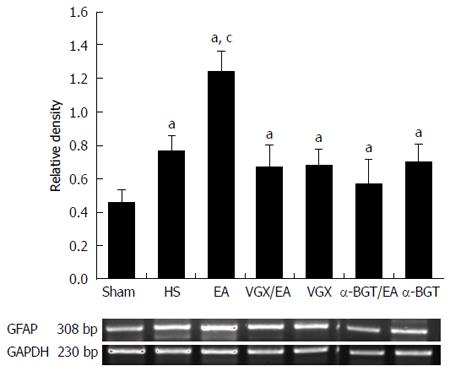
EGC activation was assessed by determination of intestinal GFAP mRNA expression via RT-PCR. Expression of GFAP in rats that underwent hemorrhagic shock was higher than that in the sham group (P < 0.05, Figure 3). Compared with the HS group, GFAP mRNA expression was increased by 61% at 6 h after EA ST36 (P < 0.05, Figure 3). After VGX or α-BGT injection, the effect of EA ST36 was abolished.
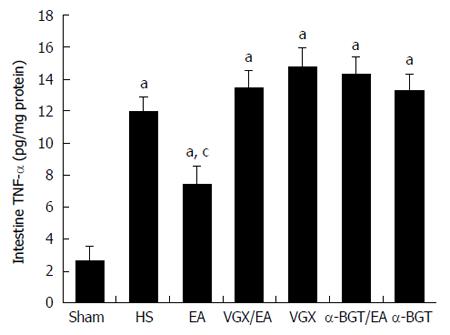
Figure 4 illustrates the effect of EA ST36 on TNF-α levels in the intestine of rats with fatal HS. Hemorrhage induced large increases in the concentration of TNF-α in intestinal homogenates. EA ST36 reduced the TNF-α level in the intestinal homogenate after blood loss, while VGX or intraperitoneal injection of α-BGT before EA ST36 reversed its anti-inflammatory effects. These results suggested that EA ST36 attenuated the release of TNF-α in the intestine.
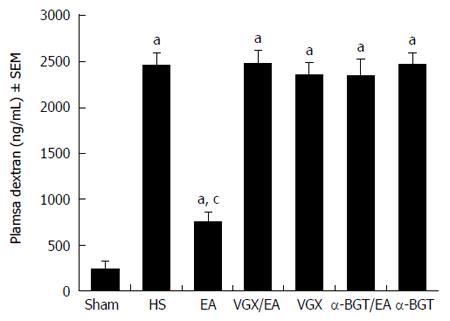
Intestinal permeability was evaluated by an in vivo assay using FITC-dextran (Figure 5). The intestinal permeability of all the animals increased compared with that in the sham group (P < 0.05). Animals in the EA group had a significantly lower level of plasma FITC-dextran compared with the HS group (760.5 ± 96.43 ng/mL vs 2466.7 ± 131.60 ng/mL, P < 0.05). However, when abdominal vagotomy or α-BGT injection was performed before EA ST36, there was no significant difference in intestinal permeability compared with the HS group. Rats in the VGX or α-BGT group showed similar levels of intestinal permeability compared with the HS group.
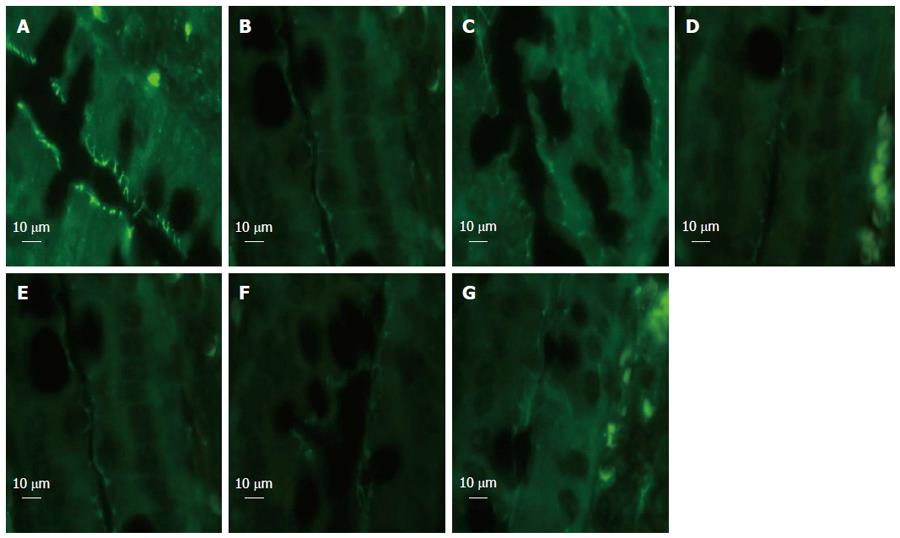
We detected changes in the expression of tight junction protein ZO-1 in response to HS. The amount of ZO-1 protein expression after immunostaining correlated with exposure-matched fluorescent intensity (Figure 6). In the sham group, ZO-1 showed strong fluorescent intensity. After blood loss, rats in the HS group showed loss and redistribution of ZO-1, as demonstrated by low fluorescent intensity at the cell periphery (Figure 6B). Rats in the EA group (Figure 6C) showed preservation of the robust structure of ZO-1. By contrast, in rats treated with EA ST36 after surgical abdominal VGX or α-BGT injection, no protection was afforded to the intestinal mucosa, as demonstrated by the interruption and partial disappearance of ZO-1 staining at the cell periphery in villous epithelial cells (Figure 6D and F).
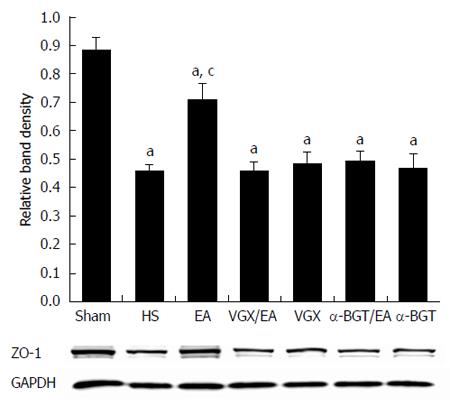
These results were confirmed by western blotting for the ZO-1 protein in intestinal tissue lysates (Figure 7). When compared with the average relative band density of rats in the sham group, ZO-1 expression was lower in all the other groups (P < 0.05). Rats treated with EA ST36 had significantly higher ZO-1 expression than the other injury groups (P < 0.05). By contrast, intestinal ZO-1 protein levels were significantly decreased in rats that underwent surgical abdominal VGX or α-BGT injection before EA ST36. ZO-1 protein levels in the VGX or α-BGT group did not differ significantly from those in the HS group.
The enteric nervous system (ENS) consists of enteric neurons and EGCs. The ENS provides local control of the gastrointestinal tract, and coordinates the multiple cellular components that make up the gut wall[19]. The ENS controls intestinal functions such as motility, blood flow, nutrient uptake, secretion, and immunological and inflammatory processes in the gut. EGCs are more abundant than neurons[20]. EGCs are the predominant cell type in the ENS and are similar in structure and function to the astrocytes of the central nervous system[21]. They express similar proteins, including the intermediate filament GFAP and the calcium-binding protein S100[22]. The number of GFAP-expressing cells increases following injury[23].
Increasing evidence shows that there is a close relationship between the EGCs and gut barrier function. The EGCs regulate the intestinal epithelial barrier function via many pathways. Laurianne et al[8] demonstrated that EGCs promote intestinal mucosal healing via activation of focal adhesion kinase and release of pro-EGF. EGCs represent a functionally important cellular component of the intestinal barrier microenvironment, and disruption of this cellular network can attenuate the mucosal healing process. Savidge et al[6] demonstrated that glial cells secrete GSNO, which modulates intestinal tight junction integrity. This was confirmed in vitro and in an in vivo model of gut inflammation, in which injection of exogenous GSNO attenuated gut inflammation, improved intestinal barrier integrity, and increased expression of the tight junction protein ZO-1. In addition to secreting GSNO, activated EGCs cause numerous changes in gene transcription within the intestinal epithelial cell, which may alter the barrier function[24]. Neunlist et al[7] demonstrated that EGCs inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Functional alterations in EGCs may therefore modify intestinal barrier functions and be involved in pathologies such as cancer or inflammatory bowel diseases.
The cholinergic anti-inflammatory pathway is a neural mechanism that inhibits the expression of proinflammatory cytokines through the interaction of the principle vagus nerve neurotransmitter, acetylcholine, and the cholinergic α-7 nicotinic acetylcholine receptor (α-7nAChR) subunit located on cytokine-expressing cells, by stimulating the vagus nerve by pharmacological or electrical methods[10,11]. Activation of the cholinergic anti-inflammatory pathway can prevent cytokine release and tissue injury[12] and prolong survival and protect against the development of hypotension in rats during HS[13]. α-7nAChR is essential to prevent proinflammatory cytokine release[25]. Ghia et al[26] showed that nicotinic cholinergic signaling attenuates intestinal inflammation in a model of colitis. Nicotine is a cholinergic agonist that decreases systemic inflammation after injury[27,28]. It does so by modulating inflammatory signals through α-7nAchR, which is expressed on neurons, microglia, and inflammatory cells[29]. Nicotine improved the barrier function and tight junction protein expression in cultured intestinal epithelial cells[30].
Recently, Costantini et al[31] proved that α-7nAchR is present on intestinal epithelial cells and EGCs. Vagus nerve stimulation (VNS) decreased circulating plasma and local intestine proinflammatory cytokine levels in an animal model[32,33]. VNS exerts its systemic anti-inflammatory effects through its ability to decrease production of proinflammatory mediators after injury viaα-7nAchRs[34]. This mechanism has been confirmed in studies showing that the protective, anti-inflammatory effects of VNS are lost in mice that lack α-7nAchRs. α-7nAchRs have been seen on cultured intestinal epithelial cells and EGCs. They have also been seen in the gastrointestinal tract of animals used in models of severe burn injury[31]. VNS activates the α-7nAchR on the EGCs to alleviate inflammatory action in the gut.
The barrier-inducing effects of EGCs have been confirmed in many studies. The latest evidence suggests that EGC activation improves intestinal barrier integrity by inhibiting the proinflammatory nuclear factor (NF)-κB pathway[35]. Gut barrier breakdown is associated with intestinal epithelial cell NF-κB activation, and activation of the NF-κB pathway increases intestinal permeability through tight-junction breakdown. Inhibition of the NF-κB pathway can preserve the enteric barrier function.
Our previous experiments in animal models showed that EA ST36 protected the intestinal barrier through a vagal anti-inflammatory mechanism that involves α-7nAChRs[12,32]. In the present study, we found that EA ST36 activated and protected EGCs, accompanied by an improvement of gut barrier function. During HS, the intestinal blood flow was sharply reduced to maintain the blood supply to the vital organs. Ischemia of the small intestine leads rapidly to impairment of the mucosal barrier function. At the same time, a large amount of inflammatory cytokines is produced[36], which aggravates the gut barrier dysfunction. In our experiments, we found that EA ST36 reduced TNF-α levels in the intestine in rats after fatal HS. EA ST36 alleviated the distortion of EGCs after HS. α-7nAchR is expressed on intestinal epithelial cells and EGCs[31], which correlates with the improvement of gut barrier function. We consider that EA ST36 activates α-7nAchR in the EGCs through a vagal anti-inflammatory pathway to downregulate the intestinal level of proinflammatory cytokines, which results in the protection of intestinal epithelial cells and EGCs. In addition, EGCs secrete TGF-β, GSNO and pro-EGF, which improve intestinal barrier integrity[7,8]. Ablation of EGCs in transgenic animals is fatal within 19 d because of hemorrhagic necrosis of the gut[9]. In the present study, we found marked morphological changes in EGCs and increased expression of GFAP after EA in rats with HS. There was a close relationship between the EGCs and the intestinal epithelial barrier. We speculated that EA ST36 exerts its protective effect on EGCs partly through vagus-induced activation of EGCs, which releases many substances for the promotion of intestinal mucosal healing and the expression of tight junction proteins.
Although there are many forms of crosstalk between EGCs and gut barrier function, the activation of EGCs plays the most pivotal role in the mechanism of EA ST36 for gut functions. EGCs improve intestinal barrier integrity, which represents a vagus-mediated anti-inflammatory pathway in response to gut injury. Our study provides a theoretical basis for the mechanism of the effect of EA ST36 on gut barrier function. It also suggests potential therapeutic strategies for intestinal diseases. For example, we could use drugs that act on EGCs to treat some intestinal diseases.
There were several limitations to our study. First, we only determined the changes of EGCs and their components at 6 h after hemorrhage, with or without EA ST36. Also, we did not study transgenic animals. Another shortcoming was the brevity of the study duration. Meanwhile, the interaction between EGCs and intestinal barrier function affected by EA ST36 requires further research.
In summary, we showed that EA ST36 alleviated gut inflammatory and barrier dysfunction, partly through activation of EGCs in rats with HS. On the one hand, EA ST36 activated the vagus anti-inflammatory mechanism of EGCs, and on the other hand, it activated EGCs to secrete substances that regulated the gut barrier function. Although the mechanisms for intestinal repair following hemorrhagic or burn injuries are multifactorial, EGCs play a novel role in this process.
The authors thank Dr. Jiang-Yang Lu, Yi-Duo Jin, Xiu-Li Ma, Hui-Zhen Ding, Yi Yang and Dong-Mei Yang from the Department of Pathology, First Hospital Affiliated to the PLA General Hospital, for their technical assistance. The authors also thank Hui-Ping Zhang, Xiang-Xi Meng and Bin Tong for their assistance with the experiments.
When severe injury occurs, the blood supply to the intestinal tract is sharply reduced, which results in barrier dysfunction. Gut barrier dysfunction increases the incidence of serious complications, which promote bacterial translocation and local production of cytokines. The bacteria and endotoxins shift to the circulation and remote organs, which contributes to subsequent local and systemic inflammation. This may lead to systemic inflammation response syndrome and multiple organ dysfunction syndrome. Thus, protecting the intestinal barrier function is important after trauma. The authors have recently found that electroacupuncture (EA) ST36 protects intestinal barrier function, and prevents remote organ injury after prolonged hemorrhagic shock (HS) in rats with delayed fluid replacement, through activating a cholinergic anti-inflammatory-dependent mechanism. They further explored the possible mechanisms involved.
EA can protect the gut barrier function. Restoration of small intestinal barrier function requires a complex set of events that are initiated within minutes of injury, and are characterized by epithelial cell restitution and reassembly of tight junction proteins to close the paracellular space. α-7 nicotinic acetylcholine receptor (α-7nAchR) in the enteric nervous system is necessary for the vagus nerve to modulate the systemic inflammatory response. The cholinergic anti-inflammatory pathway is a neural mechanism that inhibits the expression of proinflammatory cytokines through the interaction of the principle vagus nerve neurotransmitter, acetylcholine, and the cholinergic α7nAChR subunit located on cytokine-expressing cells, by stimulating the vagus nerve by electrical or pharmacological methods. The cholinergic agonist of α-7nAChR in the enteric nervous system prevents gut barrier failure after severe burn injury. Recent studies have shown that electrical stimulation of the vagus nerve can protect the intestinal barrier function and alleviate intestinal inflammatory injury in animals following burn injury, through activation of enteric glial cells (EGCs). Clinically, the loss of EGCs is implicated in the pathogenesis of inflammatory bowel disease. Increasing evidence shows that there is a close relationship between the EGCs and gut barrier function. EGCs regulate the intestinal epithelial barrier function by many pathways. EGCs promote intestinal mucosal healing via activation of focal adhesion kinase and release of pro-epidermal growth factor. EGCs represent a functionally important cellular component of the intestinal epithelial barrier microenvironment, and disruption of this cellular network attenuates the mucosal healing process. The glial cells can secrete S-nitrosoglutathione (GSNO), which modulates intestinal tight junction integrity. In addition to secreting GSNO, activated EGCs cause numerous changes in gene transcription within the intestinal epithelial cells, which may alter barrier function. Functional alterations in EGCs may therefore modify intestinal barrier functions.
The most important novel findings from this study are that EGCs were distorted following hemorrhage and showed morphological abnormalities. EA ST36 attenuated the morphological changes in EGCs and intestinal inflammation; and decreased intestinal permeability, which is considered as the possible mechanism of EA regulation of the intestinal barrier function after HS.
The study results provide evidence for the possible mechanism of acupuncture regulation of the intestinal barrier function after severe hemorrhage and trauma.
The cholinergic anti-inflammatory pathway is a neural mechanism that inhibits the expression of cytokines. It acts through the interaction of the principle vagus nerve neurotransmitter, acetylcholine, and the α7nAChR subunit located on cytokine-expressing cells, by stimulating the vagus nerve by electrical or pharmacological methods. EA is a modification of conventional acupuncture that stimulates acupoints with electrical current instead of manual manipulations, and appears to have more reproducible results in both clinical and research settings. EGCs are the predominant cell type in the enteric nervous system and are similar in structure and function to astrocytes of the central nervous system. They express similar proteins, including the intermediate filament glial fibrillary acidic protein and the calcium-blinding protein S100.
The study was particularly interesting, well designed, and the results seem to demonstrate the relationship of EGCs, intestinal barrier function, and acupuncture.
P- Reviewer: Xu JM S- Editor: Qi Y L- Editor: Stewart G E- Editor: Wang CH
| 1. | Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 399] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res. 1998;79:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Thuijls G, de Haan JJ, Derikx JP, Daissormont I, Hadfoune M, Heineman E, Buurman WA. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. 2005;21:177-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:G231-G241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Van Landeghem L, Chevalier J, Mahé MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1308-G1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Shi X, Zhong Y, Yao J, Hu S, Wang L, Litscher G. The influence of zusanli and nonmeridian acupuncture points on the survival rate and intestinal tissue features after fatal hemorrhagic shock in rats. Evid Based Complement Alternat Med. 2013;2013:750620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Du MH, Luo HM, Hu S, Lv Y, Lin ZL, Ma L. Electroacupuncture improves gut barrier dysfunction in prolonged hemorrhagic shock rats through vagus anti-inflammatory mechanism. World J Gastroenterol. 2013;19:5988-5999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72-76. [PubMed] |
| 14. | Gao W, Huang YX, Chen H, Song DY, Wang QL. Regulatory effects of electro-acupuncture at Zusanli on ir-SP content in rat pituitary gland and peripheral blood and their immunity. World J Gastroenterol. 2000;6:581-584. [PubMed] |
| 15. | Gao Z, Müller MH, Karpitschka M, Mittler S, Kasparek MS, Renz B, Sibaev A, Glatzle J, Li Y, Kreis ME. Role of the vagus nerve on the development of postoperative ileus. Langenbecks Arch Surg. 2010;395:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Marinou M, Tzartos SJ. Identification of regions involved in the binding of alpha-bungarotoxin to the human alpha7 neuronal nicotinic acetylcholine receptor using synthetic peptides. Biochem J. 2003;372:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Mandhan P, Qi BQ, Keenan JI, Ismail S, Beasley SW, Sullivan MJ. Counterstaining improves visualization of the myenteric plexus in immunolabelled whole-mount preparations. J Fluoresc. 2006;16:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Kao NR, Xenocostas A, Driman DK, Rui T, Huang W, Jiao X, Martin CM. Recombinant human erythropoietin improves gut barrier function in a hemorrhagic shock and resuscitation rat model. J Trauma. 2011;71:S456-S461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 469] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 20. | Jessen KR. Glial cells. Int J Biochem Cell Biol. 2004;36:1861-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Hanani M, Francke M, Härtig W, Grosche J, Reichenbach A, Pannicke T. Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2000;278:G644-G651. [PubMed] |
| 22. | Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res. 2001;63:109-115. [PubMed] |
| 24. | Van Landeghem L, Mahé MM, Teusan R, Léger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics. 2009;10:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Gallowitsch-Puerta M, Tracey KJ. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann N Y Acad Sci. 2005;1062:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404-408. [PubMed] |
| 28. | Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 469] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 30. | McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. The effect of nicotine in vitro on the integrity of tight junctions in Caco-2 cell monolayers. Food Chem Toxicol. 2007;45:1593-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol. 2012;181:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Hu S, Du MH, Luo HM, Wang H, Lv Y, Ma L, Lin ZL, Shi X, Gaischek I, Wang L. Electroacupuncture at Zusanli (ST36) Prevents Intestinal Barrier and Remote Organ Dysfunction following Gut Ischemia through Activating the Cholinergic Anti-Inflammatory-Dependent Mechanism. Evid Based Complement Alternat Med. 2013;2013:592127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 2966] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 34. | Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384-388. [PubMed] |
| 35. | Cheadle GA, Costantini TW, Bansal V, Eliceiri BP, Coimbra R. Cholinergic signaling in the gut: a novel mechanism of barrier protection through activation of enteric glia cells. Surg Infect (Larchmt). 2014;15:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |