Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13518
Peer-review started: October 1, 2015
First decision: November 5, 2015
Revised: November 19, 2015
Accepted: December 12, 2015
Article in press: December 14, 2015
Published online: December 28, 2015
Processing time: 84 Days and 15.7 Hours
AIM: The clinical value of second-look endoscopy (SLE) after endoscopic submucosal dissection (ESD) has been doubted continuously. The aim of this study was to assess the effectiveness of SLE based on the risk of delayed bleeding after ESD.
METHODS: A total of 310 lesions of gastric epithelial neoplasms treated by ESD were reviewed. The lesions were divided into two groups based on the risk of post-procedural bleeding estimated by Forrest classification. The high risk of rebleeding group (Forrest Ia, Ib and IIa) required endoscopic treatment, while the low risk of rebleeding group (Forrest IIb, IIc and III) did not. Delayed bleeding after ESD was investigated.
RESULTS: Sixty-six lesions were included in the high risk of rebleeding group and 244 lesions in the low risk of rebleeding group. There were no significant differences in delayed bleeding between the high risk group (1/66) and the low risk group (1/244) (P = 0.38). The high risk of rebleeding group tended to be located more often in the mid-third and had higher appearance of flat or depressed shape than the low risk group (P = 0.004 and P = 0.006, respectively).
CONCLUSION: SLE with pre-emptive prophylactic endoscopic treatment is still effective in preventing delayed bleeding after ESD.
Core tip: This is a retrospective study to assess the effectiveness of second-look endoscopy (SLE) based on the risk of delayed bleeding after endoscopic submucosal dissection (ESD). A total of 310 lesions of gastric epithelial neoplasms treated by ESD were reviewed. The lesions were divided into two groups based on the risk of post-procedural bleeding estimated by Forrest classification. The high risk of rebleeding group (Forrest Ia, Ib and IIa) required endoscopic treatment, while the low risk of rebleeding group (Forrest IIb, IIc and III) did not. Delayed bleeding after ESD was investigated. As a result, there were no significant differences in delayed bleeding between the high risk group and the low risk group. However, the high risk of rebleeding group tended to be located more often in the mid-third and had higher appearance of flat or depressed shape than the low risk group. In conclusion, SLE with pre-emptive prophylactic endoscopic treatment is still effective in preventing delayed bleeding after ESD.
- Citation: Jung JH, Kim BJ, Choi CH, Kim JG. Second-look endoscopy with prophylactic hemostasis is still effective after endoscopic submucosal dissection for gastric neoplasm. World J Gastroenterol 2015; 21(48): 13518-13523
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13518.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13518
Gastric cancer is the most common malignant neoplasm and the third most common cause of cancer related death in South Korea[1]. Endoscopy for screening of gastric cancer is widely performed in high incidence areas such as Korea and Japan. Recently, advances in endoscopic equipment have increased the early detection rate. There is a good chance of being cured when gastric cancer is diagnosed at an early state, for which surgery is the treatment of choice. Endoscopic submucosal dissection (ESD) for node-negative early gastric cancer (EGC) has been recognized as an outstanding endoscopic treatment instead of open surgery[2,3]. However, bleeding from artificial ulcers after gastric ESD has remained a major complication. Although the bleeding rate is relatively low after gastric ESD, uncontrolled bleeding is related to mortality[3]. Therefore, second-look endoscopy (SLE) continues to be performed at most institutions after gastric ESD to check for post-procedural bleeding. Nevertheless, there has been no consensus nor significant evidence of the clinical utility of SLE in patients without any sign of bleeding. In most cases of bleeding, endoscopic hemostasis effectively stops bleeding when properly performed during emergency endoscopy. For this reason, the clinical value of SLE is continuously under debate. Two retrospective analysis reported that SLE after gastric ESD may contribute little to the prevention of delayed bleeding[4,5]. In that study, gross type (IIb/IIc) was the only considerable predictor for post-ESD bleeding with a significant difference[4]. In consideration of that point, gastric ESD cases were evaluated herein to verify the clinical and pathological conditions depending on the state of the iatrogenic ulcer base of post-ESD. The aim of this study was to assess the clinical role of routine SLE after ESD based on the delayed bleeding estimated by Forrest classification, and to confirm whether SLE with prophylactic hemostasis is useful for the prevention of delayed bleeding after gastric ESD.
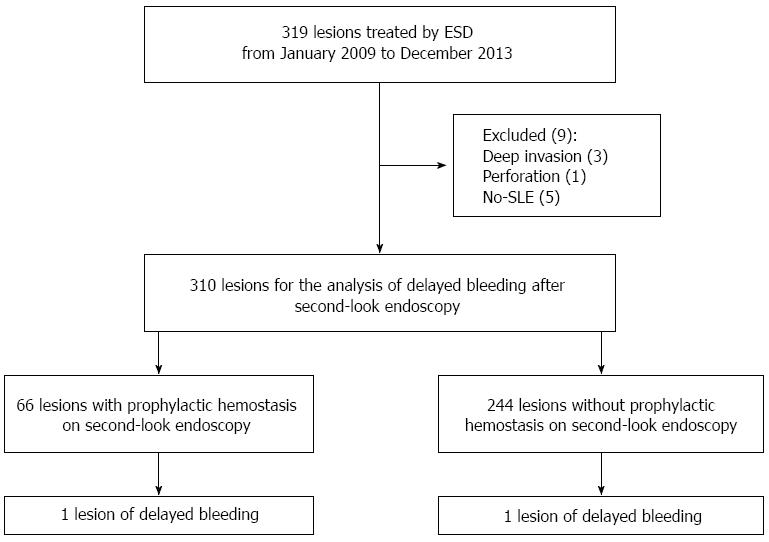
A total of 319 patients with gastric epithelial neoplasm who were consecutively treated with ESD in Chung-Ang University Hospital from January 2009 to December 2013 were retrospectively reviewed. ESD was principally indicated for node-negative EGC or gastric adenoma. A total of four cases were excluded due to deep invasion (n = 3) or perforation (n = 1). As a result, a total of 310 lesions (74 cases of EGCs and 236 cases of gastric adenoma) were explored by SLE, defined as performing elective endoscopy without any sign of bleeding within 72 h after ESD.
The 310 lesions were divided into two groups based on the risk of post-procedural bleeding (high risk group vs low risk group) estimated by Forrest classification. The high risk of rebleeding group (Forrest Ia, Ib and IIa) required endoscopic treatment, while the low risk of rebleeding group (Forrest IIb, IIc and III) did not. More specifically, the high risk of rebleeding group included cases with blood oozing and visible vessels without any sign of hemorrhage. A flow chart for inclusion in this study is shown in Figure 1. The study protocol was approved by the institutional review board of Chung-Ang University College of Medicine [IRB No. C2014088(1284)].
The ESD was performed under conscious sedation. All patients provided written informed consent before treatment. Patients fasted the morning of the treatment. After circumferential marking with an argon plasma coagulator (APC) from the tumor edge, a mixture of 0.9% saline with epinephrine, glycerol, or hyaluronic acid containing indigo carmine was injected into the submucosal layer. The muscularis mucosa was then cut and submucosal dissection was performed. An insulation-tipped knife (Olympus, Tokyo, Japan), Hook knife (Olympus, Tokyo, Japan), or Flex knife (Olympus, Tokyo, Japan) was selected as the electrosurgical knife according to the preference of the endoscopist and/or tumor characteristics. Oozing lesions or non-bleeding visible vessels in the ulcer bed were treated by hemostatic forceps (FD-410LR; Olympus) or hemoclips immediately after ESD.
In principle, treatment with antiplatelet or anticoagulant agents was stopped 1 wk before ESD. 40mg pantoprazole was administered intravenously once daily from the day of ESD to the first day of feeding. Complete blood count was checked immediately after ESD, the day after, at discharge, and after 1 and 4 wk.
The purpose of SLE was to check and prevent post-ESD bleeding from the artificial ulcer. SLE was performed within 72 h after ESD. SLE was mainly performed the day after ESD; however, emergency endoscopy was performed anytime within 24 h in patients with suspected bleeding or perforation. During SLE, prophylactic hemostasis with thermocoagulation or hemostatic clipping were performed on actively bleeding (Forrest classification Ia) or blood-oozing ulcers (Forrest classification Ib) or non-bleeding visible vessels (Forrest classification IIa). After SLE, patients without evidence of bleeding were usually allowed to have a liquid diet with an oral proton pump inhibitor. Most patients were discharged within 3 d after ESD if a bleeding event did not occur. The patients were scheduled to visit the hospital at 1 wk, 1 mo, and 3 mo after discharge. All patients were instructed to visit the hospital in case of hematemesis, hematochezia, melena or dizziness.
Post-ESD bleeding was defined as the appearance of hematemesis, hematochezia, melena, hypotension or decrease in hemoglobin of more than 2g/dL after ESD. Diagnosis was made by endoscopy. Delayed bleeding was defined as bleeding events after SLE which occurred within 30 d. The following variables were analyzed: age, sex, comorbidities with Charlson comorbidity scale, use of antiplatelet or anticoagulant agents, use of steroid or nonsteroidal anti-inflammatory drugs, procedure time, resection type (en bloc or piecemeal), lesion location (upper third-high body or cardia, middle third-mid body or lower body, lower third-antrum or angle), lesion characteristics (elevated or flat, depressed), lesion size (maximum diameter), resected specimen size (maximum diameter), histologic type, presence of Helicobacter pylori (H. pylori), Forrest classification at SLE (high risk-Ia, Ib, IIa or low risk-IIa, IIb, III), whether or not prophylactic hemostasis occurred, and presence of delayed bleeding (≤ 30 d after ESD).
Data were analyzed with comparison between the two bleeding groups. Statistical analysis was performed using the SPSS software version 18.0 (SPSS Inc., Chicago, IL, United States). Univariate analysis by the Student’s t-test was performed for age, lesion size, resected specimen size, and procedure time. χ2 test was performed for sex, cormorbidities, the use of antiplatelet or anticoagulant agents or of steroid or non-steroidal anti-inflammatory drugs, technique of resection, resection type, lesion location, lesion characteristics, histologic type, presence of H. pylori, whether or not prophylactic hemostasis occurred, and presence of delayed bleeding. The statistical significance was set at a P value < 0.05.
When the 310 lesions were classified into two groups according to the Forrest classification, 66 lesions (21.2%) were included in the high risk of rebleeding group (Forrest classification: Ia, Ib, IIa) and 244 lesions (78.8%) were included in the low risk of rebleeding group (Forrest classification: IIb, IIc, III). No bleeding occurred within 3 d after SLE in both groups. Delayed bleeding occurred in only two of the 310 lesions (0.64%). All cases of bleeding were controlled with endoscopic management (e.g. hemostatic forceps and hemoclips) without surgical intervention. Blood transfusion was not performed in delayed bleeding cases because of stable vital signs and hemoglobin levels above 9 g/dL. No rebleeding occurred after post-ESD hemostasis. There were no statistically significant differences between the two groups according to age, sex, comorbidities using the Charlson comorbidity scale (Table 1). In addition, there were no significant differences in procedure time, lesion size, resected specimen size, histologic type, presence of H. pylori, whether or not prophylactic hemostasis occurred, and presence of delayed bleeding (Table 2). The lesions of the high risk of rebleeding group tended to be located more often in the mid-third, whereas those of the low risk of rebleeding group were located more in the upper or lower third (P = 0.004). As for lesion characteristics, the high risk of rebleeding group had higher appearance of flat or depressed shape than the low risk group (P = 0.006). Delayed bleeding after SLE occurred on POD 5 in the low risk of rebleeding group and on POD 23 in the high risk of rebleeding group. There were no significant differences between the groups (P = 0.38). The three patients with double lesions had low grade dysplasia of elevated shape, and were included in the low risk of rebleeding group without delayed bleeding.
| High risk group (n = 66) | Low risk group (n = 244) | P value | |
| Forrest classfication | Ia, Ib, IIa (21.2) | IIb, IIc, III (78.8) | |
| Ia (n = 0) | IIb (n = 55) | ||
| Ib (n = 48) | IIc (n = 14) | ||
| IIa (n = 18) | III (n = 175) | ||
| Age, mean (mean ± SD, yr) | 65.2 ± 8.6 | 64.7 ± 9.4 | 0.70 |
| Sex, male/female | 43/23 | 150/94 | 0.58 |
| Comorbidities (Charlson scale) | 0.96 | ||
| 0 | 49 (74.2) | 178 (73.0) | |
| 1 | 13 (19.7) | 52 (21.3) | |
| 2 | 3 (4.5) | 9 (3.7) | |
| 3 | 1 (1.5) | 3 (1.2) | |
| 4 | 0 (0) | 2 (0.8) | |
| Use of antiplatelet or anticoagulant agents or steroid or nonsteroidal anti-inflammatory drugs | 0.03 | ||
| Yes | 10 (15.2) | 17 (7.0) | |
| None or unknown | 56 (84.8) | 227 (93.0) |
| High risk group (n = 66) | Low risk group (n = 244) | P value | |
| Prodecure time (mean ± SD, min) | 50.5 ± 27.6 | 44.2 ± 29.7 | 0.12 |
| Resection type (en bloc) | 66 (100.0) | 244 (100.0) | |
| Lesion location | 0.004 | ||
| High body, cardia | 0 (0.0) | 10 (4.1) | |
| Mid body, lower body | 9 (13.6) | 9 (3.7) | |
| Antrum, angle | 57 (86.4) | 225 (92.2) | |
| Lesion characteristics | 0.006 | ||
| Elevated | 54 (81.8) | 227 (93.0) | |
| Flat or depressed | 12 (18.2) | 17 (7.0) | |
| Lesion size (maximum diameter) | 12.0 ± 7.3 | 12.5 ± 8.4 | 0.69 |
| Resected specimen size (maximum diameter) | 32.3 ± 9.4 | 30.7 ± 10.8 | 0.25 |
| Histologic type | 0.36 | ||
| Cancer | 20 (30.3) | 54 (22.1) | |
| High grade dysplasia | 6 (9.1) | 29 (11.9) | |
| Lower grade dysplasia | 40 (60.6) | 161 (66.0) | |
| Presence of Helicobacter pylori | 0.87 | ||
| Yes | 11 (16.7) | 36 (14.8) | |
| No | 54 (81.8) | 204 (83.6) | |
| Unknown | 1 (1.5) | 4 (1.6) | |
| Endoscopic hemostasis | 1.00 | ||
| Yes | 66 (100.0) | 0 (0.0) | |
| No | 0 (0.0) | 244 (100.0) | |
| Occurrence of delayed bleeding | 0.38 | ||
| Yes | 1 (1.5) | 1 (0.4) | |
| No | 65 (98.5) | 243 (99.6) |
Performance of a routine SLE after gastric ESD was supported by several prospective, randomized trials owing to its efficacy after endoscopic hemostasis for peptic ulcer bleeding[6-8]. However, there are fundamental pathophysiological differences in ulcer formation between peptic and post-ESD ulcers. Peptic ulcers may occur due to breakdown of the mucosal defense mechanism and hyperacidic environment. On the other hand, post-ESD ulcers occur in a relatively less acidic environment. Furthermore, post-ESD ulcers are relatively shallow compared to peptic ulcers due to electively performed submucosal dissection[4,9].
Several current randomized trials suggested that routine SLE had little or no influence on the prevention of delayed bleeding[4,10]. However, most of them were limited to making generalizations because of the small scale, single center-based studies. In addition, recurrent bleeding is one of the most important risk factors for mortality, even though there are fundamental difference in the pathophysiology of peptic ulcers and post-ESD ulcers[8]. Therefore, the study of multiple aspects for predicting the likelihood of post-ESD ulcer rebleeding is in progress. According to several studies, tumor location, tumor size, ulcerative findings, and long procedure time are suggested as risk factor for delayed bleeding[4,11]. However, these factors proved not to be significant in this study. In this study, the use of antiplatelet or anticoagulant agents, steroids, and non-steroidal anti-inflammatory drugs (NSAIDs) was related with high risk of rebleeding after ESD. This result is consistent with previous studies on peptic ulcer bleeding[12].
The presence of H. pylori infection was confirmed using Giemsa staining or urea breath test, because this bacterium disturbs the healing process of gastric ulcers.
As a result, no significant differences were observed between the two groups (P = 0.88).
In this study, the post-ESD lesions were classified according to Forrest classification. Forrest classification is known to be the most important factor among various predictive variables for gastroduodenal ulcer rebleeding[13,14]. There is no doubt that higher Forrest classification has more bleeding risk than lower Forrest classification. In addition, Takizawa et al. suggested that preventive coagulation of visible vessels in the resection area after ESD may lead to a lower bleeding rate[15]. In this study, pre-emptive prophylactic endoscopic treatment for the high risk of rebleeding group during SLE was found to reduce delayed bleeding, as much as the low risk group. Although SLE did not prevent all the delayed bleeding, early detection of delayed bleeding may prevent cardiovascular complications.
From the viewpoint of probability, the high risk of rebleeding group can be recognized as a group with potential for bleeding. In fact, cases with active arterial bleeding (Forrest class Ia and Ib) have a 90% risk of rebleeding. Furthermore, non-bleeding visible vessels (Forrest class IIa) have a 50% risk of rebleeding in spite of proper initial medical management. As for the potential bleeding, theoretically, at least 50% of the high risk of rebleeding group in this study had the chance of experiencing bleeding. This estimation is by no means a negligible level in post-ESD care. In this study, the incidence of complications after ESD, such as perforation or delayed bleeding, was much lower than reported in previous studies[4,9,16]. En bloc resection rate was 100% (314/314), and curative resection rate was 99.0% (311/314) in the present study. This result may be derived from excellent outcomes owing to improvement in the techniques and therapeutic modalities. In a previous study, positive/indeterminate lateral margin was suggested as a significant risk factor for delayed bleeding from ESD[17]. Interestingly, the low risk of rebleeding group was taking more medication with bleeding tendency compared with the high risk of rebleeding group. Exposure to such drugs is well known to substantially increase the bleeding risk of peptic ulcers[13,18,19]. In particular, the low risk of bleeding group in this study included a significant number of patients with relatively higher potential bleeding risk. Thus, the imperfect organization of the low risk of rebleeding group might have led to misinterpretation. Even though the possibility of bleeding after gastric ESD is low, SLE should never be ignored.
In conclusion, based on our retrospective analysis, SLE with pre-emptive prophylactic endoscopic treatment is still effective in preventing delayed bleeding after ESD, especially when the lesion is located in upper locations and is flat or depressed in shape. In the future, large randomized controlled trials will be warranted to elucidate the effectiveness of SLE after ESD.
Owing to recent advances in endoscopic equipment enables, endoscopic submucosal dissection (ESD) for node-negative early gastric cancer has been recognized as an alternative modality of surgery. However, bleeding from artificial ulcers after gastric ESD has remained a major complication. Although the bleeding rate is relatively low after gastric ESD, uncontrolled bleeding is related to mortality. In most cases of bleeding, endoscopic hemostasis effectively stops bleeding when properly performed during emergency endoscopy. For this reason, the clinical value of second-look endoscopy (SLE) is continuously under debate. Therefore, this study evaluated the clinical efficacy of routine SLE after ESD based on the delayed bleeding estimated by Forrest classification, and to confirm whether SLE with prophylactic hemostasis is useful for the prevention of delayed bleeding after gastric ESD.
Second-look endoscopy is important for preventing delayed bleeding after gastric ESD. The results of this study contribute to clarifying the clinical efficacy of second-look endoscopy with prophylactic hemostasis.
In this study, second-look endoscopy with pre-emptive endoscopic treatment was still useful tool for preventing delayed bleeding after ESD. Especially, estimation of rebleeding risk based on the Forrest classification is also applicable to post-procedural bleeding risk assessment as well as peptic ulcer bleeding.
This study suggests that second-look endoscopy with prophylactic hemostasis is still effective for preventing delayed bleeding after gastric ESD. Especially, high risk of rebleeding represented by high grade of Forreset classification (Ia, Ib, and IIa) should be managed on the second-look endoscopy.
ESD: An endoscopic procedure that resect gastrointestinal neoplasm in en bloc fashion with circumferential mucosal incision and submucosal dissection. SLE: An endoscopic technique that confirm recurred or residual bleeding from the gastrointestinal tract after endoscopic procedure.
The author of this paper evaluated the efficacy of second-look endoscopy with prophylactic hemostasis based on the Forrest classification and compared the results of the high risk of rebleeding group with the low risk of rebleeding group. As a result, the high risk of rebleeding group tended to be located more often in the mid-third and had higher appearance of flat or depressed shape than the low risk group. In conclusion, SLE with pre-emptive prophylactic endoscopic treatment is still effective in preventing delayed bleeding after ESD.
P- Reviewer: Herbella FAM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 2. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 519] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 3. | Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913-2917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Takahashi F, Yoshitake N, Akima T, Kino H, Nakano M, Tsuchida C, Tsuchida K, Tominaga K, Sasai T, Masuyama H. A second-look endoscopy may not reduce the bleeding after endoscopic submucosal dissection for gastric epithelial neoplasm. BMC Gastroenterol. 2014;14:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Villanueva C, Balanzó J, Torras X, Soriano G, Sáinz S, Vilardell F. Value of second-look endoscopy after injection therapy for bleeding peptic ulcer: a prospective and randomized trial. Gastrointest Endosc. 1994;40:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Saeed ZA, Cole RA, Ramirez FC, Schneider FE, Hepps KS, Graham DY. Endoscopic retreatment after successful initial hemostasis prevents ulcer rebleeding: a prospective randomized trial. Endoscopy. 1996;28:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Chiu PW, Lam CY, Lee SW, Kwong KH, Lam SH, Lee DT, Kwok SP. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003;52:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Ryu HY, Kim JW, Kim HS, Park HJ, Jeon HK, Park SY, Kim BR, Lang CC, Won SH. Second-look endoscopy is not associated with better clinical outcomes after gastric endoscopic submucosal dissection: a prospective, randomized, clinical trial analyzed on an as-treated basis. Gastrointest Endosc. 2013;78:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Kim JS, Chung MW, Chung CY, Park HC, Ryang DY, Myung DS, Cho SB, Lee WS, Joo YE. The need for second-look endoscopy to prevent delayed bleeding after endoscopic submucosal dissection for gastric neoplasms: a prospective randomized trial. Gut Liver. 2014;8:480-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Kim HH, Park SJ, Park MI, Moon W. Clinical impact of second-look endoscopy after endoscopic submucosal dissection of gastric neoplasms. Gut Liver. 2012;6:316-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 622] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Guglielmi A, Ruzzenente A, Sandri M, Kind R, Lombardo F, Rodella L, Catalano F, de Manzoni G, Cordiano C. Risk assessment and prediction of rebleeding in bleeding gastroduodenal ulcer. Endoscopy. 2002;34:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Kim BJ, Park MK, Kim SJ, Kim ER, Min BH, Son HJ, Rhee PL, Kim JJ, Rhee JC, Lee JH. Comparison of scoring systems for the prediction of outcomes in patients with nonvariceal upper gastrointestinal bleeding: a prospective study. Dig Dis Sci. 2009;54:2523-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Goto O, Fujishiro M, Oda I, Kakushima N, Yamamoto Y, Tsuji Y, Ohata K, Fujiwara T, Fujiwara J, Ishii N. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci. 2012;57:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Nakamura M, Nishikawa J, Hamabe K, Nishimura J, Satake M, Goto A, Kiyotoki S, Saito M, Fukagawa Y, Shirai Y. Risk factors for delayed bleeding from endoscopic submucosal dissection of gastric neoplasms. Scand J Gastroenterol. 2012;47:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open. 2014;4:e004587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (35)] |
| 19. | Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 798] [Article Influence: 23.5] [Reference Citation Analysis (0)] |