Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13473
Peer-review started: June 3, 2015
First decision: July 14, 2015
Revised: July 23, 2015
Accepted: November 9, 2015
Article in press: November 9, 2015
Published online: December 28, 2015
Processing time: 44 Days and 19.1 Hours
AIM: To investigate the effect of Golgi phosphorylation protein 3 (GOLPH3) expression on cell apoptosis, angiogenesis and prognosis in colorectal cancer (CRC).
METHODS: The expression of GOLPH3 in CRC tissues and normal colorectal mucosae was determined by immunohistochemistry in 62 patients. In addition, immunohistochemistry was also carried out to detect the expression of vascular endothelial growth factor (VEGF), CD34 and microvessel density (MVD). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay was used to determine the apoptotic index (AI). The Kaplan-Meier method was used to analyze the relationship between GOLPH3 expression and survival in another 123 CRC cases.
RESULTS: Compared with normal colorectal mucosae, a notably higher level of GOLPH3 protein expression was identified in CRC tissues (53.2% vs 24.2%, P < 0.05). Positive GOLPH3 expression was significantly associated with tumor invasion depth, TNM stage, and lymph node metastasis (P = 0.001; P = 0.020; P = 0.020; P < 0.05, respectively), but not with tumor length, tumor site, and age (P = 0.363; P = 0.819; P = 0.599; P > 0.05, respectively). VEGF expression and MVD in GOLPH3-positive CRC was significantly higher than in GOLPH3-negative CRC (VEGF: 69.7% vs 31.0%; MVD: 21.45 ± 9.39 vs 14.24 ± 8.97; P < 0.05). GOLPH3 expression was negatively correlated with AI in CRC as shown by Spearman correlation analysis (r = -0.320, P < 0.05). The 5-year survival rate in GOLPH3-negative CRC (69.4%) was significantly higher than in GOLPH3-positive CRC (48.6%) (log-rank test, P < 0.05).
CONCLUSION: High expression of GOLPH3 is found in CRC tissues. GOLPH3 expression may be a novel prognostic marker for CRC patients.
Core tip: Golgi phosphorylation protein 3 (GOLPH3) was identified as a Golgi membrane protein initially and as a new oncogene in recent years. This study reports a notably higher level of GOLPH3 expression in colorectal cancer (CRC) tissues compared with normal colorectal mucosae. Positive GOLPH3 expression was significantly associated with tumor invasion depth, TNM stage, and lymph node metastasis. In addition, GOLPH3 expression was correlated with cell apoptosis and angiogenesis. Our data suggest that GOLPH3 expression may be a novel prognostic marker for patients with CRC.
- Citation: Guo YT, Qiu CZ, Huang ZX, Yu WS, Yang XF, Wang MZ. Correlational research of Golgi phosphorylation protein 3 expression in colorectal cancer. World J Gastroenterol 2015; 21(48): 13473-13479
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13473.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13473
Colorectal cancer (CRC) is a serious threat to humans, and the incidence and mortality rate of CRC are high in many countries. At present, complete resection is still the main treatment option, however, despite continuing improvements in surgical techniques, radiotherapy and chemotherapy, almost half of patients who receive radical surgery die of tumor metastasis and recurrence. Thus, it is imperative to identify a predictive index of CRC recurrence or metastasis. In addition, an understanding of the molecular regulatory mechanism associated with CRC invasion and metastasis is of great importance to guide clinical diagnosis and treatment[1].
The occurrence and development of CRC requires the participation of multiple genes, but there is still a lack of objective molecular markers to help in the early diagnosis of tumor and prognosis. Thus, it would be of great significance to further explore the pathogenesis of CRC and the resistance mechanism, and identify new targets for tumor targeting therapy.
Golgi phosphorylation protein 3 (GOLPH3), which was identified as a Golgi membrane protein initially, and as a new oncogene in recent years, is found in a number of organisms and plays a decisive part in morphology and Golgi trafficking[2-4]. The mammalian target of rapamycin (mTOR) is a key point in the PI3K/AKT/mTOR signaling pathway, and is associated with tumor growth and proliferation[3,5]. Recent research showed that GOLPH3 can promote the proliferation of cancer cells by activating mTOR and may be a new oncogene[5]. However, research on the GOLPH3 gene and apoptosis in CRC is relatively rare.
In this study, we measured the expression of GOLPH3 in CRC tissues to investigate its relationship with cell apoptosis and angiogenesis, and explored the effects of GOLPH3 on the development and prognosis of CRC.
Group I: This group included 62 CRC patients who underwent surgical resection from February 2012 to July 2012 in Fujian Medical University the Second Affiliated Hospital, and had available tumor samples. A total of 62 CRC tissues were acquired from resected normal mucosae and tumors. The samples were then frozen in liquid nitrogen and stored at -80 °C. The TNM stages were determined using classification guidelines by the American Joint Committee on Cancer in the 7th edition. The clinicopathological characteristics of the patient cohort are summarized in Table 1. Each patient had a complete medical record and did not receive chemoradiotherapy before surgery.
| Clinicopathologic parameters | Total | GOLPH3 expression | χ2 | Pvalue | |
| Negative | Positive | ||||
| Age (yr) | |||||
| ≤ 60 | 30 | 13 | 17 | 0.276 | 0.599 |
| > 60 | 32 | 16 | 16 | ||
| Tumor site | |||||
| Right | 11 | 6 | 5 | 0.399 | 0.819 |
| Hemicolon | |||||
| Left hemicolon | 12 | 5 | 7 | ||
| Rectum | 39 | 18 | 21 | ||
| Tumor length (cm) | |||||
| ≤ 5 | 40 | 17 | 23 | 0.827 | 0.363 |
| > 5 | 22 | 12 | 10 | ||
| Differentiation | |||||
| G1-G2 | 51 | 27 | 24 | 4.391 | 0.036 |
| G3 | 11 | 2 | 9 | ||
| Clinical stage | |||||
| I-II | 33 | 20 | 13 | 5.422 | 0.020 |
| III | 29 | 9 | 20 | ||
| Depth of invasion | |||||
| Inside serous membrane | 14 | 12 | 2 | 11.014 | 0.001 |
| Outside serous membrane | 48 | 17 | 31 | ||
| Lymph node metastasis | |||||
| Yes | 29 | 9 | 20 | 5.422 | 0.020 |
| No | 33 | 20 | 13 | ||
Group II: This group included 123 CRC patients who underwent surgical resection from January 2005 to December 2009 in the Second Affiliated Hospital of Fujian Medical University. All patients (72 males and 51 females) had complete medical records and did not receive chemoradiotherapy before surgery. The age range of the CRC patients was 28-87 years (mean 64.04 ± 12.52 years). All patients were followed up by phone or clinic visit for 1 to 93 mo, and the median follow-up period was 62 mo.
Rabbit anti-GOLPH3 (ab98023) polyclonal antibody (Abcam); mouse anti-vascular endothelial growth factor (VEGF) monoclonal antibody (VGl) (Abcam); and mouse anti-CD34 monoclonal antibody (QBEnd/10) (Abcam) were used. The secondary antibody was biotin-labeled Goat anti-rabbit IgG (Abcam). The immunohistochemical kit was from Beijing Zhongshan Biotechnology Co., Ltd (Bejing, China). The in situ apoptosis detection kit was from Roche in Shanghai, China.
Immunohistochemistry was performed according to standard protocols, and was used to detect GOLPH3, VEGF and CD34 protein expression in 62 CRC cases, and GOLPH3 protein expression was detected in another 123 CRC cases. Sections from the paraffin-embedded samples were dried overnight at 37 °C, and then deparaffinized with xylene and rehydrated. The sections were treated with 3% hydrogen peroxide for 20 min to inhibit the activity of endogenous peroxidase and then microwaved for antigenic retrieval using ethylene diamine tetraacetic acid (EDTA) buffer. Nonspecific antibody binding was blocked. Subsequently, the sections were incubated with anti-GOLPH3 antibody (1:100), anti-VEGF antibody and anti-CD34 antibody overnight at 4°C. After rinsing, the sections were incubated with biotin-labeled secondary antibody bound to a streptavidin-horseradish peroxidase complex. The peroxidase reaction was developed in DAB buffer substrate for visualization. The sections were then counterstained, mounted, and observed under microscope. Phosphate buffered saline (PBS) replaced the primary antibody as a negative control.
We chose the “no dead zone” of the 62 CRC tissues, and followed the instructions in the apoptosis detection kit.
Immunohistochemical results were evaluated by two pathologists independently who were blinded to patient data, and any disagreement was resolved by consensus. The protein expression of GOLPH3 and VEGF was evaluated by combining the intensity of staining and the proportion of positively stained tumor cells, and the final score was calculated as follows. The scores for the proportion of positively stained tumor cells were: 0, < 5%; 1, 5%-25%; 2, 25%-50%; 3, 50%-75%; and 4, ≥ 75%; and the scores for staining intensity were: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellow brown); and 3, strong staining (brown). The staining index of GOLPH3 and VEGF in CRC tissues was obtained by multiplying the two scores for each sample. The final scores of 0, 1, 2, 3, 4, 6, 9, or 12[6] were obtained. A maximum score of 4 was defined as negative expression.
Microvessels were recorded by counting CD34 positively stained endothelial cells. The microvessel density (MVD) was assessed by two pathologists independently, and any disagreements were resolved by consensus. In the few instances of discrepant scoring, two pathologists recounted and an average was obtained. The highest microvascular density areas were selected under low microscope power (100 × magnification) and the vessels in five fields were counted at high microscope power (400 × magnification). MVD measurements were obtained by calculating the average of the counts in five fields. Vessels were excluded if they had thick muscular walls or a large lumen. The calculated results were rounded.
The cell nucleus stained yellow-brown was considered positive for the corresponding protein expression. The apoptosis index (AI) was defined as the ratio of positively stained tumor cells to all tumor cells. For each case, 1000 randomly selected tumor cells in 5 areas were counted under 400 × magnification.
Statistical analysis was performed using the statistical package for Social Sciences, version 19.0 (SPSS, Inc., Chicago, IL, United States). The relationships between GOLPH3 expression and both clinicopathological features and VEGF were analyzed by the χ2 test. Pearson correlation analysis was used to determine the relationship between intratumoral MVD and GOLPH3 protein expression, whereas the factors associated with AI were analyzed using the t test and Spearman correlation analysis. The Kaplan-Meier method was used to estimate the relationship between GOLPH3 expression and prognosis of CRC, and the differences were compared using the log-rank test. P < 0.05 was considered statistically significant.
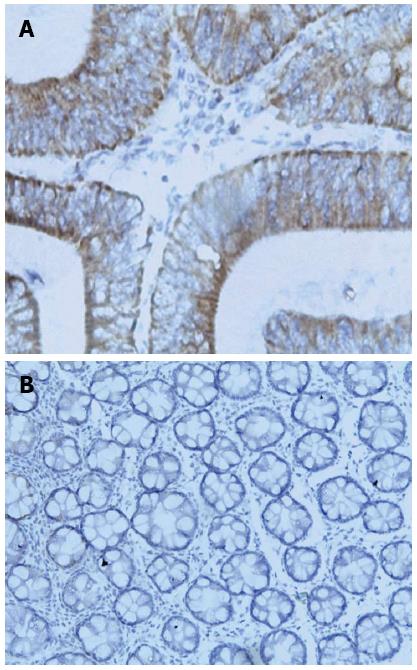
GOLPH3 expression in CRC tissues and normal colorectal mucosae: Immunohistochemistry results indicated that GOLPH3 expression was mainly located in the cytoplasm of CRC tissues (Figure 1A). The positive expression rate of GOLPH3 was 53.2% (33/62) in CRC tissues, and was markedly higher than that in normal colorectal mucosae [24.2% (15/62); P < 0.01] (Figure 1B).
GOLPH3 expression and its association with clinicopathological characteristics in CRC tissues: Compared with moderately-to-well differentiated, no metastatic lymph nodes, and stage I-II CRCs, GOLPH3 expression level was significantly higher in poorly differentiated, metastatic lymph nodes, and stage III CRCs (P < 0.05), respectively. However, GOLPH3 expression was not significantly correlated with other clinicopathologic parameters, such as age, site, length of the invasive tumor (P > 0.05) (Table 1).
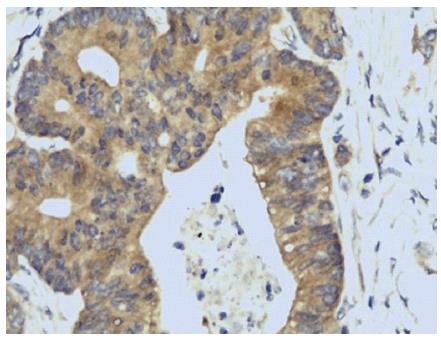
Correlation between GOLPH3 and VEGF expression in CRC tissues: Immunohistochemistry results indicated that VEGF expression was mainly located in the cytoplasm, and showed tan-grains (Figure 2). The rate of VEGF positive expression was 69.7% (23/33) in CRC with GOLPH3 positive expression, and was significantly higher than that in CRC with GOLPH3 negative expression (31.0%, 9/29) (I2 = 9.239, P = 0.002). Thus, immunohistochemistry method showed that GOLPH3 expression was associated with VEGF expression (P = 0.05).
Correlation between GOLPH3 and MVD in CRC tissues: The expression of CD34 was mainly located in vascular endothelial cytoplasm. The MVD in the GOLPH3 negative expression group (29/62) was 14.24 ± 8.97 in CRC tissues, and was significantly lower than that in the GOLPH3 positive expression group (33/62) (MVD = 21.45 ± 9.39) (t = -3.090, P < 0.01).
Relationship between GOLPH3 expression and apoptosis in CRC tissues. AI was 1.531 ± 0.118 in CRC tissues with GOLPH3 positive expression, and was significantly lower than that in CRC tissues with GOLPH3 negative expression (2.138 ± 0.186) (t = 2.824, P < 0.01). The staining score for GOLPH3 expression was negatively correlated with AI in CRC tissues using Spearman correlation analysis (r = -0.320, P < 0.05).
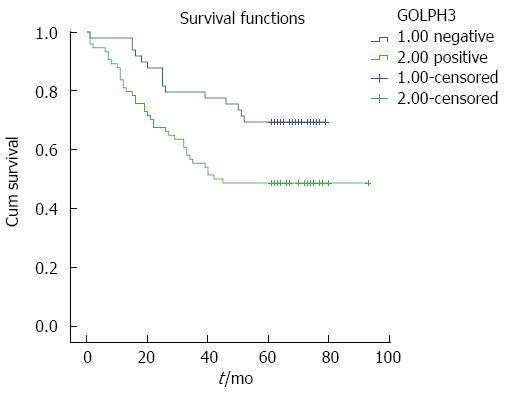
High GOLPH3 expression was related to poor prognosis in CRC. In this group, the overall 5-year survival rate was 56.9%. The CRC patients were divided into two groups according to GOLPH3 expression levels: 5-year survival rate was 48.6% in the GOLPH3 positive expression group, and was 69.4% in the GOLPH3 negative expression group (Figure 3). The log-rank test showed that the survival time in the GOLPH3 positive expression group was significantly lower than that in the GOLPH3 negative expression group (P = 0.014).
Cancer progression is a very complex process, and includes tumor cell transformation, growth, invasion, angiogenesis, dissemination and survival in the circulation, and subsequent adhesion and colonization in the distant organ or tissue. Among these events, aberrant cell apoptosis, proliferation, and angiogenesis play crucial roles in the growth and dissemination of tumors, and cancer progression[7,8].
GOLPH3, also known as GMx33, is located on chromosome 5p13, and its encoded protein is a highly conserved 34 kDa protein initially identified through proteomic characterization of the Golgi apparatus, and plays a role in protein transmission[9]. In recent years, high expression of GOLPH3 has been shown to be associated with poor prognosis in many cancers, such as breast cancer[10], esophageal squamous cell carcinoma[11], oral tongue cancer[12], gastric cancer[6], prostate cancer[13], glioblastoma multiforme[14], gliomas[15], and rhabdomyosarcoma[16]. In the present study, it was similarly demonstrated that the overexpression of GOLPH3 in CRC tissues could reflect the degree of malignancy of CRC, and was correlated with poor prognosis, which may be a factor in the biological behavior and prognosis of CRC.
At present, great progress has been made in the research on the mechanism of GOLPH3. Phosphatidylinositol-4-phosphate (PI4P) is known to be highly enriched at the trans-Golgi[17] and is required for Golgi-to-plasma membrane trafficking[18-20]. GOLPH3 plays a critical role in Golgi-to-plasma membrane trafficking as a novel effector of PI4P[3]. In addition, GOLPH3 tightly interacts with an unconventional myosin, MYO18A, recruiting it to the Golgi. MYO18A binds to F-actin and the complex applies a tensile force that pulls on the Golgi membrane[3,21,22]. A series of studies indicated that GOLPH3 protein could cause abnormal secretion of glycoprotein by adjusting the function of glycosyltransferases[23,24]. At present, glycosylation has been confirmed to be related to the growth, adhesion, migration, invasion and immune recognition of tumor cells. Abnormal glycosylation can increase the aggressiveness of tumors. Moreover, some authors observed that overexpression of GOLPH3, by driving the Golgi DNA damage response, confers resistance to killing by DNA damaging therapeutic agents, and may explain its role in determining the poor prognosis of a variety of cancers[25].
Angiogenesis is essential for cancer growth and metastasis, and provides tumor cells with enough nutrients and oxygen, which is regulated by various factors. VEGF is an important factor, which induces tumor vessel formation. Research shows that the expression of VEGF is closely related to the development and infiltration of CRC[26,27]. Our results showed that the level of GOLPH3 protein expression in VEGF negative-expression cases was significantly lower than that in VEGF positive-expression cases, and the GOLPH3 expression level was positively correlated with MVD. These findings demonstrated that GOLPH3 expression can upregulate VEGF expression, which may promote tumor angiogenesis and growth in CRC. GOLPH3 overexpression can upregulate HIF-1 expression by activating the PI3K/AKT/mTOR signaling pathway and promote VEGF overexpression, which results in the migration of endothelial cells to generate new blood vessels to increase the blood flow to tumor cells. Moreover, activation of the AKT signaling pathway can also activate endothelial nitric oxide synthase by affecting neural phospholipase, which results in persistent nitric oxide production and the promotion of CRC angiogenesis.
Our results showed that GOLPH3 expression was negatively correlated with AI in CRC tissues and may inhibit cell apoptosis. GOLPH3 protein can activate the AKT signaling pathway[9], and the activated AKT signaling pathway can inhibit the activity of caspase-9 by a phosphorylating reaction, resulting in a cascade reaction, including caspase-2, 3, 6, 8, and 10[28]. This series of reactions plays a role in resistance to apoptosis.
In conclusion, high expression of GOLPH3 was found in CRC. GOLPH3 overexpression may participate in the occurrence and development of CRC by inhibiting apoptosis and promoting angiogenesis, and may be used as a prognostic predictor for CRC patients.
The authors would like to thank Jian-Long Qiu from the Pathology Department of the Second Affiliated Hospital of Fujian Medical University, who offered help with the paraffin sections.
Golgi phosphorylation protein 3 (GOLPH3) was initially identified as a Golgi membrane protein and as a new oncogene in recent years, and plays a crucial role in Golgi trafficking and morphology. Recent research showed that GOLPH3 can promote the proliferation of cancer cells by activating mTOR. Colorectal cancer (CRC) is one of the most common malignant tumors of the human digestive system, thus, it is important to identify a predictive index of CRC recurrence or metastasis.
High expression of GOLPH3 has been shown to be associated with poor prognosis in breast cancer, esophageal squamous cell carcinoma, oral tongue cancer, gastric cancer, prostate cancer, gliomas, and rhabdomyosarcoma. Some authors observed that overexpression of GOLPH3, by driving the Golgi DNA damage response, confers resistance to killing by DNA damaging therapeutic agents, and may explain its role in determining the poor prognosis of a variety of cancers.
This study reports, for the first time, the relationship between the expression of GOLPH3 and CRC by detecting cell apoptosis and angiogenesis. A notably higher level of GOLPH3 protein expression was found in CRC tissues compared with normal colorectal mucosae. GOLPH3 expression was correlated with cell apoptosis and angiogenesis. The 5-year survival rate in GOLPH3-negative CRC was significantly higher than that in GOLPH3-positive CRC.
High expression of GOLPH3 was positively associated with CRC. Therefore, GOLPH3 expression may be a novel prognostic marker for patients with CRC.
Vascular endothelial growth factor (VEGF) is secreted by certain tumor cells, inducing the formation of tumor blood vessels and stimulating tumor growth. VEGF is a strong angiogenesis factor. TUNEL is a special technique used to determine cell apoptosis, which is necessary for the growth and dissemination of tumors, and cancer progression.
The authors demonstrate the urgency for discovery of novel molecular markers for CRC; and their work strives to address this need. Notably, the role of GOLPH3 in cancer has only been recognized recently, and there is need to further explore how its expression affects the behavior of different types of cancer, including CRC.
P- Reviewer: Zou XF S- Editor: Yu J L- Editor: FIlipodia E- Editor: Zhang DN
| 1. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1176] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 2. | Bell AW, Ward MA, Blackstock WP, Freeman HN, Choudhary JS, Lewis AP, Chotai D, Fazel A, Gushue JN, Paiement J. Proteomics characterization of abundant Golgi membrane proteins. J Biol Chem. 2001;276:5152-5165. [PubMed] |
| 3. | Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 503] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 4. | Scott KL, Chin L. Signaling from the Golgi: mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res. 2010;16:2229-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Scott KL, Kabbarah O, Liang MC, Ivanova E, Anagnostou V, Wu J, Dhakal S, Wu M, Chen S, Feinberg T. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature. 2009;459:1085-1090. [PubMed] |
| 6. | Hu BS, Hu H, Zhu CY, Gu YL, Li JP. Overexpression of GOLPH3 is associated with poor clinical outcome in gastric cancer. Tumour Biol. 2013;34:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Kim ER, Kim YH. Clinical application of genetics in management of colorectal cancer. Intest Res. 2014;12:184-193. [PubMed] |
| 9. | Snyder CM, Mardones GA, Ladinsky MS, Howell KE. GMx33 associates with the trans-Golgi matrix in a dynamic manner and sorts within tubules exiting the Golgi. Mol Biol Cell. 2006;17:511-524. [PubMed] |
| 10. | Zeng Z, Lin H, Zhao X, Liu G, Wang X, Xu R, Chen K, Li J, Song L. Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin Cancer Res. 2012;18:4059-4069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Wang JH, Chen XT, Wen ZS, Zheng M, Deng JM, Wang MZ, Lin HX, Chen K, Li J, Yun JP. High expression of GOLPH3 in esophageal squamous cell carcinoma correlates with poor prognosis. PLoS One. 2012;7:e45622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Li H, Guo L, Chen SW, Zhao XH, Zhuang SM, Wang LP, Song LB, Song M. GOLPH3 overexpression correlates with tumor progression and poor prognosis in patients with clinically N0 oral tongue cancer. J Transl Med. 2012;10:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Hua X, Yu L, Pan W, Huang X, Liao Z, Xian Q, Fang L, Shen H. Increased expression of Golgi phosphoprotein-3 is associated with tumor aggressiveness and poor prognosis of prostate cancer. Diagn Pathol. 2012;7:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Xu T, Qin R, Yan Y, Chen C, Chen Y, Yu H, Xia C, Lu Y, Ding X. Overexpression of Golgi phosphoprotein-3 (GOLPH3) in glioblastoma multiforme is associated with worse prognosis. J Neurooncol. 2012;110:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Li XY, Liu W, Chen SF, Zhang LQ, Li XG, Wang LX. Expression of the Golgi phosphoprotein-3 gene in human gliomas: a pilot study. J Neurooncol. 2011;105:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Kunigou O, Nagao H, Kawabata N, Ishidou Y, Nagano S, Maeda S, Komiya S, Setoguchi T. Role of GOLPH3 and GOLPH3L in the proliferation of human rhabdomyosarcoma. Oncol Rep. 2011;26:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393-404. [PubMed] |
| 18. | Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294-34300. [PubMed] |
| 19. | Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523-525. [PubMed] |
| 20. | Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299-310. [PubMed] |
| 21. | Bishé B, Syed GH, Field SJ, Siddiqui A. Role of phosphatidylinositol 4-phosphate (PI4P) and its binding protein GOLPH3 in hepatitis C virus secretion. J Biol Chem. 2012;287:27637-27647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Ng MM, Dippold HC, Buschman MD, Noakes CJ, Field SJ. GOLPH3L antagonizes GOLPH3 to determine Golgi morphology. Mol Biol Cell. 2013;24:796-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Schmitz KR, Liu J, Li S, Setty TG, Wood CS, Burd CG, Ferguson KM. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev Cell. 2008;14:523-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol. 2009;187:967-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Buschman MD, Rahajeng J, Field SJ. GOLPH3 links the Golgi, DNA damage, and cancer. Cancer Res. 2015;75:624-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Jang MJ, Jeon YJ, Kim JW, Cho YK, Lee SK, Hwang SG, Oh D, Kim NK. Association of VEGF and KDR single nucleotide polymorphisms with colorectal cancer susceptibility in Koreans. Mol Carcinog. 2013;52 Suppl 1:E60-E69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Yin Y, Cao LY, Wu WQ, Li H, Jiang Y, Zhang HF. Blocking effects of siRNA on VEGF expression in human colorectal cancer cells. World J Gastroenterol. 2010;16:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209-3214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |