Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1424
Peer-review started: August 16, 2014
First decision: September 15, 2014
Revised: October 7, 2014
Accepted: November 11, 2014
Article in press: November 11, 2014
Published online: February 7, 2015
Processing time: 177 Days and 10.7 Hours
The high mobility group box 1 (HMGB1), which belongs to the subfamily of HMG-1/-2, is a highly conserved single peptide chain consisting of 215 amino acid residues with a molecular weight of approximately 24894 Da. HMGB1 is a ubiquitous nuclear protein in mammals and plays a vital role in inflammatory diseases. Acute pancreatitis is one of the most common causes of acute abdominal pain with a poor prognosis. Acute pancreatitis is an acute inflammatory process of the pancreas (duration of less than six months), for which the severe form is called severe acute pancreatitis (SAP). More and more studies have shown that HMGB1 has a bidirectional effect in the pathogenesis of SAP. Extracellular HMGB1 can aggravate the pancreatic inflammatory process, whereas intracellular HMGB1 has a protective effect against pancreatitis. The mechanism of HMGB1 is multiple, mainly through the nuclear factor-κB pathway. Receptors for advanced glycation end-products and toll-like receptors (TLR), especially TLR-2 and TLR-4, are two major types of receptors mediating the inflammatory process triggered by HMGB1 and may be also the main mediators in the pathogenesis of SAP. HMGB1 inhibitors, such as ethyl pyruvate, pyrrolidine dithiocarbamate and Scolopendra subspinipes mutilans, can decrease the level of extracellular HMGB1 and are the promising targets in the treatment of SAP.
Core tip: The newly discovered high mobility group box 1 protein is a ubiquitous nuclear protein that exists extensively in mammals. More and more studies have shown its vital role in inflammation. This paper is the first to reveal the bidirectional effect of this protein in the pathogenesis of severe acute pancreatitis and its role as a potential treatment target.
- Citation: Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol 2015; 21(5): 1424-1435
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1424
The high mobility group box 1 protein (HMGB1), an important chromatin protein, is encoded by the Hmgb1 gene in humans[1,2]. HMGB1 is also called amphoterin and was discovered 40 years ago[3]. This protein belongs to the high mobility group family and has an important role in mediating inflammation[3,4]. It has been shown that serum levels of HMGB1 are elevated in several inflammatory diseases, including sepsis, mechanical trauma, acute myocardial infarction, acute respiratory distress syndrome, hepatic injury, rheumatoid arthritis and stroke[5-9].
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas, and severe acute pancreatitis (SAP) is a severe type of acute pancreatitis associated with high mortality rates[10]. Recently, more and more studies have shown that HMGB1 may have a role in the SAP process. The aim of this review is to clarify the relationship between HMGB1 and SAP and to determine how HMGB1 affects the pathogenesis of SAP.
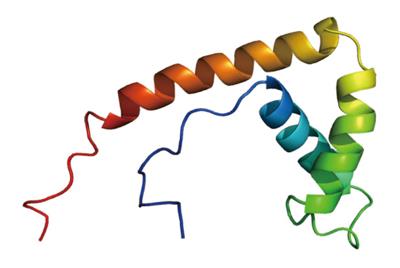
High mobility group (HMG) proteins are a family of non-histone nuclear proteins that have a role in transcription, replication, recombination, repair, and other DNA-associated activities. HMG-1/-2, HMG-I/-Y, and HMG-14/-17 are three subfamilies of HMG proteins[2]. HMGB1, which belongs to the subfamily of HMG-1/-2, is a highly conserved single peptide chain consisting of 215 amino acid residues with a molecular weight of approximately 24894 Da (Figure 1). The N terminal of the protein is composed of lysine that is rich in positive charge. The C terminal, also known as the acidic tail, is composed of aspartic acid and glutamic acid that are rich in negative charge. HMGB1 consists of the following three domains: A box (amino acid residues 9-79), B box (amino acid residues 95-163) and an acidic C-terminal tail (the receptor binding site, amino acid residues 186-215)[2,11-14]. Functional analysis has shown that the B box plays a major role in inflammation, and that the A box is the antagonistic site of the B box[15]. Both A and B boxes are able to bind to DNA and have a role in folding and distorting the double-stranded DNA. Generally, HMGB1 is ubiquitous in mammalian cells, and it is highly expressed in the liver, thymus, lymph tissue, testis, and in neonates[15].
HMGB1 belongs to the family of damage-associated molecular pattern molecules, which can be recognized by pattern recognition receptors and initiate an immune response in the noninfectious inflammatory response[16]. As a nuclear protein, HMGB1 plays a vital role in nucleosome stabilization and DNA transcription. However, HMGB1 can also be released extracellularly under stress. Extracellular HMGB1 is known to affect certain cellular signal transduction pathways[17-19]. It is well known that extracellular HMGB1 is an important pro-inflammatory cytokine[20]. Although the exact intracellular signaling transduction mechanism of HMGB1 is not clear, it has been reported that receptors for advanced glycation end-products (RAGE) and toll-like receptors (TLR) are two major types of receptors mediating the inflammatory process triggered by HMGB1[21].
AP is defined as an acute inflammatory process of the pancreas (duration less than six months) that affects other regional tissues or remote organ systems[10]. Of these, the lungs and kidneys are the most affected organs. Acute lung injury or acute respiratory distress syndrome can occur immediately or during the later course of pancreatitis, as well as acute kidney injury or acute renal failure. AP is often caused by biliary tract diseases, alcohol abuse, trauma, surgery, overeating, metabolic disorders (e.g., hypercalcemia and hyperlipidemia), infection, or other related factors. The typical symptoms of AP are sudden-onset upper abdominal pain often radiating to the back, and a significant elevation of serum lipase or amylase (three times above normal)[22]. According to the severity of the disease, AP can be divided into the following three degrees: mild , moderately severe, and severe. The latest classification also includes a new categorization of critical AP, which describes infected pancreatitis with persistent organ failure[23].
SAP is characterized by the existence of either infected pancreatic necrosis or persistent organ failure, and has a global mortality of 15%-30%[23,24]. Organ failure is defined as shock (systolic blood pressure < 90 mmHg), pulmonary insufficiency (PaCO2≤ 60 mmHg), renal failure (serum creatinine level > 177 μmol/L or 2 mg/dL after resuscitation[25]), or gastrointestinal bleeding > 500 mL per 24 h. In the recent updated classification of AP, the definition of organ failure differs slightly and is based on the failure of the following three organ systems: cardiovascular (need for inotropic agent), renal (serum creatinine level ≥ 171 μmol/L or ≥ 2 mg/dL) and respiratory (PaO2/FiO2≤ 300 mmHg or 40 kPa[23]). Organ failure lasting more than 48 h is considered to be persistent and is the main feature of SAP. Organ failure can be either single or multiple. Patients with organ failure are more likely to develop local complications[22]. Patients with SAP always develop systemic inflammatory response syndrome (SIRS). SIRS can be diagnosed with the presence of two or more manifestations[26,27] (listed in Table 1).
| Finding | Value |
| Temperature | < 36 °C or > 38 °C |
| Heart rate | > 90 beats/min |
| Respiratory rate | > 20/min or PaCO2 < 32 mmHg (4.3 kPa) |
| WBC | < 4 × 109/L (< 4000/mm³), > 12 × 109/L (> 12000/mm³), or > 10% bands |
The main feature of SAP is necrosis of the pancreas. Regardless of the etiology, the pathogenesis of SAP is mainly due to the autodigestion of the pancreas by pancreatic juice and trypsinogen activation. Normally, the pancreas uses the following defense mechanisms against autodigestion: (1) the protective layer in the epithelial of pancreatic duct composed of mucopolysaccharides; (2) pancreatic acini can prevent the invasion of pancreatic enzymes inside of the cells; and (3) the blood flow into the pancreas contains substances that can neutralize pancreatic enzymes. Furthermore, the majority of pancreatic enzymes, such as trypsins, are secreted in the form of zymogens (non-activated pancreatic enzymes). However, these aforementioned defense mechanisms can be destroyed in a number of pathologic conditions, for example, the obstruction of the pancreatic duct or the invasion of acini by infected bile. Both of these situations can lead to increased pressure in the pancreatic duct, rupture of pancreatic acini and a sudden, explosive release of all the pancreatic enzymes, including trypsin, pancrelipase and amylopsin. These mechanisms result in the autodigestion of the pancreas[28-31].
Autophagy is the primary cellular degradative pathway in AP. Recent studies have shown that autophagy is impaired in pancreatitis as a result of defective lysosomes, involving mainly the following three major autophagic pathways: chaperone-mediated autophagy, microautophagy, and macroautophagy[32,33]. In addition, the following enzyme systems are also activated in AP: (1) collagenases allow the spread of inflammation; (2) elastases can damage the walls of blood vessels and cause bleeding; (3) ubiquitin-proteasome complex can further extend tissue necrosis; and (4) lipases can cause necrosis of adipose tissue around the pancreas (such as mesenteric root, lesser omental bursa, retroperitoneal space, renal artery, both sides of aorta, pelvic cavity, etc.). Calcium can combine with the fat necrosis and lead to the formation of saponification spots, which is one of the reasons for hypocalcemia in patients. Meanwhile, the decomposed and necrotic pancreatic tissue can produce vasoactive substances, including kallikrein, bradykinin and prostaglandin. These substances can decrease the tension of pericardial blood vessels, and coupled with a substantial amount of peripancreatic exudation and a sharp drop in blood volume as well as blood pressure, the pre-existing circulatory disorder and renal damage can further deteriorate. In addition, the myocardial depressant factor in the necrotic toxin can cause further damage to cardiopulmonary function. Organ dysfunction may also involve the liver and central venous system. All of these lesions can be referred to “enzymatic shock.” As a result of activation of all the enzymes, the damaged acinar cells will consequently lead to necrosis and inflammation[34].
The progression of AP can be divided into the following three phases: local acinar injury, systemic response, and generalized sepsis[35]. Although the exact pathogenesis has not been completely revealed, activation of nuclear factor (NF)-κB is a key link[36]. NF-κB plays a vital role in various stages of pancreatitis via mediation of the inflammatory process[37], and NF-κB activation is considered to be independent of trypsinogen activation in the pathogenesis of AP[38,39]. Moreover, the intracellular Ca2+ signaling pathway and protein kinase C may trigger the early activation of NF-κB in pancreatic acini[40]. A great amount of pro-inflammatory mediators can be released as a result of NF-κB activation during pancreatitis, including numerous types of cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-6 and IL-18, various chemokines such as IL-8, macrophage inflammatory protein-1, growth-related oncogene-α and monocyte chemoattractant protein-1, reactive oxygen species, reactive nitrogen species, platelet-activating factor and adhesion molecules[40-44]. Extra-pancreatic NF-κB activation can also be seen in the liver, lungs, endothelium and peripheral blood monocyte-macrophages[45-47].
In mild AP, inflammatory reaction as well as pro-inflammatory mediators is always confined to the pancreas. In SAP, the inflammation of the pancreas can be further exacerbated and cause a SIRS, which is an amplified and overwhelming inflammatory response. Major pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, are then released into the circulation and can cause remote organ injury[48,49]. Activated circulating neutrophils and monocytes can cause damage to vascular endothelial cells and organ parenchymal cells and increase the permeability of the vessels by releasing proteolytic enzymes and oxygen radicals, thereby leading to tissue edema and organ injury[50]. Furthermore, the emergence of microcirculatory dysfunction can further aggravate the injury of important organs[51,52]. Coagulation disorder is another important component of the inflammatory response in SAP and is also associated with the severity of pancreatitis[53-55].
HMGB1 was first reported by Wang et al[4] as a mediator for endotoxin lethality in animal models. Later, the authors considered HMGB1 to be a potential late inflammatory mediator involved in the pathogenesis of sepsis[56]. HMGB1 is derived from the secretions of certain immune cells (such as monocytes) and non-immune cells (such as epithelial cells), as well as passive release from necrotic and apoptotic cells[4,19,57-60]. HMGB1 can mediate inflammation via receptors of innate immune systems such as TLR and RAGE in the pathogenesis of many inflammatory diseases, including sepsis, pancreatitis and arthritis[61,62].
TLRs are a family of proteins that play a vital role in the innate immune system. TLRs are named for their resemblance to the protein encoded by the Toll gene discovered by Christiane in 1985[63]. TLRs belong to the family of pattern recognition receptors and respond to the structurally conserved molecules derived from various germs, the so-called pathogen-associated and damage-associated molecular pattern. Thirteen TLRs (named TLR1 to TLR13) have been found in mammalian species[25,64,65].
TLR4 is the first target activated by extracellular HMGB1[66-68]. TLR4 is the only TLR that uses four adaptors, myeloid-differentiation primary response protein 88 (MyD88), MAL, Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF), and TRAM and mediates their effects through the NF-κB and MAPK pathways. After TLR4 is combined by HMGB1 or forms a complex with HMGB1 and exogenous or endogenous molecules, it initially recruits MyD88 and thus activates the downstream NF-κB. Activated NF-κB is then transported from the cytoplasm to the nucleus and induces the expression of major inflammatory factors such as IL-1β and IL-6, interferon (IFN)-γ, and TNF-α in the early phase[66]. The aforementioned HMGB1-TLR4 pathway can also trigger a second downstream pathway mediated by TRIF and leads to activation of type I interferon as well as a delayed activation of NF-κB[69], resulting in the release of cytokine and activation of macrophages. IL-1β, IL-6 and TNF-α are always released during the early stage of inflammation (the development of systemic inflammatory response) whereas HMGB1 is often released by macrophages 12-18 h after the onset of the inflammatory response and only mediates the late stage of the inflammatory process[4]. The HMGB1 released extracellularly spurs a cascade of inflammatory reactions both in local and distant organs, ultimately resulting in multi-organ dysfunction. Other TLRs such as TLR2 and TLR9 also have a role in the inflammatory mediation by HMGB1[17,70]. There are many overlaps between the TLR4-mediated pathway and TLR-2-mediated pathway, and the mechanism is almost the same[17].
RAGE is another important receptor of HMGB1. It is expressed on a variety of cells, such as monocytes and belongs to the immunoglobulin superfamily[71,72]. Other than HMGB1, RAGE can also interact with diverse ligands, including S100 protein[73,74]. Two major signaling pathways that are activated by RAGE are p38 MAPK and ERK1/2 pathways[17]. Both pathways can lead to the phosphorylation and degradation of inhibitors of κB by IκB kinase and thus activate NF-κB. Activated NF-κB then transfers to the nucleus and results in increase in NF-κB DNA binding, expression of various pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6), proliferation of cells and chemotaxis.
Extracellular HMGB1 was already known as a novel pro-inflammatory cytokine in humans[56]. In the last decade, many studies have claimed a positive correlation between extracellular HMGB1 and SAP severity (Table 2).
| Ref. | Journal | Country | Subject | Method | Result |
| Yasuda et al[75] | Pancreas | Japan | Patients with SAP | Control group: healthy volunteers (n = 8); Experimental group: patients with SAP (n = 45) | Serum HMGB1 levels were significantly increased in patients with SAP and were correlated with disease severity |
| Kocsis et al[78] | Pancreatol | Hungary | Patients with AP | Control group: healthy volunteers (n = 20); AP group: patients with pancreatitis and divided into mild (n = 32) and severe (n = 30) subgroups; Sepsis group: patients with sepsis (n = 20) | HMGB1 was significantly elevated in the plasma of SAP patients compared with healthy and mild pancreatitis patients, and was correlated with procalcitonin concentrations. There was an inverse correlation between the levels of sRAGE and HMGB1 in patients with SAP. Circulating DNA was significantly elevated in patients with severe pancreatitis or sepsis and was related to the severity scores |
| Lindström et al[80] | Pancreas | Finland | Patients with AP | Grade 0: mild AP (n = 282); Grade 1: SAP without organ failure (n = 135); Grade 2: SAP with organ failure (n = 38) | Serum HMGB1 level is comparable in three groups, but sRAGE is significantly higher in AP patients who develop organ failure compared to AP patients who recover without organ failure |
| Yuan et al[84] | Pancreas | China | Male ICR mice | Control group: SAP mice (n = 24); Treatment group: SAP mice treated with recombinant HMGB1 A box protein 12 (n = 12) and 24 h (n = 12) after the modeling injection | HMGB1 A box can decrease the serum HMGB1 levels, attenuate organ dysfunction and improve the survival of SAP mice. Thus, it has a remarkable protective effect against pancreatitis and associated organ injury |
| Luan et al[82] | Immunobiol | China | Male Wistar rats | Control group: sham operation; SAP group: SAP-induced rats; Treated groups: SAP-induced rats treated with pRNA-U6.1/Neo-HMGB1 (containing siRNA targeting human HMGB1) | Downregulation of HMGB1 by using siRNA could inhibit the activation of NF-κB in SAP rats so as to decrease the levels of downstream inflammatory cytokines, alleviate endothelial permeability and attenuate severe pancreatitis-associated acute lung injury |
| Kang et al[86] | Gastroenterol | United States | HMGB1 flox/flox and Pdx1-Cre transgenic mice | AP group: AP-induced mice; Control group: administered with saline as a control | Deficiency of endogenous HMGB1 could escalate local inflammation through destabilization of the nucleus and enable rapid DNA and histone release, resulting in accelerated tissue injury and lethality, indicating that intracellular HMGB1 appears to have a protective effect against inflammation |
Yasuda and colleagues[75] were the first to report that the serum HMGB1 level was significantly increased within 72 h in patients with SAP. They compared forty-five SAP patients with eight healthy controls and found that the mean level of serum HMGB1 was nearly three times higher in patients with SAP and was positively related to the severity of SAP as well as organ dysfunction and infection. Furthermore, serum HMGB1 levels were positively correlated with serum lactate dehydrogenase, C-reactive protein, and total bilirubin and could estimate the prognosis of SAP patients; i.e., the higher the serum HMGB1 level, the worse the outcome. This result indicates that HMGB1 may be an important mediator in the pathogenesis of pancreatitis and organ dysfunction. To confirm this result further, they designed an experiment in mice. They chose thirty-eight female C3H/HeN mice and divided them into the following three groups: group A included eight mice with sham operations and intraperitoneal injections of normal saline; group B included twenty mice with SAP (induced by duodenal loop closure) and intraperitoneal injections of normal saline; group C included twelve mice with SAP and intraperitoneal injections of anti-HMGB1 neutralizing antibody[76]. Serum amylase levels were significantly decreased in mice injected with anti-HMGB1 neutralizing antibody compared with those injected with normal saline twelve hours after induction of SAP. The morphology of the pancreas and lungs changed significantly in SAP mice, but was ameliorated in groups injected with anti-HMGB1 neutralizing antibody. Similar changes were also seen in the liver and kidneys. These results demonstrated that blockade of HMGB1 attenuated the development of SAP and associated organ dysfunction. Subsequently, they conducted another small experiment and proposed a hypothesis called “HMGB1 circulation”[77]; HMGB1 is first produced by pancreatic and peritoneal macrophages during early SAP in response to inflammation and then partially released to the blood, thereby causing damage to remote organs. In turn, damaged organs can also release HMGB1 and cause a vicious circle. Kocsis et al[78] also found a decrease in serum soluble RAGE in patients with severe pancreatitis compared with healthy controls and mild pancreatitis patients and revealed an inverse correlation between serum levels of soluble RAGE and HMGB1. In addition, they also found significantly elevated circulating DNA levels in patients with SAP and sepsis. This result was in contrast to the result of the study conducted by Bagul et al[79] and Lindström et al[80]. Moreover, HMGB1 also contributes to the development of intestinal barrier dysfunction secondary to SAP, and HMGB1 levels in intestine were correlated with the severity of intestinal barrier dysfunction[81]. The study by Luan et al[82] was consistent with that by Sawa et al[76] and showed that downregulating HMGB1 levels using siRNA could inhibit NF-κB activation, reduce inflammatory reaction and protect against SAP-associated lung injury.
In summary, serum and pancreatic levels of HMGB1 are increased significantly in patients with SAP and SAP-induced animal models. There are two possible mechanisms[75]. First, this phenomenon can be explained by the theory of “HMGB1 circulation.” Second, as HMGB1 levels in the lungs and intestine were also increased in animal models and patients with SAP-associated acute lung injury and intestinal injury, HMGB1 may be released directly by the pancreas as well as damaged organs in SAP. Further research is needed to reveal the exact function of HMGB1 in SAP.
As mentioned before, HMGB1 has two functional structures: the A box and B box. The A box is the main site mediating the anti-inflammatory process, while the B box is the main site for the pro-inflammatory response[83]. To study the effect of the A box in pancreatitis further, Yuan et al[84] designed a study in male mice. The SAP groups were divided into control and treatment groups. In the treatment group, the SAP mice were treated with a recombinant HMGB1 A box protein 12 and 24 h after modeling. The HMGB1 A box significantly improved the elevation of the serum levels of HMGB1 and pancreatic injury and alleviated other organ injury more than that in control group. As a result, the HMGB1 A box showed a protective effect against SAP and improved the survival rate. The latest study conducted by Kong et al[85] also confirmed the protective effect of HMGB1 A box in lung injury induced by AP.
A more recent study by Kang et al[86] demonstrated a protective effect of intracellular HMGB1 against inflammation, limiting AP in HMGB1 knockout mice. This finding indicates the complex role of HMGB1 in AP, i.e., endogenous HMGB1 derived from the pancreas itself can protect cells from activation of NF-κB, release of nucleosomes and DNA damage, thereby limiting the severity of pancreatic injury. In contrast, extracellular HMGB1 released from innate immune cells including macrophages and monocytes would aggravate the inflammatory response and increase pancreatic and remote organ damage.
Recently, some researchers studied the effect of HMGB1 inhibitors in preventing against SAP (Table 3). They thought HMGB1 inhibitors might be potential targets in ameliorating SAP. Anti-HMGB1 neutralizing antibody is an inhibitor for HMGB1. Ethyl pyruvate (EP), pyrrolidine dithiocarbamate (PDTC) and Scolopendra subspinipes mutilans (SSM) are three potential HMGB1 inhibitors that may attenuate pancreatitis.
| Ref. | Journal | Country | Subject | HMGB1 inhibitor | Method | Result |
| Sawa et al[76] | World J Gastroenterol | Japan | Female C3H/HeN mice | Anti-HMGB1 neutralizing antibody | Group A: sham, laparotomy with saline injection (n = 6); Group B: SAP, SAP with saline injection (n = 20); Group C: HMGB1 Ab + SAP, SAP with anti-HMGB1 antibody injection (n = 12) | Blockade of HMGB1 in the early phase is useful as a new therapeutic option against the inflammatory response and MODS in patients with SAP |
| Yang et al[96] | Crit Care Med | United States | Male C57BL/6 mice | EP | RLS group: mice were injected with RLS; REPS group: mice were treated with REPS; Control group: mice were injected with PBS | Delayed treatment with EP downregulated the inflammatory response through decreasing the release of pro-inflammatory cytokines and attenuated the development of both local and distant organ dysfunction, improving survival in a murine model of severe necrotizing pancreatitis |
| Cheng et al[97] | Pancreas | China | Male Wistar rats | EP | Group A: SAP-induced rats; Group B: moderate pancreatitis-induced rats; Group C: mild pancreatitis-induced rats; Control group: rats received the same dose of vehicle solution | A strong correlation between levels of HMGB1 and severity of acute pancreatitis. Treatment with EP significantly protected against SAP lethality and ameliorated extrapancreatic tissue and organ injury or dysfunction in rats with SAP |
| Yang et al[112] | World J Gastroenterol | China | Male Wistar rats | EP | Group I: sham operation (n = 32); Groups II: SAP-induced rats and treated with EP (n = 32); Groups III: SAP-induced rats (n = 32) | Serum HMGB1 evaluated significantly in SAP rats, whereas delayed EP administration can significantly reduce the serum level of HMGB1 as well as AST, ALT and Cr level and prolong the survival time in rats |
| Yang et al[99] | J Surg Res | United States | Male C57Bl/6 mice | EP | Control group: injected with PBS; EP group: SAP-induced mice and treated with EP; RLS group: SAP-induced mice and treated with RLS | EP is able to inhibit NF-κB DNA binding, decrease the level of both early inflammatory cytokines such as TNF-α, IL-6, COX-2, and iNOS and late proinflammatory mediator (HMGB1), reduce inflammatory cells infiltration, and reverse hepatic oxidative stress, thus protecting hepatocytes from SAP-induced injury. Thus, treatment with EP ameliorates hepatocellular injury and redox stress in the setting of SAP |
| Luan et al[81] | Pancreas | China | Male Wistar rats | EP | Control group: sham operation (n = 20); SAP group: SAP-induced rats (n = 20); EP-treated group: SAP-induced rats and treated with EP (n = 20) | HMGB1 contributes to the development of gut barrier dysfunction after SAP. Intestinal HMGB1 levels were significantly increased in rats with SAP and were correlated with the severity of intestinal barrier dysfunction |
| Luan et al[100] | Pancreas | China | Male Wistar rats | EP | Control group: sham operation (n = 8); SAP group: SAP-induced rats (n = 8); EP-treated group: SAP-induced rats and treated with EP (n = 8) | EP administration inhibits NF-κB activation to suppress the expression of both early (TNF-α, IL-1β) and late (HMGB1) cytokines that mediate liver injury after SAP and reduces liver injury in SAP rats. Thus EP can provide durable protection against the deleterious effects of proinflammatory cytokines and HMGB1 |
| Luan et al[98] | J Surg Res | China | Male Wistar rats | EP | Control group: sham procedure (n = 48); SAP group: SAP-induced rats (n = 48); EP-treated group: SAP-induced rats and treated with EP (n = 48) | EP attenuates taurocholate-induced pancreatitis and pancreas injury, decreases the taurocholate-induced pancreatic expression of TNF-α and HMGB1, alleviates neutrophil infiltration and lipid peroxidation in the pancreas and decreases NF-κB DNA binding activity as well |
| Luan et al[113] | Clin Exp Immunol | China | Male Wistar rats | EP | Control group: sham procedure (n = 48); SAP group: SAP-induced rats (n = 48); EP-treated group: SAP-induced rats and treated with EP (n = 48) | EP can reduce the lung permeability index in mice with LPS-induced acute lung injury in a dose-dependent way. The protective effect is associated with a reduction in both early (TNF-α and IL-1β) and late (HMGB1) cytokine levels by inhibiting NF-κB activity |
| Zhang et al[103] | Dig Dis Sci | China | Male Sprague-Dawley rats | antioxidant PDTC | Sham operation group (n = 48); SAP group (n = 48); PDTC-treated group (n = 48) | HMGB1 is a late cytokine mediator that plays an important role in the pathogenesis of SAP. PDTC pre-administration might inhibit NF-κB activation to inhibit the production of HMGB1 and reduce pancreas injury in SAP rats; but PDTC was less effective when it was given 2 h after the induction of pancreatitis |
| Jo et al[107] | World J Gastroenterol | Korea | C57BL/6 mice | SSM | Control group: mice were treated with saline; AP group: mice with induced AP; SSM group: divided into three subgroups: SSM 0.1 g/kg + AP; SSM 0.5 g/kg + AP; SSM 1 g/kg + AP | SSM pre-treat decreased HMGB1 and other cytokines such as TNF-α and IL-1β in AP mice. It also played a protective role during the development of AP and pancreatitis-associated lung injury via deactivating c-Jun NH2-terminal kinase, p38 and NF-κB |
Anti-HMGB1 neutralizing antibody was reported to ameliorate the inflammatory reaction in the airway, liver and intestine and could even prevent bacterial translocation[87,88]. Sawa et al[76] reported that blockade of the high mobility group box-1 protein using the anti-HMGB1 antibody could attenuate SAP in the mouse model.
EP, which is derived from pyruvic acid, is an important intermediate product in glucose metabolism[89,90]. EP was first used as a potential treatment in rat models of reactive oxygen species-mediated acute renal failure[91]. EP is reported to inhibit lipopolysaccharide-induced NF-κB activation and then decrease HMGB1 levels in sepsis-induced mice and show its protective effect in all types of organ dysfunction[90,92-95]. Yang et al[96] first demonstrated that delayed treatment with EP could downregulate the inflammatory reaction and ameliorate the development of both local and distant organ dysfunction in an animal model of severe necrotizing pancreatitis. Subsequently, Cheng et al[97] and Yang et al[96] found that the anti-inflammatory effect of EP was via modulating HMGB1 and other inflammatory cytokine responses. Further research found that the mechanism of EP was by inhibiting the activation of NF-κB and downregulating serum HMGB1, TNF-α, IL-1β and other cytokines to ameliorate tissue injury and organ dysfunction in AP[98]. Acute lung injury is the most common extra-pancreatic complication leading to death in SAP patients. Studies showed that EP protected against the development of lung injury in SAP-induced mice[82]. Moreover, EP can attenuate other SAP-associated organ dysfunctions, such as liver and intestinal barrier injury in murine models[81,82,99,100].
PDTC is an antioxidant that can prevent induction of nitric oxide synthase. Some studies have shown that PDTC may be a potent inhibitor of NF-κB and play a role in suppressing the inflammatory process[101,102]. A recent study has shown that PDTC pre-administration can decrease HMGB1 levels and alleviate the inflammatory reaction in SAP rats by inhibiting NF-κB activation[103]. However, it was less effective when it was given 2 h after the induction of pancreatitis, therefore indicating that PDTC may indirectly inhibit HMGB1.
SSM is a polysaccharide that is extracted from Scolopendra. It was reported that SSM has many biologic effects including anti-inflammation as a traditional medicine[104-106]. A study by Jo et al[107] showed that SSM pre-treatment decreased cytokines, including HMGB1, TNF-α and IL-1β, by inhibiting c-Jun NH2-terminal kinase, p38 and NF-κB in AP mice. Therefore, SSM can attenuate the development of AP and related lung injury.
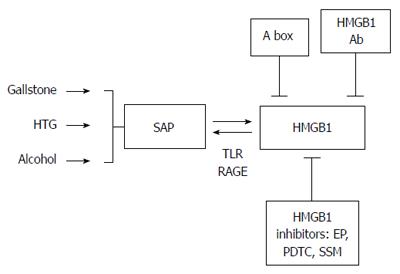
We reviewed all the studies on HMGB1 and SAP and drew several conclusions (Figure 2). First, extracellular HMGB1 is a vital mediator of inflammation and plays a major role in many inflammatory-related diseases. A number of studies have reported the increased levels of serum HMGB1 in SAP patients or models and showed its positive correlation with the severity of the disease. Second, decreasing HMGB1 levels by HMGB1 antibodies, the A Box or specific inhibitors can significantly decrease the release of related cytokines and reduce the inflammatory reaction in pancreatitis, thereby attenuating organ dysfunction and improving prognosis. Delayed EP administration is known to be an effective way to inhibit HMGB1 release in the setting of SAP, whereas PDTC and SSM work only when administered in advance. A possible mechanism of these components is to inhibit the activation of NF-κB and reduce extracellular HMGB1 levels; hence, ameliorating the development of SAP. However, the exact mechanisms of these inhibitors still need verification through more fundamental studies. Lastly, the latest study detected the role of intracellular HMGB1 in inflammation and demonstrated its protective effect against pancreatitis in HMGB1 knockout mice. This research indicates that HMGB1 may have a bidirectional effect in the pathogenesis of SAP.
In addition to inflammation, HMGB1 also plays a regulatory role in angiogenesis. It is now known that HMGB1 affects many angiogenesis-related conditions, such as cancer, proliferative diabetic retinopathy and wound healing, via the p53 pathway and is said to be a promising therapeutic target in many tumors including epidermal tumors, prostate cancer and colon cancer[20,108-111]. The exact function of HMGB1 and its mechanism still need to be elucidated.
P- Reviewer: Stocco G, Shen HN S- Editor: Qi Y L- Editor: AmEditor E- Editor: Wang CH
| 1. | Melvin VS, Edwards DP. Coregulatory proteins in steroid hormone receptor action: the role of chromatin high mobility group proteins HMG-1 and -2. Steroids. 1999;64:576-586. [PubMed] |
| 2. | Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237-5246. [PubMed] |
| 3. | Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14-19. [PubMed] |
| 4. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [PubMed] |
| 5. | Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17-22. [PubMed] |
| 6. | Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res. 2009;81:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld AN, Wang H. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Nakamura T, Fujiwara N, Sato E, Kawagoe Y, Ueda Y, Yamada S, Koide H. Effect of polymyxin B-immobilized fiber hemoperfusion on serum high mobility group box-1 protein levels and oxidative stress in patients with acute respiratory distress syndrome. ASAIO J. 2009;55:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 10. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [PubMed] |
| 11. | Paonessa G, Frank R, Cortese R. Nucleotide sequence of rat liver HMG1 cDNA. Nucleic Acids Res. 1987;15:9077. [PubMed] |
| 12. | Wen L, Huang JK, Johnson BH, Reeck GR. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989;17:1197-1214. [PubMed] |
| 13. | Rauvala H, Merenmies J, Pihlaskari R, Korkolainen M, Huhtala ML, Panula P. The adhesive and neurite-promoting molecule p30: analysis of the amino-terminal sequence and production of antipeptide antibodies that detect p30 at the surface of neuroblastoma cells and of brain neurons. J Cell Biol. 1988;107:2293-2305. [PubMed] |
| 14. | Ferrari S, Finelli P, Rocchi M, Bianchi ME. The active gene that encodes human high mobility group 1 protein (HMG1) contains introns and maps to chromosome 13. Genomics. 1996;35:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 302] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 16. | Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care. 2011;15:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis. 2008;11:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 404] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepäntalo M, Carpén O, Parkkinen J, Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1). Blood. 2004;104:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Mullins GE, Sunden-Cullberg J, Johansson AS, Rouhiainen A, Erlandsson-Harris H, Yang H, Tracey KJ, Rauvala H, Palmblad J, Andersson J. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand J Immunol. 2004;60:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Weng H, Deng Y, Xie Y, Liu H, Gong F. Expression and significance of HMGB1, TLR4 and NF-κB p65 in human epidermal tumors. BMC Cancer. 2013;13:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 1016] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 22. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4315] [Article Influence: 359.6] [Reference Citation Analysis (45)] |
| 23. | Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 24. | Forsmark CE, Toskes PP. Acute pancreatitis. Medical management. Crit Care Clin. 1995;11:295-309. [PubMed] |
| 25. | Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516-3521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 714] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 26. | Gille-Johnson P, Hansson KE, Gårdlund B. Severe sepsis and systemic inflammatory response syndrome in emergency department patients with suspected severe infection. Scand J Infect Dis. 2013;45:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3170] [Article Influence: 264.2] [Reference Citation Analysis (0)] |
| 28. | Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Barry RE. The pathogenesis of acute pancreatitis. Br Med J (Clin Res Ed). 1988;296:589. [PubMed] |
| 31. | Singh VP, Saluja AK, Bhagat L, van Acker GJ, Song AM, Soltoff SP, Cantley LC, Steer ML. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993-G1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Grasso D, Ropolo A, Lo Ré A, Boggio V, Molejón MI, Iovanna JL, Gonzalez CD, Urrutia R, Vaccaro MI. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem. 2011;286:8308-8324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 35. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Kylänpää L, Rakonczay Z, O’Reilly DA. The clinical course of acute pancreatitis and the inflammatory mediators that drive it. Int J Inflam. 2012;2012:360685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Rakonczay Z, Hegyi P, Takács T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Hietaranta AJ, Saluja AK, Bhagat L, Singh VP, Song AM, Steer ML. Relationship between NF-kappaB and trypsinogen activation in rat pancreas after supramaximal caerulein stimulation. Biochem Biophys Res Commun. 2001;280:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280:C465-C472. [PubMed] |
| 40. | Booth DM, Mukherjee R, Sutton R, Criddle DN. Calcium and reactive oxygen species in acute pancreatitis: friend or foe? Antioxid Redox Signal. 2011;15:2683-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Rau BM, Krüger CM, Schilling MK. Anti-cytokine strategies in acute pancreatitis: pathophysiological insights and clinical implications. Rocz Akad Med Bialymst. 2005;50:106-115. [PubMed] |
| 42. | Gullo L, Migliori M, Oláh A, Farkas G, Levy P, Arvanitakis C, Lankisch P, Beger H. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223-227. [PubMed] |
| 43. | Hegyi P, Rakonczay Z. The role of nitric oxide in the physiology and pathophysiology of the exocrine pancreas. Antioxid Redox Signal. 2011;15:2723-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Escobar J, Pereda J, López-Rodas G, Sastre J. Redox signaling and histone acetylation in acute pancreatitis. Free Radic Biol Med. 2012;52:819-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Gray KD, Simovic MO, Chapman WC, Blackwell TS, Christman JW, Washington MK, Yull FE, Jaffal N, Jansen ED, Gautman S. Systemic nf-kappaB activation in a transgenic mouse model of acute pancreatitis. J Surg Res. 2003;110:310-314. [PubMed] |
| 46. | Masamune A, Shimosegawa T, Kimura K, Fujita M, Sato A, Koizumi M, Toyota T. Specific induction of adhesion molecules in human vascular endothelial cells by rat experimental pancreatitis-associated ascitic fluids. Pancreas. 1999;18:141-150. [PubMed] |
| 47. | Liu HS, Pan CE, Liu QG, Yang W, Liu XM. Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World J Gastroenterol. 2003;9:2513-2518. [PubMed] |
| 48. | Montravers P, Chollet-Martin S, Marmuse JP, Gougerot-Pocidalo MA, Desmonts JM. Lymphatic release of cytokines during acute lung injury complicating severe pancreatitis. Am J Respir Crit Care Med. 1995;152:1527-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Malmstrøm ML, Hansen MB, Andersen AM, Ersbøll AK, Nielsen OH, Jørgensen LN, Novovic S. Cytokines and organ failure in acute pancreatitis: inflammatory response in acute pancreatitis. Pancreas. 2012;41:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Repo H, Harlan JM. Mechanisms and consequences of phagocyte adhesion to endothelium. Ann Med. 1999;31:156-165. [PubMed] |
| 51. | Foitzik T, Eibl G, Hotz B, Hotz H, Kahrau S, Kasten C, Schneider P, Buhr HJ. Persistent multiple organ microcirculatory disorders in severe acute pancreatitis: experimental findings and clinical implications. Dig Dis Sci. 2002;47:130-138. [PubMed] |
| 52. | Lasson A, Ohlsson K. Consumptive coagulopathy, fibrinolysis and protease-antiprotease interactions during acute human pancreatitis. Thromb Res. 1986;41:167-183. [PubMed] |
| 53. | Okamura D, Starr ME, Lee EY, Stromberg AJ, Evers BM, Saito H. Age-dependent vulnerability to experimental acute pancreatitis is associated with increased systemic inflammation and thrombosis. Aging Cell. 2012;11:760-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Lasson A, Ohlsson K. Disseminated intravascular coagulation and antiprotease activity in acute human pancreatitis. Scand J Gastroenterol Suppl. 1986;126:35-39. [PubMed] |
| 55. | Lindstrom O, Kylanpaa L, Mentula P, Puolakkainen P, Kemppainen E, Haapiainen R, Fernandez JA, Griffin JH, Repo H, Petaja J. Upregulated but insufficient generation of activated protein C is associated with development of multiorgan failure in severe acute pancreatitis. Crit Care. 2006;10:R16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 379] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 57. | Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551-5560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1018] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 58. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3283] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 59. | Yamada Y, Fujii T, Ishijima R, Tachibana H, Yokoue N, Takasawa R, Tanuma S. The release of high mobility group box 1 in apoptosis is triggered by nucleosomal DNA fragmentation. Arch Biochem Biophys. 2011;506:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Bell CW, Jiang W, Reich CF, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318-C1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 415] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 61. | Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010;1799:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 62. | Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014;18:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Hansson GK, Edfeldt K. Toll to be paid at the gateway to the vessel wall. Arterioscler Thromb Vasc Biol. 2005;25:1085-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362-371. [PubMed] |
| 65. | Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372-378. [PubMed] |
| 66. | Asavarut P, Zhao H, Gu J, Ma D. The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol Taiwan. 2013;51:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Vande Walle L, Kanneganti TD, Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2:162-165. [PubMed] |
| 68. | Miyake Y, Yamasaki S. Sensing necrotic cells. Adv Exp Med Biol. 2012;738:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6726] [Article Influence: 448.4] [Reference Citation Analysis (0)] |
| 70. | Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 641] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 71. | Schmidt AM, Hofmann M, Taguchi A, Yan SD, Stern DM. RAGE: a multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin Thromb Hemost. 2000;26:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Park IH, Yeon SI, Youn JH, Choi JE, Sasaki N, Choi IH, Shin JS. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol Immunol. 2004;40:1203-1211. [PubMed] |
| 73. | Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99-111. [PubMed] |
| 74. | Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 405] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 75. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 76. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. [PubMed] |
| 77. | Yasuda T, Ueda T, Shinzeki M, Sawa H, Nakajima T, Takeyama Y, Kuroda Y. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas. 2007;34:487-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Kocsis AK, Szabolcs A, Hofner P, Takács T, Farkas G, Boda K, Mándi Y. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology. 2009;9:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Bagul A, Pushpakom S, Boylan J, Newman W, Siriwardena AK. Quantitative analysis of plasma DNA in severe acute pancreatitis. JOP. 2006;7:602-607. [PubMed] |
| 80. | Lindström O, Tukiainen E, Kylänpää L, Mentula P, Rouhiainen A, Puolakkainen P, Rauvala H, Repo H. Circulating levels of a soluble form of receptor for advanced glycation end products and high-mobility group box chromosomal protein 1 in patients with acute pancreatitis. Pancreas. 2009;38:e215-e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Role of high-mobility group box 1 protein in the pathogenesis of intestinal barrier injury in rats with severe acute pancreatitis. Pancreas. 2010;39:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Luan ZG, Zhang XJ, Yin XH, Ma XC, Zhang H, Zhang C, Guo RX. Downregulation of HMGB1 protects against the development of acute lung injury after severe acute pancreatitis. Immunobiology. 2013;218:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 930] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 84. | Yuan H, Jin X, Sun J, Li F, Feng Q, Zhang C, Cao Y, Wang Y. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Kong X, Zhang C, Jin X, Wu X, Zhang S, Zhong Z, Feng Q, Liu T, Yuan H. The effect of HMGB1 A box on lung injury in mice with acute pancreatitis. Biofactors. 2011;37:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 86. | Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 87. | Zhang F, Huang G, Hu B, Fang LP, Cao EH, Xin XF, Song Y, Shi Y. Anti-HMGB1 neutralizing antibody ameliorates neutrophilic airway inflammation by suppressing dendritic cell-mediated Th17 polarization. Mediators Inflamm. 2014;2014:257930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 88. | Yang R, Zou X, Tenhunen J, Zhu S, Kajander H, Koskinen ML, Tonnessen TI. HMGB1 neutralization is associated with bacterial translocation during acetaminophen hepatotoxicity. BMC Gastroenterol. 2014;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Fink MP. Ethyl pyruvate. Curr Opin Anaesthesiol. 2008;21:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351-12356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 490] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 91. | Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991;88:1886-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Bünger R, Mallet RT, Hartman DA. Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile failure. Eur J Biochem. 1989;180:221-233. [PubMed] |
| 93. | Cicalese L, Lee K, Schraut W, Watkins S, Borle A, Stanko R. Pyruvate prevents ischemia-reperfusion mucosal injury of rat small intestine. Am J Surg. 1996;171:97-100; discussion 100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Sileri P, Schena S, Morini S, Rastellini C, Pham S, Benedetti E, Cicalese L. Pyruvate inhibits hepatic ischemia-reperfusion injury in rats. Transplantation. 2001;72:27-30. [PubMed] |
| 95. | Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 96. | Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med. 2004;32:1453-1459. [PubMed] |
| 97. | Cheng BQ, Liu CT, Li WJ, Fan W, Zhong N, Zhang Y, Jia XQ, Zhang SZ. Ethyl pyruvate improves survival and ameliorates distant organ injury in rats with severe acute pancreatitis. Pancreas. 2007;35:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Luan ZG, Ma XC, Zhang H, Zhang C, Guo RX. Protective effect of ethyl pyruvate on pancreas injury in rats with severe acute pancreatitis. J Surg Res. 2013;181:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Yang R, Shaufl AL, Killeen ME, Fink MP. Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis. J Surg Res. 2009;153:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Therapeutic treatment with ethyl pyruvate attenuates the severity of liver injury in rats with severe acute pancreatitis. Pancreas. 2012;41:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Virlos I, Mazzon E, Serraino I, Di Paola R, Genovese T, Britti D, Thiemerman C, Siriwardena A, Cuzzocrea S. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock. 2003;20:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253-258. [PubMed] |
| 103. | Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen TK, Zhu YF, Wu L. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig Dis Sci. 2010;55:2529-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | You WK, Sohn YD, Kim KY, Park DH, Jang Y, Chung KH. Purification and molecular cloning of a novel serine protease from the centipede, Scolopendra subspinipes mutilans. Insect Biochem Mol Biol. 2004;34:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Pemberton RW. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999;65:207-216. [PubMed] |
| 106. | Ren WH, Zhang SQ, Song DX, Zhou KY. [Antibacterial activity of water soluble fraction from Scolopendra subspinipes mutilans]. Zhong Yao Cai. 2007;30:10-14. [PubMed] |
| 107. | Jo IJ, Bae GS, Park KC, Choi SB, Jung WS, Jung SY, Cho JH, Choi MO, Song HJ, Park SJ. Scolopendra subspinipes mutilans protected the cerulein-induced acute pancreatitis by inhibiting high-mobility group box protein-1. World J Gastroenterol. 2013;19:1551-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene. 2013;32:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 109. | Gnanasekar M, Kalyanasundaram R, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A. HMGB1: A Promising Therapeutic Target for Prostate Cancer. Prostate Cancer. 2013;2013:157103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Kikuchi H, Yagi H, Hasegawa H, Ishii Y, Okabayashi K, Tsuruta M, Hoshino G, Takayanagi A, Kitagawa Y. Therapeutic potential of transgenic mesenchymal stem cells engineered to mediate anti-high mobility group box 1 activity: targeting of colon cancer. J Surg Res. 2014;190:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014;95:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 112. | Yang ZY, Ling Y, Yin T, Tao J, Xiong JX, Wu HS, Wang CY. Delayed ethyl pyruvate therapy attenuates experimental severe acute pancreatitis via reduced serum high mobility group box 1 levels in rats. World J Gastroenterol. 2008;14:4546-4550. [PubMed] |
| 113. | Luan ZG, Zhang J, Yin XH, Ma XC, Guo RX. Ethyl pyruvate significantly inhibits tumour necrosis factor-α, interleukin-1β and high mobility group box 1 releasing and attenuates sodium taurocholate-induced severe acute pancreatitis associated with acute lung injury. Clin Exp Immunol. 2013;172:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |