Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13438
Peer-review started: October 20, 2015
First decision: November 9, 2015
Revised: November 12, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: December 28, 2015
Processing time: 65 Days and 14.8 Hours
AIM: To investigate the role of protein kinase C (PKC)-δ activation in the pathogenesis of acute liver failure (ALF) in a well-characterized mouse model of D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced ALF.
METHODS: BALB/c mice were randomly assigned to five groups, and ALF was induced in mice by intraperitoneal injection of D-GaIN (600 mg/kg) and LPS (10 μg/kg). Kaplan-Meier method was used for survival analysis. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels at different time points within one week were determined using a multiparameteric analyzer. Serum levels of high-mobility group box 1 (HMGB1), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-10 as well as nuclear factor (NF)-κB activity were determined by enzyme-linked immunosorbent assay. Hepatic morphological changes at 36 h after ALF induction were assessed by hematoxylin and eosin staining. Expression of PKC-δ in liver tissue and peripheral blood mononuclear cells (PBMCs) was analyzed by Western blot.
RESULTS: The expression and activation of PKC-δ were up-regulated in liver tissue and PBMCs of mice with D-GalN/LPS-induced ALF. Inhibition of PKC-δ activation with rottlerin significantly increased the survival rates and decreased serum ALT/AST levels at 6, 12 and 24 h compared with the control group (P < 0.001). Rottlerin treatment also significantly decreased serum levels of HMGB1 at 6, 12, and 24 h, TNF-α, IL-6 and IL-1 β at 12 h compared with the control group (P < 0.01). The inflammatory cell infiltration and necrosis in liver tissue were also decreased in the rottlerin treatment group. Furthermore, sphingosine kinase 1 (SphK1) dependent PKC-δ activation played an important role in promoting NF-κB activation and inflammatory cytokine production in ALF.
CONCLUSION: SphK1 dependent PKC-δ activation plays an important role in promoting NF-κB activation and inflammatory response in ALF, and inhibition of PKC-δ activation might be a potential therapeutic strategy for this disease.
Core tip: In this study, we found protein kinase C (PKC)-δ expression and activation in liver tissue or peripheral blood mononuclear cells of mice with acute liver failure (ALF). Inhibition of PKC-δ activation attenuated ALF in this animal model. Furthermore, sphingosine kinase 1 (SphK1) was required for PKC-δ activation in LPS-stimulated macrophages and the ALF mouse model. Our findings suggest that SphK1 dependent PKC-δ activation plays an important role in ALF, and inhibition of PKC-δ activation might be a potential therapeutic strategy for this disease.
- Citation: Lei YC, Yang LL, Li W, Luo P. Sphingosine kinase 1 dependent protein kinase C-δ activation plays an important role in acute liver failure in mice. World J Gastroenterol 2015; 21(48): 13438-13446
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13438
Acute liver failure (ALF) is a severe clinical syndrome characterized by sudden and massive death of liver cells resulting from a variety of hepatic diseases, and can lead to hepatic encephalopathy, coagulopathy and multi-organ failure[1]. Despite advances in the development of new treatments for ALF, little progress has been made in seeking efficient interventions that can improve the outcome of this disease. Currently, ALF is still associated with an extremely high mortality and often demands urgent liver transplantation due to limited therapeutic options[1-3]. Although liver cells are the major target cells in ALF, inflammatory cells, especially macrophages and neutrophils, dominate the manifestation of liver injury. Increasing the level of bacterial lipopolysaccharide (LPS) in the blood may induce inflammatory cell activation. There is increasing evidence suggesting that the predominant mechanism responsible for the development of ALF is activation of the systemic immune response, through release of proinflammatory cytokines and damage-associated molecular patterns (DAMPs) as a result of massive hepatocyte necrosis, which in turn plays a pivotal role in the clinical course and outcome in ALF patients[4]. Large studies have demonstrated that the presence of systemic inflammatory response syndrome (SIRS) in ALF is associated with a poor prognosis[5]. Patients with ALF have high circulating concentrations of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6[6,7].
Protein kinase C (PKC)-δ has been identified as a critical inflammatory regulator and is instrumental in neutrophil recruitment, sequestration, and activation. In neutrophils, PKC-δ controls proinflammatory events and regulates cytokine-elicited oxygen radical production, degranulation, and activation of nuclear factor-κB (NF-κB)[8,9]. PKC-δ inhibition prevented neutrophil adherence and migration[10]. In PKC-δ null mice, neutrophil adhesion, migration, oxygen radical generation, and degranulation are limited[11]. Thus, PKC-δ may have an important regulatory role in the inflammatory response. PKC-δ is activated by multiple proinflammatory stimuli, including cytokines, such as TNF-α and IL-1, and PAMPs, such as LPS[12,13]. PKC-δ is an important component of proinflammatory signaling pathways that regulate activation of the transcription factor NF-κB. NF-κB regulates gene expression of chemokines, adhesion molecules, and cytokines, which can initiate and perpetuate inflammation and thus function in a positive-feedback loop.
Sphingosine kinases are intracellular signaling enzymes that catalyze the formation of the lipid mediator sphingosine-1-phosphate[14]. Several proinflammatory stimuli, including LPS, TNF-α, anaphylatoxin C5a and immune complexes, activate sphingosine kinase 1 (SphK1) on human neutrophils and macrophages and promote several proinflammatory responses[15-17]. A recent study demonstrated that SphK1 plays a critical role in endotoxin signaling and sepsis-induced inflammatory responses by activating NF-κB[18]. SphK1 expression was strongly up-regulated and rapidly activated in macrophages stimulated with LPS. SphK1 was required to activate NF-κB and to induce the secretion of proinflammatory cytokines or high mobility group box 1 (HMGB1) in LPS-stimulated macrophages, and SphK1 mediated TLR-triggered NF-κB activation in macrophages through PKC-δ activation[18]. The ability of PKC-δ to mediate the secretion of proinflammatory mediators prompted us to investigate its role in systemic inflammatory response in ALF. Based on the fact that NF-κB activation is the common pathway in proinflammatory cytokine secretion, we speculated that SphK1-mediated PKC-δ activation may play an important role in ALF.
In this study, we demonstrated PKC-δ expression and activation in liver tissue or peripheral blood mononuclear cells (PBMCs) of mice with D-galactosamine (D-GalN)/LPS-induced ALF. Inhibition of PKC-δ with rottlerin ameliorated ALF in this animal model. Furthermore, SphK1 was required for PKC-δ activation in LPS-stimulated macrophages and the ALF mouse model. These results indicate that SphK1 dependent PKC-δ activation plays an important role in ALF.
Male BALB/c mice aged 8 wk and weighing 20 ± 0.5 g were obtained from the Experimental Animal Center of Nanchang University, Nanchang, China. The mice were handled and treated in accordance with the strict guiding principles of the National Institution of Health for experimental care and use of animals. ALF was induced in mice by intraperitoneal injection of D-GalN (600 mg/kg; Sigma-Aldrich) and LPS (10 μg/kg; Sigma-Aldrich) as previously described. At 36 h following ALF induction, a portion of the animals were sacrificed to harvest liver tissue for hematoxylin and eosin (HE) staining. N,N-dimethylsphingosine (DMS; Sigma-Aldrich), a PKC-δ specific chemical inhibitor, and rottlerin (Sigma-Aldrich), a SphK1 specific chemical inhibitor, were intraperitoneally injected 0.5 h prior to ALF induction.
Serum samples were stored at -80 °C until analysis. Serum ALT and AST levels were measured using a multiparameteric analyzer (AU 5400, Olympus, Japan). Serum levels of TNF-α, IL-1β, IL-6, IL-10 and HMGB1 were determined using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, United States).
Proteins (40 μg) from total tissue or cell lysates/samples were resolved on 10% polyacrylamide gels under denaturing conditions and then transferred to 0.45 μm nitrocellulose membranes. The blots were probed using a polyclonal anti-PKC-δ antibody (Santa Cruz Biotechnology, United States), and an anti-β-actin antibody (Santa Cruz Biotechnology) was used for confirming equal protein loading. Bands were visualized using a horseradish peroxidase-conjugated anti-IgG secondary antibody and the ECL Western Blotting Detection System (GE Healthcare, United Kingdom).
Frozen lung tissue (0.15 g) was homogenized in 3 mL buffer containing 10 mmol/L Hepes (pH 7.4), 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L Na-orthovanadate, 20 μmol/L 4-(2-aminoethyl)-benzenesulfonyl fluoride, 1% Triton X-100, 5 μg/mL leupeptin, phosphatase inhibitor cocktail and protease inhibitor cocktail (Sigma Chemical, St. Louis, MO, United States). Protein concentrations of the cell lysates were determined using a bicinchoninic acid protein assay kit, according to the manufacturer’s instructions (Thermo Scientific, Rockford, IL, United States). Proteins (30 μg/lane) were separated on 4%-12% SDS-PAGE gels and transferred to nitrocellulose membranes. PKC-δ (Thr505) phosphorylation was determined by immunoblotting using a phospho-specific PKC-δ (Thr505) antibody (Cell Signaling Technology, Beverly, MA, United States) as described previously. Equal protein loading was confirmed by reprobing membranes using a PKC-δ antibody that recognizes both phosphorylated and nonphosphorylated forms of PKC-δ (Santa Cruz Biotechnology).
Following LPS stimulation, NF-κB activity was analyzed using the Mercury TransFactor Profiling Kit-Inflammation kit (BD), following the manufacturer’s instructions. The enzymatic product was analyzed with a standard plate reader.
For morphological investigation, the livers of mice were carefully dissected out at 36 h following ALF induction and immersed in 10% phosphate-buffered formalin for 1 d. The specimens were then dehydrated through an ascending series of ethanol and cleared in toluene, before being embedded in paraffin. The tissue blocks were cut at 4 μm thickness using a Leica rotary microtome (Model 2165). Paraffin sections were mounted on albuminized glass slides by floating and flattening the sections in a water bath at 45 °C. The mounted sections were drained until dry and kept in an incubator at 30 °C. Paraffin sections were first dewaxed in two changes of xylene, passed through a descending series of alcohol, and were finally washed in deionized water, before staining with hematoxylin and eosin for 1-2 min at room temperature. The sections were rinsed three times in deionized water, dehydrated quickly through an ascending series of ethanol, and passed through xylene before being mounted with Permount. Slides were viewed with an Olympus microscope using 10 ×, 40 × and 100 × Uplan Apo lenses.
Data are expressed as the mean ± standard error of the mean. Statistical significance was determined by a two-tailed Student’s t-test or one-way analysis of variance (ANOVA), and, specifically, a log-rank test for survival analysis. A P-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 13.0 for Windows.
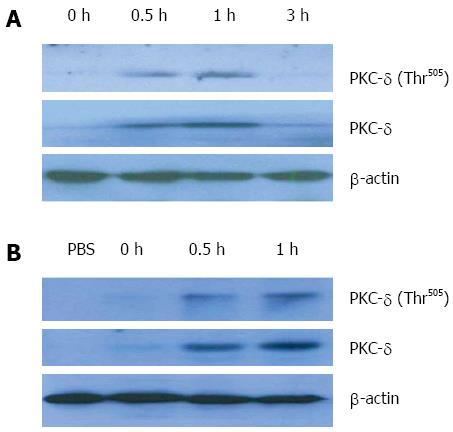
To dissect the role of PKC-δ in ALF, we first detected whether PKC-δ expression is triggered in GalN/LPS-induced ALF in vivo. Liver tissue samples were harvested at 0, 0.5, 1 and 3 h, respectively, after D-GalN/LPS injection. Compared with the negative control, the expression of PKC-δ in liver tissue was promptly increased at 0.5 h and 1 h, and then declined to the basal level after 3 h (Figure 1A). Activation of PKC-δ is a multistep process that can be assessed by quantifying phosphorylation of Thr505 in the PKC-δ activation loop. There was little phosphorylation of PKC-δ in liver tissue homogenates obtained at 0 and 3 h after ALF induction in mice. In contrast, liver tissues collected at 0.5 h and 1 h after GalN/LPS administration had a significant increase in PKC-δ phosphorylation. As expected, PKC-δ expression and phosphorylation in PBMCs of mice were also increased at 0.5 h and 1 h after ALF induction (Figure 1B). These results suggest that PKC-δ expression and activation in liver tissue and PBMCs are an early event in the acute phase of ALF in mice.
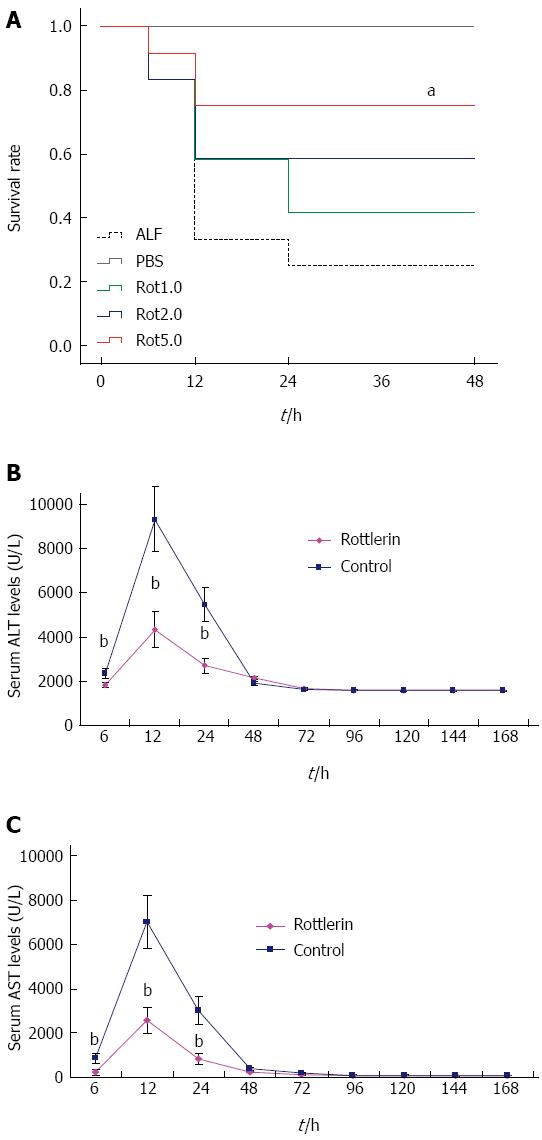
For survival analysis, five treatment groups of mice were used; group I received PBS only, and groups II to V were pretreated with four doses (0, 1, 2, and 5 μmol/L) of rottlerin (a PKC-δ specific chemical inhibitor), respectively, 0.5 h after D-GalN/LPS challenge. In group II, the mice began to die from 6 h, and only 25.0% (3/12) survived at 48 h. However, pretreatment with rottlerin increased the survival rate in a dose-dependent manner. The survival rates were 41.7% (5/12), 58.3% (7/12), and 75.0% (9/12) within 48 h in mice pretreated with 1, 2, and 5 μmol/L rottlerin, respectively. In group V, the survival rates were significantly increased compared with those of group II (P = 0.003) (Figure 2A). Since rottlerin at 5 μmol/L significantly protected the liver from ALF, we selected 5 μmol/L rottlerin for the following study.
We next investigated whether rottlerin decreases serum ALT/AST levels. In the surviving mice, the peak levels of ALT/AST were detected at 12 h, both in the control and rottlerin treatment groups. However, the maximum ALT/AST levels were reduced significantly in the rottlerin treatment group than in the control group (Figure 2B and C). The maximum ALT/AST levels at 12 h were reduced by 63.8% (P < 0.001) and 63.2% (P < 0.001), respectively, in the rottlerin treated group. The ALT/AST levels at 6 h and 24 h were also decreased significantly in the rottlerin treated group compared with the control group (P < 0.01). No significant differences were observed at any of other time points from 24 h to one week.
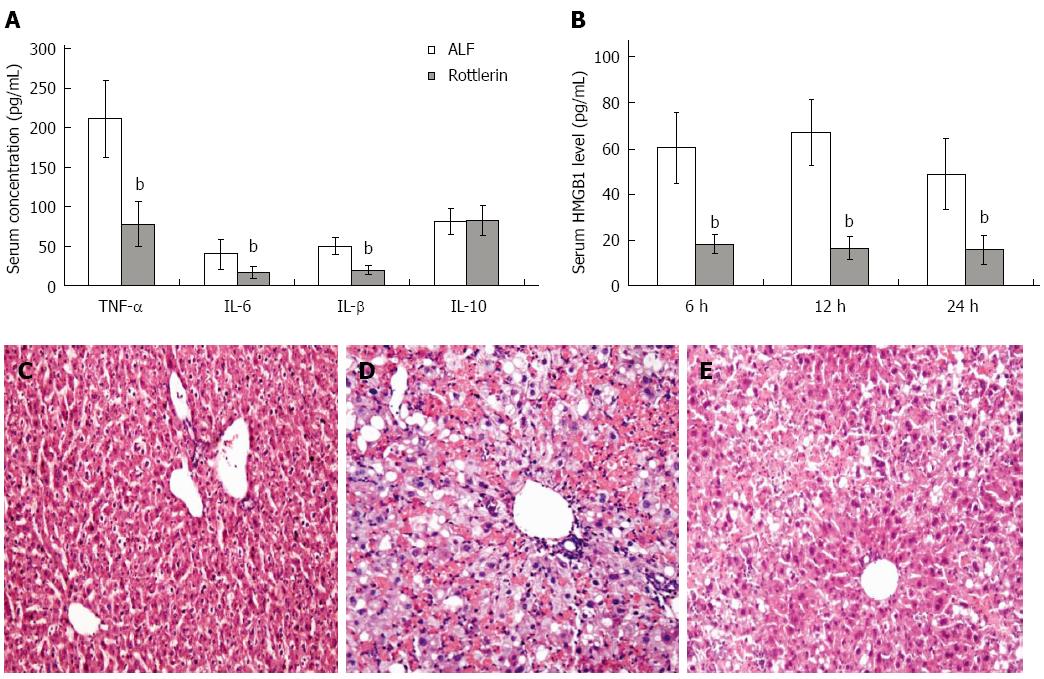
Analysis of serum cytokine levels revealed a significant decrease for TNF-α, IL-1β and IL-6 after rottlerin treatment (P < 0.01). However, level of the anti- inflammatory cytokine IL-10 was not increased in the rottlerin treatment group (Figure 3A). HMGB1 released by macrophages and damaged or necrotic cells functions as a DAMP and contributes to the pathogenesis of ALF. In the control group, serum HMGB1 level increased at 6 h after ALF induction, reached the peak at 12 h, and then fall down rapidly. Serum concentrations of HMGB1 were decreased at above three time points after rottlerin treatment (Figure 3B). These data demonstrate that inhibition of PKC-δ activity with rottlerin reduced liver inflammation and necrosis and decreased proinflammatory cytokine and HMGB1 levels.
Liver histology was normal in the PBS treated normal mice (Figure 3C). D-GalN/LPS treatment alone caused significant hepatic injury in mice at 36 h, where the necrosis areas were more than 50% of almost all lobules examined, and panlobular mononuclear leukocyte infiltration with cytoplasmic vacuolization and severe distortion of tissue architecture was observed (Figure 3D). However, rottlerin treated mice showed only small areas of necrotic and inflammatory cell infiltration (Figure 3E).
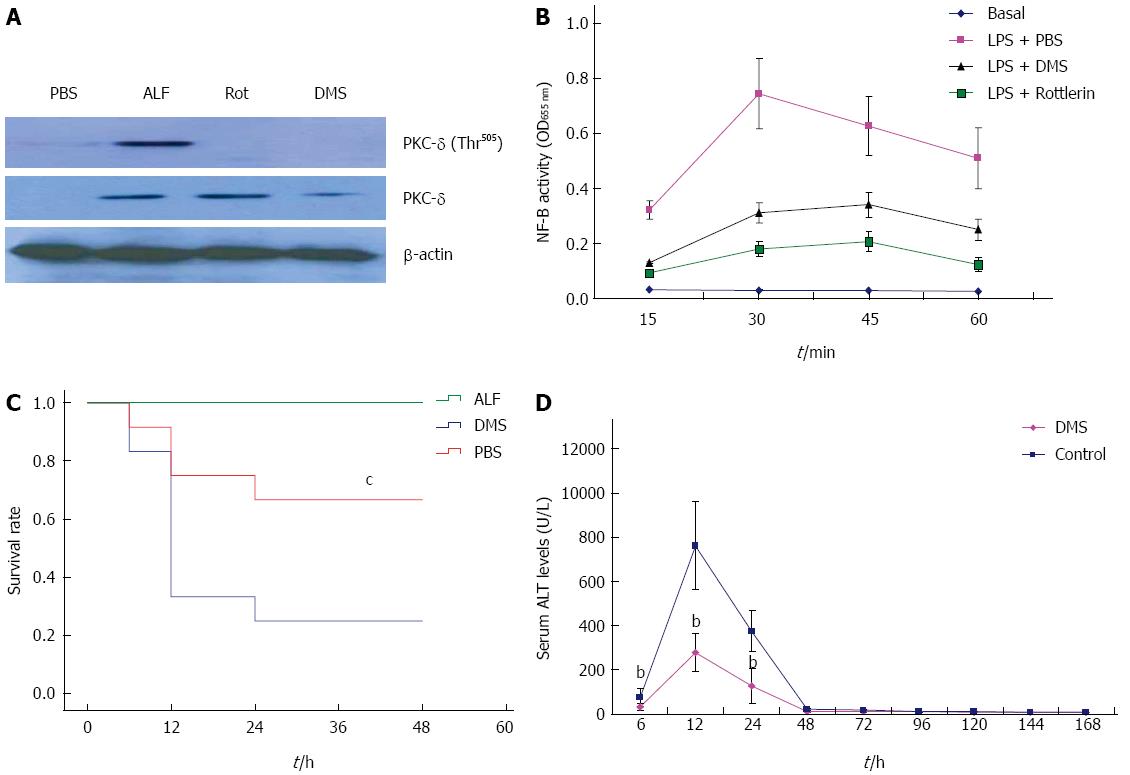
Sphk1 plays a critical role in LPS-induced proinflammatory cytokine release through NF-κB activation as well as PKC-δ. We speculated that SphK1 is the upstream regulator of PKC-δ activation. In order to verify this hypothesis, we first examined whether inhibition of SphK1 down-regulates PKC-δ expression and activation in liver tissue of mice with ALF. As expected, PKC-δ expression and activation were indeed inhibited in liver tissue of mice pretreated with DMS, a SphK1 specific inhibitor (Figure 4A). We next decided to examine whether SphK1 dependent PKC-δ activation results in NF-κB activation in macrophages. In LPS-stimulated macrophages, DMS as well as PKC-δ inhibitor, rottlerin, largely inhibited LPS-triggered NF-κB activation (Figure 4B). These data suggest that LPS sequentially activate SphK1 and PKC-δ, which then leads to the activation of NF-κB and subsequent generation and release of proinflammatory molecules. Finally, we examined whether SphK1 dependent PKC-δ activation plays an important role in ALF in mice. Blockade of SphK1 with 50 μmol/L DMS significantly decreased mortality (Figure 4C) and serum ALT level (Figure 4D) compared with the control group (P < 0.01). Taken together, these results indicate that PKC-δ activation is dependent on SphK1 activation in the ALF mouse model.
ALF is an acute inflammatory process of the liver and may lead to SIRS[15]. It can be caused by hepatotoxic drugs and diseases such as hepatitis associated cirrhosis, and complicated with other diseases[19,20]. PKC-δ has been implicated in the inflammatory response and regulates macrophage cytokine production through NF-κB activation. However, the potential role for PKC-δ in the systemic inflammatory response in ALF has not been explored. In in vivo experiments, compared with PBS treated normal mice, administration of LPS together with a sub-lethal dose of D-GalN induced more severe hepatic damage accompanied by necrotic changes and sever inflammation in the liver, which is similar to the manifestation of human liver failure[14]. Therefore, it is of interest to further investigate the hepatoprotective potential and mechanism of PKC-δ inhibition in D-GalN/LPS-induced ALF.
PKC-δ is mainly expressed in neutrophils and macrophages. Kupffer cells (KCs) are the liver resident macrophages and constitute most of tissue macrophages. During ALF, there is a remarkable increase of activated hepatic macrophages, and partial deletion of KCs with GdCL3 attenuates D-GalN/LPS-induced ALF[21].
PKC-δ has been identified as a critical inflammatory regulator and it controls proinflammatory response through NF-κB activation[8,9]. PKC-δ inhibition prevented neutrophil adherence and migration[10]. Thus, PKC-δ may have an important regulatory role in the inflammatory response. Our current study demonstrates that expression and activation (Thr505 phosphorylation) of PKC-δ in the liver tissue and PBMCs were promptly increased at 0.5 and 1 h after ALF induction. These results suggest that PKC-δ expression and activation in the liver are an early event in the acute phase of ALF.
To address the functional significance of PKC-δ in ALF, we treated mice with rottlerin, a PKC-δ specific chemical inhibitor. Pretreatment with rottlerin increased the survival rate in a dose-dependent manner in the D-GalN/LPS-induced ALF model. In the surviving mice, peak liver enzyme levels were detected at 12 h, and the maximum ALT/AST levels were reduced significantly in the rottlerin treatment group. Furthermore, rottlerin treated mice showed only small areas of necrotic and inflammatory cell infiltration at 36 h. However, mice treated with D-GalN/LPS alone had significant hepatic injury at the same time points, where the necrosis areas were more than 50% of almost all lobules examined, and panlobular mononuclear leukocyte infiltration with cytoplasmic vacuolization and severe distortion of tissue architecture was observed. These data demonstrate that inhibition of PKC-δ activity with rottlerin improved the survival rate, attenuated liver enzyme release, and reduced liver inflammation and necrosis in ALF mice. To the best of our knowledge, this is the first time that PKC-δ activation was found to be critical for the development of ALF.
Although etiology of ALF varies in different areas, the resulting clinical manifestations are remarkably similar, and this reflects common patterns of innate immune responses[22]. Among many others, proinflammatory cytokines (TNF-α, IL-1β and IL-6) may play a common role in the pathophysiology of ALF. In this study, inhibition of PKC-δ decreased proinflammatory cytokine levels, indicating that PKC-δ may positively participates in innate immune responses in liver injury. HMGB1 is a non-histone nuclear protein ubiquitously expressed in eukaryotes and exerts distinct functions at different subcellular localizations. Within the nucleus, HMGB1 plays an important role in the regulation of gene transcription[23]. Upon release by phagocytes and damaged/necrotic cells[24-27], extracellular HMGB1 functions as a DAMP and contributes to the pathogenesis of various inflammatory diseases[28,29]. HMGB1 exerts its effects through a number of the Toll-like receptors (TLR2/4)[27] and leads to the activation of immune cells and consequent release of multiple proinflammatory cytokines[30]. In animal models of infection or local tissue injury, HMGB1 functions as a critical mediator of systemic or local inflammatory injury[31]. As a result, HMGB1 has been established as a late mediator of lethal systemic inflammatory disease. In the clinical setting, elevated serum HMGB1 levels have been described in patients with sepsis, pneumonia and acute pancreatitis[24,32-35], as well as ALF[36]. In this study, PKC-δ inhibitor attenuated serum HMGB1 level, indicating that HMGB1 positively participates in inflammatory responses in ALF.
Sphk1 play a critical role in LPS-induced release of proinflammatory cytokines through NF-κB activation as well as PKC-δ. This led us to speculate that SphK1 is the upstream regulator of PKC-δ activation. In our study, inhibition of SphK1 indeed down-regulated PKC-δ expression and activation in liver tissue of mice with ALF. Furthermore, DMS, a SphK1 inhibitor, as well as rottlerin, a PKC-δ inhibitor, largely inhibited LPS-triggered NF-κB activation in LPS-stimulated macrophages. These data suggest that LPS sequentially activates SphK1 and PKC-δ, which then leads to NF-κB activation and subsequently promotes proinflammatory cytokine production. Finally, blockade of SphK1 with 50 μmol/L DMS significantly decreased mortality and serum ALT level in ALF mice. Taken together, these results indicate that PKC-δ activation is dependent on SphK1 in the ALF mouse model.
In summary, PKC-δ expression and activation are up-regulated in the liver of mice with ALF and are an early event in the acute phase of ALF. PKC-δ inhibition represents a potent strategy for inhibiting the inflammatory response in ALF. This approach may attenuate liver enzyme release, reduce liver inflammation and necrosis, decrease proinflammatory cytokine levels, and ultimately improves survival in ALF. Furthermore, SphK1 dependent PKC-δ activation may play an important role in ALF. Further preclinical studies with PKC-δ inhibitors may result in the development of a new clinically applicable therapeutic strategy for ALF in the future.
Acute liver failure is an acute inflammatory process of the liver and may lead to systemic inflammatory response syndrome. Protein kinase C (PKC)-δ and sphingosine kinase 1 (SphK1) have been implicated in the inflammatory response and regulate macrophage cytokine production through nuclear factor (NF)-κB activation.
Activation of the systemic immune response is the predominant mechanism responsible for the development of acute liver failure. PKC-δ is an important component of proinflammatory signaling pathways that regulate activation of NF-κB, which initiates inflammation in a positive-feedback loop. SphK1 is required to activate NF-κB and to induce the secretion of proinflammatory cytokines through PKC-δ activation.
PKC-δ activation was observed in liver tissue or PBMCs of mice with acute liver failure, and inhibition of PKC-δ ameliorated acute liver failure in mice. Furthermore, SphK1 was required for PKC-δ activation in acute liver failure.
Further preclinical studies with PKC-δ inhibitors may result in the development of a new clinically applicable therapeutic strategy for acute liver failure in the future.
In this study, the authors demonstrated PKC-δ activation in liver tissue or PBMCs and that inhibition of PKC-δ ameliorated acute liver failure in a mouse model of acute liver failure. Furthermore, SphK1 was found to be required for PKC-δ activation in acute liver failure. It is the first time that the authors demonstrated that SphK1 dependent PKC-δ activation plays an important role in acute liver failure. PKC-δ and SphK1 inhibitors may represent a new clinically applicable therapeutic strategy for acute liver failure in the future.
P- Reviewer: Chandrakesan P, Theiss AL S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | Atillasoy E, Berk PD. Fulminant hepatic failure: pathophysiology, treatment, and survival. Annu Rev Med. 1995;46:181-191. [PubMed] |
| 4. | Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 5. | Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, Lee WM, Blei AT. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755-764. [PubMed] |
| 6. | Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72-74. [PubMed] |
| 7. | Wu Z, Han M, Chen T, Yan W, Ning Q. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int. 2010;30:782-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Kilpatrick LE, Sun S, Li H, Vary TC, Korchak HM. Regulation of TNF-induced oxygen radical production in human neutrophils: role of delta-PKC. J Leukoc Biol. 2010;87:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Chakrabarti S, Zee JM, Patel KD. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J Leukoc Biol. 2006;79:214-222. [PubMed] |
| 10. | Woo CH, Lim JH, Kim JH. VCAM-1 upregulation via PKCdelta-p38 kinase-linked cascade mediates the TNF-alpha-induced leukocyte adhesion and emigration in the lung airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2005;288:L307-L316. [PubMed] |
| 11. | Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49-56. [PubMed] |
| 12. | Puneet P, Yap CT, Wong L, Lam Y, Koh DR, Moochhala S, Pfeilschifter J, Huwiler A, Melendez AJ. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290-1294. [PubMed] |
| 13. | Vancurova I, Miskolci V, Davidson D. NF-kappa B activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cdelta. Correlation to nuclear Ikappa Balpha. J Biol Chem. 2001;276:19746-19752. [PubMed] |
| 14. | Melendez AJ. Sphingosine kinase signalling in immune cells: potential as novel therapeutic targets. Biochim Biophys Acta. 2008;1784:66-75. [PubMed] |
| 15. | Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol. 2013;718:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Mottillo EP, Zhao J, Gartung A, VanHecke GC, Lee JF, Maddipati KR, Xu H, Ahn YH, Proia RL. Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. J Biol Chem. 2014;289:32178-32185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Melendez AJ, Harnett MM, Pushparaj PN, Wong WS, Tay HK, McSharry CP, Harnett W. Inhibition of Fc epsilon RI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375-1381. [PubMed] |
| 18. | Lufrano M, Jacob A, Zhou M, Wang P. Sphingosine kinase-1 mediates endotoxemia-induced hyperinflammation in aged animals. Mol Med Rep. 2013;8:645-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Laeeq SM, Luck NH, Wadhwa RK, Abbas Z, Hasan SM, Younus M, Mubarak M. Left liver lobe diameter albumin ratio as a predictor of esophageal varices in patients with cirrhosis: A preliminary report. J Transl Intern Med. 2014;2:164-167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Manzoor M, Wadhwa RK, Abbas Z, Hasan SM, Luck NH, Mubarak M. Unusual presentation of nonalcoholic steatohepatitis-related cirrhosis in a patient with celiac disease and microscopic colitis. J Transl Intern Med. 2014;2:172-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-β. Hepatology. 2011;53:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Leifeld L, Dumoulin FL, Purr I, Janberg K, Trautwein C, Wolff M, Manns MP, Sauerbruch T, Spengler U. Early up-regulation of chemokine expression in fulminant hepatic failure. J Pathol. 2003;199:335-344. [PubMed] |
| 23. | Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237-5246. [PubMed] |
| 24. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [PubMed] |
| 25. | Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950-2954. [PubMed] |
| 26. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [PubMed] |
| 27. | Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331-342. [PubMed] |
| 28. | Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774-778. [PubMed] |
| 29. | Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1-5. [PubMed] |
| 30. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [PubMed] |
| 31. | Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538-R1544. [PubMed] |
| 32. | Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564-573. [PubMed] |
| 33. | Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007;33:1347-1353. [PubMed] |
| 34. | Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061-1067. [PubMed] |