Published online Dec 21, 2015. doi: 10.3748/wjg.v21.i47.13309
Peer-review started: May 15, 2015
First decision: July 10, 2015
Revised: July 21, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: December 21, 2015
Processing time: 215 Days and 13.7 Hours
AIM: To investigate the progression rate of small pancreatic cystic lesions and identify characteristics associated with their progression.
METHODS: Patients with pancreatic cystic lesions with at least 1-year of follow-up were evaluated retrospectively. We excluded patients with cysts larger than 3 cm or with features that were a concern for malignancy. In total, 135 patients were evaluated. The interval progression of the cysts was examined. Characteristics were compared between patients with and without progression.
RESULTS: The pancreatic cysts ranged from 3 to 29 mm. The mean follow-up period was 4.5 ± 2.3 years and the mean progression rate was 1.0 ± 1.3 mm/year. Ninety patients showed interval progression and were divided into two groups; the minimal-change group (n = 41), who had cyst progression at less than 1 mm/year, and the progression group (n = 49), who had a progression rate of more than 1 mm/year. Compared with the cysts without progression, the lesions of the progression group were more frequently associated with tubular cyst, septation or a prominent pancreatic duct (P < 0.05). The odds ratio for progression was 5.318 for septation and 4.582 for tubular cysts.
CONCLUSION: Small pancreatic cysts progress slowly. Lesions with tubular shape, septa, or prominent pancreatic duct were more likely to progress, and required further diagnostic intervention or shorter surveillance interval.
Core tip: Observation is advised for small pancreatic cyst without features that are a concern for malignancy. Our study determined that small pancreatic cysts with borderline pancreatic duct dilation, tubular shape, or septa were associated with risk of progression. Our findings may be helpful to stratify patients for different management planning according to their risk of progression.
- Citation: Tsai HM, Chuang CH, Shan YS, Liu YS, Chen CY. Features associated with progression of small pancreatic cystic lesions: A retrospective study. World J Gastroenterol 2015; 21(47): 13309-13315
- URL: https://www.wjgnet.com/1007-9327/full/v21/i47/13309.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i47.13309
The increased utilization of cross-sectional imaging, such as multi-detector computed tomography (MDCT) and magnetic resonance (MR) imaging, has resulted in the increased detection of pancreatic cystic lesions, generally for unrelated reasons[1,2]. Pancreatic cystic lesions are encountered in up to 3% of computed tomography (CT) examinations and 20% of MR imaging studies among individuals with no prior history of pancreatic disease[3,4]. It is estimated that 10% of individuals have a pancreatic cystic lesions by the age of 70[4].
Pancreatic cystic lesions not only have diverse histologies and imaging features, but also differ in terms of clinical presentation, biological behavior and risk of malignancy[5]. According to the risk of malignancy, lesions can be categorized into: (1) those with no malignant potential (pseudocysts and serous cystadenomas); and (2) those that are precancerous or cancerous (mucinous cystic neoplasms and intraductal papillary mucinous neoplasms)[6]. Patients with imaging features that are considered to be associated with increased risk of malignancy, including cystic lesions larger than 3 cm, a dilated pancreatic duct and the presence of solid mural nodules[7-10], are advised to undergo further pathology diagnosis or surgical resection. Conversely, emerging data support observation as the preferred approach for patients who have small cystic lesions detected incidentally[11-13]. Nevertheless, approximately 3.3% of patients with small pancreatic cystic lesions were found to have occult malignancy after surgical resection[10]. Subjecting these patients to unnecessary testing and treatment can result in a potentially harmful and expensive cascade of tests and procedures. However, lesions left for observation can cause anxiety for both patients and clinicians because of the potential specter of a lethal malignancy.
The shape and number of cysts have been used to differentiate low or no malignant potential for serous oligocystic adenoma from lesions with malignant potential, including mucinous cystic neoplasm and IPMN[14]. By reviewing the images of our patients with small pancreatic cystic lesions, we aimed to determine the progression rate of these small lesions and to find the characteristic features that could differentiate progressive from non-progressive lesions. Hopefully, our findings will help in risk stratification and decision-making for the appropriate management and follow-up strategy in patients with small pancreatic cystic lesions.
Institutional review board approval was obtained for this retrospective study; informed patient consent was not obtained. From January 2004 through December 2011, we identified 523 patients with cystic lesions of the pancreas that had been detected by sonography, CT or MR imaging. One hundred and sixty seven patients with prior clinical and laboratory evidence of pancreatitis were excluded. Eight patients with co-existing pancreatic malignancies, including four cases of adenocarcinoma and four cases of lymphoma, were excluded. Of the remaining 348 patients, 41 (11.8%) underwent surgical resection because of presence of symptoms, high-risk of stigmata (such as dilated main pancreatic duct or mural nodule), or at the patient’s request. To focus on the progression of small pancreatic cystic lesions without features that are a concern for malignancy, we further excluded 172 patients with lesions larger than 3 cm (n = 12), a dilated pancreatic duct greater than 5 mm (n = 5), less than 1-year of follow-up (n = 123), or those who had only been evaluated by ultrasonography (n = 32). Finally, data from the remaining 135 patients with incidentally detected small pancreatic cystic lesions (94 men, 41 women; age range, 20-92 years; mean age, 67 years) were evaluated. Of these patients, 90 had undergone CT only, 22 had undergone MR imaging only and 23 had undergone CT and MR imaging. The interval progression was examined by using the initial and the last imaging of the cyst, not necessarily using a the same imaging modality. None of our patients were associated with features that were a concern malignancy; therefore, none of them had been studied by endoscopic ultrasonography.
Most of our patients underwent the imaging examination to study other abdominal disease; therefore, the protocols we used were not specific to pancreatic disease but were those that we routinely used for abdomen examination.
Helical CT with two different multi-detector helical CT scanners (Sensation-16, Siemens Medical Systems, Forchheim, Germany or Definition Flash, Siemens Medical Systems, Forchheim, Germany) were performed in all patients. A Routine abdominal CT beginning 60-70 s after intravenous injection of contrast material was the most commonly performed examination. For routine CT scanning, 120-150 mL of nonionic contrast material (300-350 mg/mL) was injected at a rate of 2.5-3.0 mL/s, and images were acquired at a 5-mm section thickness after a 70 s delay. The field of view was adjusted according to the size of the patient.
MR imaging was performed with a 1.5-T system (Achieva, Philips Medical Systems, Netherland B.V.) using a phased-array torso coil. T2 weighted axial (TR/TE/FA 558/150/90) and coronal fat suppression images (TR/TE/FA 686/150/90), as well as two-dimensional (TR/TE/FA 8000/800/90) and three-dimensional MR cholangiopancreatography (TR/TE/FA 2000/700/90) were obtained. T1-weighted in-phase (TR/TE/FA 175/4.6/80) and opposed-phase (TR/TE/FA 175/2.3/80) images were acquired. Subsequently, axial breath hold three-dimensional T1-weighted high resolution isotropic volume examinations (TR/TE/FA 4.3/2.1/14; reconstructed slide thickness, 2.5 mm) were taken before and after intravenous contrast injection during the arterial (15 and 50 s), portal venous (90 s), and equilibrium (150 and 300 s) phases.
The CT and MR images were reviewed retrospectively by two radiologists (Tsai HM and Liu YS, with 20 and 10 years of experience, respectively). The shape (spherical or tubular), number, size and location of the cysts, the existence of a prominent pancreatic duct (3-4 mm in diameter), and the morphological features of the cysts, such as presence or absence of calcifications, and/or septa on CT and MR images, were recorded. When multiple cysts were present within the pancreas, the diameter and morphological features of the largest lesion were recorded. When the shape was not spherical, the longest diameter was recorded.
The sizes of the cysts on the patient’s first and last follow-up images were measured. The cyst progression rate was determined by dividing the change in size by the follow-up time in years. Accordingly, patients were first divided into those without progression of cystic lesions and those with interval progression. Patients with interval progression of cysts were further divided into the minimal-change and the progression groups, determined by a progression rate that was lower or higher than the mean cyst progression rate. Accordingly, the patients were divided into three groups: non-progression, minimal-change, and the progression.
The demographic data and imaging features were compared among the three groups. Comparisons were made using a Fisher’s exact test or χ2 test for nominal variables and a Student’s t-test for continuous variables. A univariate analysis was performed to identify risk factors for progression, using logistic regression analysis. To evaluate independent predictors of malignancy, a multivariate analysis was performed with a model using factors identified as being significant in the univariate analysis or those with a P-value less than 0.20. A P-value less than 0.05 was considered statistically significant. Results are presented as means ± standard deviation.
The mean cyst progression rate was 1 mm per year (1.0 ± 1.3 mm/year). The patients were divided into three groups, the non-progression (n = 45), the minimal change (n = 41), and the progression groups (n = 49). Although the age of our patients ranged from 20 to 92 years, most of them were elderly (mean age, 67.3 ± 12.4 years). The mean age of the progression group was the highest among the three groups, but it was not significantly different from that of the non-progression group (Table 1). Male gender was predominant in all three groups. The follow-up period of our patients ranged from 1.1 to 10.1 years, with a mean of 4.5 ± 2.3 years. Although the progression group had a longer follow-up time than the non-progression group, it was not statistically significant (Table 1).
The pancreatic cystic lesions ranged from 3 to 29 mm (mean, 12.5 ± 6.5 mm), based on the longest diameter. The initial size was not related to future progression and was similar among the three groups (Table 2). Although a trend for increasing multiple-cystic lesions was noted from the non-progression group to the progression group, the difference was not statistically significant (Table 2). Few cystic lesions were detected over the uncinate process. The remaining cystic lesions were evenly distributed across all the other sites of the pancreas and there was no predilection site for progressive pancreatic cystic lesions (Table 2).
| Non-progression group (n = 45) | Minimal-change group (n = 41) | Progression group(n = 49) | |
| Lesion characteristics | |||
| Initial size (mm) | 12.0 ± 5.4 | 11.6 ± 6.5 | 13.8 ± 7.4 |
| Multiple cystic lesions | 3 (6.7) | 5 (12.2) | 9 (18.4) |
| Location | |||
| Uncinate | 1 (0.2) | 1 (2.4) | 4 (8.2) |
| Head | 9 (20.0) | 9 (22.0) | 13 (26.5) |
| Neck | 17 (37.8) | 7 (17.1) | 9 (18.4) |
| Body | 10 (22.2) | 14 (34.1) | 12 (24.5) |
| Tail | 8 (17.8) | 10 (24.4) | 11 (22.4) |
| Imaging features | |||
| Septation | 4 (8.9)1 | 5 (12.2) | 13 (26.5)2 |
| Tubular cyst | 5 (11.1)1 | 8 (19.5) | 14 (28.6)2 |
| Calcification | 2 (4.5) | 1 (2.5) | 1 (2.0) |
| Prominent pancreatic duct | 0 (0)1 | 3 (7.3) | 9 (18.4)2 |
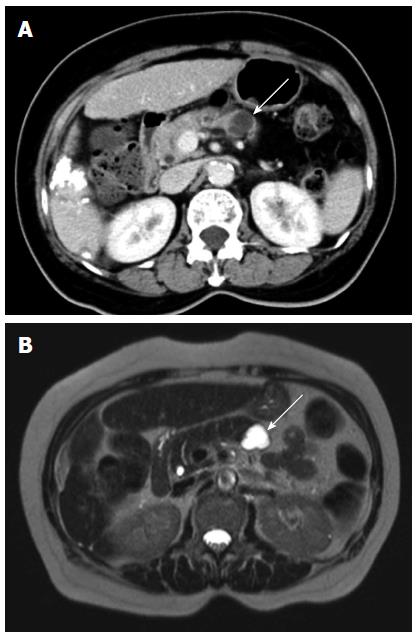
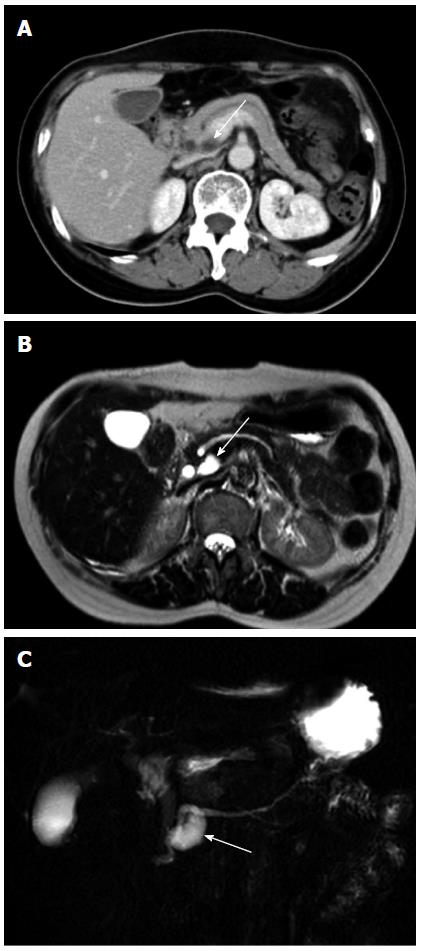
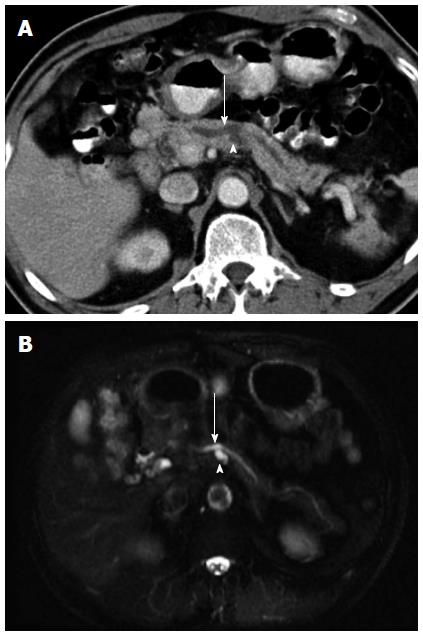
As shown in Table 2, more patients in the progression group had a septated (Figure 1) or a tubular (Figure 2) cyst than those in the non-progression group (septated: 26.5% vs 8.9%, P < 0.05, tubular: 28.6% vs 11.1%, P < 0.05). Nine patients (18.4%) in the progression group had a prominent pancreatic duct (Figure 3), which was in marked contrast to none observed in the non-progression group (P < 0.01). E could not check the calcification of pancreatic cysts in 22 of our patients because they underwent MR imaging only. In the other 113 patients who had a CT scan examined during the follow-up, only four patients had a calcified pancreatic cystic lesions and all calcifications were located in the periphery.
Cystic lesions with septa or a tubular shape were included for the regression analysis; whereas, cysts with a prominent pancreatic duct were not analyzed, because this feature was exclusively found in lesions with interval progression. By multivariate regression analysis, lesions with septa had 5.318-fold increased odds of interval progression than lesions without septa; the odds ratio of progression was 4.582 for the tubular shape lesion (Table 3).
| Univariate | Multivariate | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Septation | 3.701 (1.107-2.372) | 0.034 | 5.318 (1.539-18.374) | 0.008 |
| Tubular content | 3.200 (1.047-9.782) | 0.041 | 4.582 (1.450-14.480) | 0.010 |
The interval progression diameter ranged from 0 to 54 mm (average, 4.1 ± 7.2 mm) with a progression rate ranging from 0 to 6.2 mm per year (average 1.0 ± 1.3 mm per year). No mural nodules were found during the follow-up period. Twelve patients had cysts that progressed to more than 3 cm in diameter (range from 6.4 cm to 3.0 cm) within a mean follow-up period of 5.9 ± 2.8 years (range from 1.5 to 10.0 years). Two patients underwent surgery because of concern of malignant potential based on the growth characteristics. The pathologies of these two lesions were serous adenoma and serous oligocystic adenoma, respectively. The remaining patients were kept under observation according to the patients’ preference.
The decision to proceed with surgical resection for a small pancreatic cystic lesion is difficult because of the significant morbidity and mortality associated with pancreatic surgery, especially in the elderly. Accordingly, the Sendai guidelines recommend surveillance only for cysts less than 3 cm without “worrisome features” or “high-risk stigmata”[15]. Despite the good performance of the Sendai surveillance guidelines, which have been validated recently in a report showing that patients who met the criteria had a 97% probability of benign follow-up for up to 7 years and 8 mo[16], they are not prefect, because some small cysts were still found to have malignant outcomes on long-term follow-up. Surveillance, therefore, is associated with concern, anxiety and fear about the uncertainty of the diagnosis and the natural history of these cysts. Our study revealed the slow progression rate of small pancreatic cystic lesions and indicated that tubular cysts or cysts associated with prominent pancreatic ducts or septa tended to be progressive. Our study may provide more information to ease the uncertainty associated with the follow-up strategy chosen for patients with a small pancreatic cystic lesions.
Septa can be associated both with a malignant or potentially malignant mucinous cystic neoplasm and a benign serous cystadenoma[17,18]. Sahani et al[12] reported that small pancreatic cysts without septa or solid components are almost always benign; however, since 20% of their septated cystic lesions were associated with malignancies or borderline malignancies, detecting septa within small cysts should raise a concern for malignancy. Our study found that small septated cysts had an increased risk of interval progression, further supporting the observation that septa in a small cyst increase the level of suspicion of malignancy.
IPMN has a malignant potential and tends to be progressive. As the tumor produces mucin content, dilatation of the main pancreatic duct is frequently found with main duct IPMN. A dilated main pancreatic duct of 5-9 mm is considered a “worrisome feature”, while a diameter of more than 10 mm is one of the “high-risk stigmata”[15] that warrant further diagnosis or surgery. In our study, cysts with these features were initially excluded; however, we found that cysts with a prominent pancreatic duct of 3 to 4 mm in diameter still showed a tendency for interval progression. In addition, cysts with a tubular shape could be caused by the dilation of the pancreatic duct branch and are frequently associated with branch duct IPMN[14]. As both features are characteristics of IPMN, which possesses malignant potential, the more tubular cysts or cysts with prominent pancreatic ducts found in our progression group may indicate the presence of more IPMN cases in this group.
Peripheral calcification was prevalent in mucinous cystic neoplasms, while head location or multiple cysts were more frequently associated with branch duct IPMN[5]; therefore, cysts with these features would be expected to be progressive. However, calcification was rarely found in our cases. While the number of cases with multi-cystic lesions showed an increasing trend in the progression group, it did not reach statistical significance. The above-mentioned imaging features, although also characteristic features of mucinous cystic lesions, were less valuable to distinguish small pancreatic cystic lesions with different progression rates in our study.
Male gender was predominant across all three groups in this study, which was contradictory to previous studies reporting that female gender was predominant in serous and mucinous cystic neoplasms[19]. All our cystic lesions were asymptomatic and were found coincidentally during imaging examinations for abdominal organs other than the pancreas; therefore, this finding might simply reflect the male predominance of liver diseases in this liver diseases endemic country.
This was a retrospective study; therefore, the follow-up intervals of imaging were not the same for all patients. It may be argued that the progressive lesions observed in our patients could simply result from a longer follow-up period. Undeniably, the longest follow-up period seen in our minimal-change group may actually reflect this possibility. However, the similar follow-up periods between the non-progression and progression groups indicated there were factors other than “time” that determined the risk of progression in pancreatic cystic lesions.
We suspected that the progression rate of cyst might not be constant and could be accelerated when the cyst becomes larger, which challenged the progression rate of 1 mm/year that was determined by observing cyst progression at different follow-up intervals instead of year-by-year. Such suspicion might be reasonable in those lesions larger than 3 cm, but it should be trivial in our study, because all our lesions were smaller than 3 cm and the mean initial size was similar among groups with different interval changes.
As observed from the benign pathology in our two patients who had cysts growing greater than 3 cm and who underwent surgery, the increase in the size of the cyst was not necessarily associated with malignancy. However, cyst progression remains a key point to be followed on surveillance images[15,20]. Our study determined the mean growth rate as 1 mm per year after a mean follow-up period of about 6 years, which supports the low incidence of malignant transformation; i.e., of 0.4% per year during surveillance[20], and the current proposed annual surveillance strategy for small cysts without high risk stigmata or worrisome features[15]. The addition of molecular profiling, cytology and chemistry of pancreatic cystic fluid to imaging studies, has defined an integrated approach to molecular pathology testing that has increased the accuracy of assessing the malignant potential of pancreatic cysts[16]. Our study explored imaging features associated with the risk of progression in small pancreatic cystic lesions that might be helpful to stratify patients into those who require cystic fluid testing and those who merely require observation.
Pancreatic cystic lesions are encountered in up to 3% of computed tomography examinations and 20% of magnetic resonance imaging studies among individuals with no prior history of pancreatic disease. Pancreatic cystic lesions not only have diverse histologies and imaging features, but also differ in terms of clinical presentation, biological behavior and risk of malignancy. Emerging data supports observation as the preferred approach for patients who have small cystic lesions without features that are a concern for malignancy. Nevertheless, approximately 3.3% of patients with small pancreatic cystic lesions were found to have occult malignancy after surgical resection. The authors aimed to determine the progression rate of these small lesions and to identify the characteristic features that can differentiate progressive from non-progressive lesions.
There was little data dealing with the progression and characteristic findings of small pancreatic cysts. The results of this study contributed to the identification of the progression rate of small pancreatic cysts and the characteristics associated with such progressive cysts.
This study determined the mean growth rate of small pancreatic cyst as 1 mm per year after a mean follow-up period of about 6 years. This study indicated that tubular cysts or cysts with prominent pancreatic ducts or septa were imaging features associated with risk of progression in small pancreatic cystic lesions.
This study provides more information to ease the uncertainty associated with the follow-up strategy chosen for patients with a small pancreatic cyst and to select patients at risk of progressive disease to undergo EUS-FNA for cystic fluid analysis.
High-risk stigmata: pancreatic cyst with enhanced solid components and a main pancreatic duct dilation ≥ 10 mm. Worrisome features: pancreatic cyst ≥ 3 cm, with thickened enhanced cyst walls, non-enhanced mural nodules, main pancreatic duct of 5-9 mm, an abrupt change in the main pancreatic duct caliber with distal pancreatic atrophy, and lymphadenopathy.
This valuable paper deals with the imaging features that are associated with meaningful progression of pancreatic cysts. Although there is not histological correlation, I believe that interesting data are derived from this research, and that it could be published after minor revision.
P- Reviewer: Luna A, Mathew S S- Editor: Yu J L- Editor: Stewart G E- Editor: Ma S
| 1. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 656] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 2. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 4. | de Jong K, Nio CY, Mearadji B, Phoa SS, Engelbrecht MR, Dijkgraaf MG, Bruno MJ, Fockens P. Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas. 2012;41:278-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S, Fernandez-del Castillo C. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol. 2013;200:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | de Castro SM, Houwert JT, van der Gaag NA, Busch OR, van Gulik TM, Gouma DJ. Evaluation of a selective management strategy of patients with primary cystic neoplasms of the pancreas. Int J Surg. 2011;9:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Donahue TR, Hines OJ, Farrell JJ, Tomlinson JS, Eibl G, Reber HA. Cystic neoplasms of the pancreas: results of 114 cases. Pancreas. 2010;39:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Huang ES, Turner BG, Fernandez-Del-Castillo C, Brugge WR, Hur C. Pancreatic cystic lesions: clinical predictors of malignancy in patients undergoing surgery. Aliment Pharmacol Ther. 2010;31:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, Kochman ML, Foley PJ, Drebin J, Oh YS. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Walsh RM, Vogt DP, Henderson JM, Zuccaro G, Vargo J, Dumot J, Herts B, Biscotti CV, Brown N. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665-670; discussion 670-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez-Del Castillo C. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Soejima R. [Intractable airway infections and its management]. Nihon Naika Gakkai Zasshi. 1991;80:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1613] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 16. | Al-Haddad MA, Kowalski T, Siddiqui A, Mertz HR, Mallat D, Haddad N, Malhotra N, Sadowski B, Lybik MJ, Patel SN. Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts. Endoscopy. 2015;47:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Hammond N, Miller FH, Sica GT, Gore RM. Imaging of cystic diseases of the pancreas. Radiol Clin North Am. 2002;40:1243-1262. [PubMed] |
| 18. | Sarr MG, Murr M, Smyrk TC, Yeo CJ, Fernandez-del-Castillo C, Hawes RH, Freeny PC. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7:417-428. [PubMed] |
| 19. | Talukdar R, Nageshwar Reddy D. Treatment of pancreatic cystic neoplasm: surgery or conservative? Clin Gastroenterol Hepatol. 2014;12:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Wu BU, Sampath K, Berberian CE, Kwok KK, Lim BS, Kao KT, Giap AQ, Kosco AE, Akmal YM, Difronzo AL. Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol. 2014;109:121-129; quiz 130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |