Published online Dec 21, 2015. doi: 10.3748/wjg.v21.i47.13302
Peer-review started: August 5, 2015
First decision: September 9, 2015
Revised: September 20, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: December 21, 2015
Processing time: 132 Days and 23.7 Hours
AIM: To validate the association between atypical endoscopic features and lymph node metastasis (LNM).
METHODS: A total of 247 patients with rectal neuroendocrine tumors (NETs) were analyzed. Endoscopic images were reviewed independently by two endoscopists, each of whom classified tumors by sized and endoscopic features, such as shape, color, and surface change (kappa coefficient 0.76 for inter-observer agreement). All of patients underwent computed tomography scans of abdomen and pelvis for evaluation of LNM. Univariate and multivariate analyses were performed to identify the factors associated with LNM. Additionally, the association between endoscopic atypical features and immunohistochemical staining of tumors was analyzed.
RESULTS: Of 247 patients, 156 (63.2%) were male and 15 (6.1%) were showed positive for LNM. On univariate analysis, tumor size (P < 0.001), shape (P < 0.001), color (P < 0.001) and surface changes (P < 0.001) were significantly associated with LNM. On multivariate analysis, tumor size (OR = 11.53, 95%CI: 2.51-52.93, P = 0.002) and atypical surface (OR = 27.44, 95%CI: 5.96-126.34, P < 0.001) changes were independent risk factors for LNM. The likelihood of atypical endoscopic features increased as tumor size increased. Atypical endoscopic features were associated with LNM in rectal NETs < 10 mm (P = 0.005) and 10-19 mm (P = 0.041) in diameter. Immunohistochemical staining showed that the rate of atypical endoscopic features was higher in non L-cell tumors.
CONCLUSION: Atypical endoscopic features as well as tumor size are predictive factors of LNM in patients with rectal NETs.
Core tip: We were studied about association between endoscopic atypical features in rectal neuroendocrine tumor and metastasis in 2008. Thus, our study was designed to validate the association between atypical endoscopic features and lymph node metastasis (LNM). Our study showed that the atypical endoscopic features, such as size > 10 mm, surface changes, were risk factors for LNM. Additionally, rectal neuroendocrine tumors which showed atypical endoscopic features were associated with non L-cell tumors. When we examined rectal neuroendocrine tumor using colonoscopy, atypical endoscopic features help to predict the treatment plan.
- Citation: Hyun JH, Lee SD, Youk EG, Lee JB, Lee EJ, Chang HJ, Sohn DK. Clinical impact of atypical endoscopic features in rectal neuroendocrine tumors. World J Gastroenterol 2015; 21(47): 13302-13308
- URL: https://www.wjgnet.com/1007-9327/full/v21/i47/13302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i47.13302
Increases in rate of screening colonoscopy have resulted in increases in incidence and prevalence of rectal neuroendocrine tumors (NETs). Most rectal NETs are slow growing tumors that originate from enterochromaffin cells and rarely metastasize[1,2]. A recent consensus guideline suggests that tumor size is the most powerful predictor of lymph node metastasis (LNM) of rectal NETs[3,4]. Guideline of the National Comprehensive Cancer Network recommend that NETs ≤ 2 cm in diameter be excised transanally or endoscopically excision and that NETs > 2 cm in diameter undergo radical resection[5]. However, LNMs have been reported in patients with NETs < 6 mm in diameter[6], suggesting that tumor size alone is not predictive of LNM.
Colonoscopy is the most useful method of diagnosing and treating rectal NETs. Although typical NETs appear as yellowish, sessile, submucosal tumors[7,8], some are morphologically unusual, having irregular surfaces or being pedunculated or hyperemic. These unusual features have been associated with LNM, suggesting an association between endoscopic findings and LNM[9].
Study was designed to validate the association between endoscopically atypical features and LNM. In addition, the association between endoscopically atypical features and the immunohistochemistry of these tumors was analyzed[10].
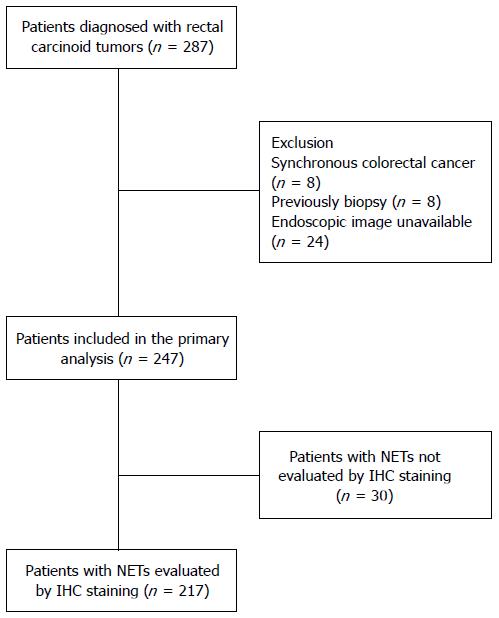
Data from 287 patients with rectal NETs diagnosed and treated at the National Cancer Center (Goyang, South Korea) and Daehang Hospital (Seoul, South Korea) between January 2008 and December 2010 were retrospectively reviewed[10]. Eight patients with synchronous colorectal cancer, eight who underwent multiple biopsies before visiting our institutions and 24 whose endoscopic images were unavailable were excluded from this study. Finally, 247 patients with rectal NETs were analyzed (Figure 1). Of these 247 lesions, 208 were endoscopically resected, 22 were removed transanally, and 16 were treated with radical surgery. One patient received only palliative chemotherapy, because he had multiple unresectable liver and peritoneal metastases. Clinicopathologic variables were retrospectively collected from the patients’ medical records. This study was approved by the Institutional Review Board of the National Cancer Center of Korea (NCC2014-0104).
All patients underwent endoscopic examination with video colonoscopes (Olympus CF-Q240, CF-Q260 or CF-H260; Olympus, Tokyo, Japan) for diagnosis and treatment. Endoscopic images were reviewed independently by two endoscopists, resulting in kappa coefficient of 0.76 for interobserver agreement. Any disagreements between the two endoscopists were resolved by open discussions with all expert endoscopists.
All patients underwent computed tomography (CT) scans of the abdomen and pelvis for evaluation of LNM. Patients were considered positive for LNM if CT scans revealed nodes > 3 mm in diameter in the perirectal area or nodes > 1 cm in diameter in the pelvis[10-12]. Tumor sizes were confirmed by pathology reports, except for the one patient who did not undergo curative resection because of extensive liver metastases. All tumors were classified by size (longest diameter), and then by endoscopic features such as shape, color, and surface changes, including depression, erosion and ulceration. Of the 247 lesions, 217 were also assessed immunohistochemically.
Interobserver agreement on endoscopic findings was analyzed by calculating the kappa coefficient. The associations between endoscopic findings and LNM were analyzed by χ2 or Fisher’s exact tests. Multivariate analysis using a logistic regression model was performed to identify associations between all potential parameters and LNM. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 14.0 (SPSS Inc., Chicago, IL). A two-sided P < 0.05 was considered statistically significant.
Table 1 shows the baseline clinicopathological characteristics of the 247 patients with rectal NETs. Of these patients, 91 (36.8%) were male and 156 (63.2%) were female. Mean age at diagnosis was 51.6 ± 10.7 years and mean tumor size was 5.76 ± 2.65 mm. Two patients had liver metastases at diagnosis, with one also having peritoneal seeding and 15 (6.1%) were diagnosed with LNM.
| Value | |
| Age, mean ± SD (range), yr | 51.56 ± 10.69 (27-76) |
| Sex | |
| Male | 91 (36.8) |
| Female | 156 (63.2) |
| Tumor size, mean ± SD (mm) | 5.76 ± 2.76 |
| Resection type | |
| Endoscopic resection | 208 (84.2) |
| TEM or TAE | 22 (8.9) |
| Radical resection | 16 (6.5) |
| None | 1 (0.4) |
| Distant organ metastasis at diagnosis | |
| Negative | 245 (99.2) |
| Positive | 2 (0.8) |
| LN metastasis | |
| Negative | 232 (93.9) |
| Positive | 15 (6.1) |
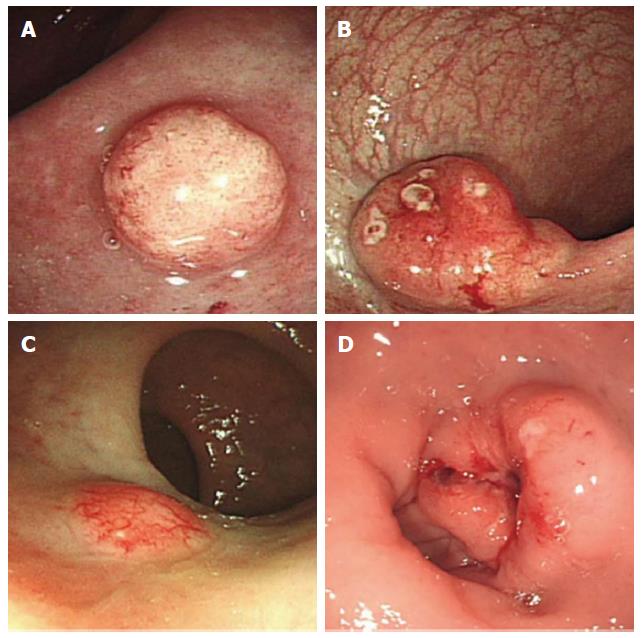
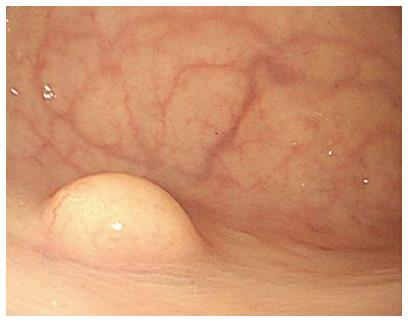
Fifty-five patients (22.3%) had rectal NETs with one or more atypical features (Figure 2), whereas the other 192 patients (77.7%) had rectal NETs with endoscopically typical features such as being sessile and having a smooth surface covered with normal or yellowish mucosa (Figure 3). On univariate analysis, tumor size, tumor shape, surface changes, and color were significantly associated with LNM. On multivariate analysis, tumor size (OR = 11.53, 95%CI: 2.51-52.93), atypical surface changes (OR = 27.44, 95%CI: 5.96-126.34), and any type of atypical feature (OR = 4.38, 95%CI: 0.92-20.80) were independent risk factors for LNM (Table 2). Moreover, atypical features correlated with increased tumor size (Table 3).
| Univariate analysis | Multivariate analysis | ||||
| Metastasis (-) | Metastasis (+) | P value | OR (95%CI) | P value | |
| Gender | 0.183 | - | - | ||
| Male | 144 (92.3) | 12 (7.7) | |||
| Female | 88 (96.7) | 3 (3.3) | |||
| Age (yr) | 1.000 | - | - | ||
| ≤ 50 | 113 (94.2) | 7 (5.8) | |||
| > 50 | 119 (93.7) | 8 (6.3) | |||
| Size (mm) | < 0.001 | 11.53 (2.51-52.93) | 0.002 | ||
| < 10 | 219 (98.6) | 3 (1.4) | |||
| ≥ 10, < 20 | 13 (59.1) | 9 (40.9) | |||
| ≥ 20 | 0 (0) | 3 (100) | |||
| Tumor shape | < 0.001 | - | - | ||
| Sessile | 205 (97.6) | 5 (2.4) | |||
| Semipedunculated | 27 (77.1) | 8 (22.9) | |||
| Ulcerofungating | 0 (0) | 2 (100) | |||
| Surface change | < 0.001 | 27.44 (5.96-126.34) | < 0.001 | ||
| Smooth | 222 (97.8) | 5 (2.2) | |||
| Depressed/eroded | 10 (55.6) | 8 (44.4) | |||
| Ulcerated | 0 (0) | 2 (100) | |||
| Color | < 0.001 | - | - | ||
| Normal or yellow | 210 (96.8) | 7 (3.2) | |||
| Hyperemia | 22 (73.3) | 8 (26.7) | |||
| Atypical features, any | < 0.001 | 4.38 (0.92-20.80) | 0.064 | ||
| Typical features | 189 (98.4) | 3 (1.6) | |||
| Atypical features | 43 (78.2) | 12 (21.8) | |||
| Typical (n = 192) | Atypical (n = 55) | P value | |
| Tumor size (mm) | < 0.001 | ||
| < 10 | 186 (83.8) | 36 (16.2) | |
| ≥ 10, < 20 | 6 (27.3) | 16 (72.7) | |
| ≥ 20 | 0 (0) | 3 (100) |
Table 4 shows the association between endoscopic features and metastasis in rectal NETs < 10 mm and 10-19 mm in diameter were evaluated in Table 4, respectively. Tumor shape and color were not associated with LNM for either size range of rectal NETs. However, tumor surface changes were associated with LNM in patients with NETs < 10 mm (P = 0.005) and 10-19 mm (P = 0.041) in diameter. Ulceration was not observed in any tumor < 20 mm in diameter.
| Metastasis (-) | Metastasis (+) | P value | |
| < 10 mm in diameter | |||
| Shape | 0.155 | ||
| Sessile | 199 (99.0) | 2 (1.0) | |
| Semipedunculated | 20 (95.2) | 1 (4.8) | |
| Surface change | 0.005 | ||
| Smooth | 212 (99.1) | 2 (0.9) | |
| Depression/erosion | 7 (87.5) | 1 (12.5) | |
| Color | 0.627 | ||
| Yellow | 203 (98.5) | 3 (1.5) | |
| Hyperemia | 16 (100) | 0 (0) | |
| 10-19 mm in diameter | |||
| Shape | 0.548 | ||
| Sessile | 6 (66.7) | 3 (33.3) | |
| Semipedunculated | 7 (53.8) | 6 (46.2) | |
| Surface change | |||
| Smooth | 10 (76.9) | 3 (23.1) | 0.041 |
| Depression/erosion | 3 (33.3) | 6 (66.7) | |
| Color | 0.665 | ||
| Yellow | 7 (63.6) | 4 (36.4) | |
| Hyperemia | 6 (54.5) | 5 (45.5) |
Table 5 shows the association between atypical features and the results of immunohistochemical staining results. L-cell phenotype and GLP1 were associated with atypical features, whereas non-L cell phenotype was associated with surface changes and color of NETs (Table 6).
| Typical feature | Atypical feature | P value | |
| L-cell | 0.008 | ||
| (-) | 27 (62.8) | 16 (37.2) | |
| (+) | 142 (81.6) | 32 (18.4) | |
| EC-cell | 0.464 | ||
| (-) | 162 (78.3) | 45 (21.7) | |
| (+) | 7 (70.0) | 3 (30.0) | |
| Serotonin | 0.306 | ||
| (-) | 161 (78.5) | 44 (21.5) | |
| (+) | 8 (66.7) | 4 (33.3) | |
| Somatostatin | 1.000 | ||
| (-) | 161 (77.8) | 46 (22.2) | |
| (+) | 8 (80.0) | 2 (20.0) | |
| GLP1 | 0.021 | ||
| (-) | 57 (69.5) | 25 (30.5) | |
| (+) | 112 (83.0) | 23 (17.0) | |
| PYY1 | 0.513 | ||
| (-) | 72 (75.8) | 23 (24.2) | |
| (+) | 97 (79.5) | 25 (20.5) | |
| PPY1 | 0.443 | ||
| (-) | 88 (75.9) | 28 (24.1) | |
| (+) | 81 (80.2) | 20 (19.8) |
| L-cell (-) | L-cell (+) | P value | |
| Tumor shape | 0.107 | ||
| Sessile | 34 (18.4) | 151 (81.6) | |
| Semipedunculated | 8 (25.8) | 23 (74.2) | |
| Ulcerofungating | 1 (100) | 0 (0) | |
| Surface change | 0.022 | ||
| Smooth | 36 (18.0) | 164 (82.0) | |
| Depression/erosion | 6 (37.5) | 10 (62.5) | |
| Ulceration | 1 (100) | 0 (0) | |
| Color | < 0.001 | ||
| Normal or yellow | 31 (16.1) | 162 (83.9) | |
| Hyperemia | 12 (50.0) | 12 (50.0) |
Risk factors predictive of LNM of rectal NETs were assessed by univariate and multivariate analyses, with the latter showing that tumor size and atypical surface changes were significant independent predictors of LNM. The ability to predict the likelihood of LNM is important for managing patients requiring radical surgery to prevent tumor progression. Recent studies have reported that risk factors for LNM of rectal NETs include tumor size > 10 mm; atypical features; pathologic T stage; and muscular, perineural or lymphovascular invasion[9,13-16]. Two studies recommended radical lymph node dissection for patients with rectal NETs > 10 mm and lymphatic invasion[16,17]. Lymphatic invasion, however, cannot be evaluated before resection of rectal NETs. On colonoscopy, the size of rectal NETs was the only predictor of LNM. We previously reported an association between atypical features of rectal NETs and LNM[9]. Moreover, the incidence of atypical features was found to be associated with increased tumor size, suggesting that atypical features may be useful in determining treatment for tumors 11-19 mm in diameter. This study was performed to validate the predictive value of atypical features of NETs in a separate patient cohort.
The cutoff value for carcinoid tumor size that can determine the treatment plan and assess patient prognosis has not been definitively established. Tumors ≤ 10 mm in diameter are locally resected, by methods such as endoscopic resection, transanal excision or transanal endoscopic microsurgery. The American Joint Committee on Cancer staging system has recommended that patients with tumors ≥ 20 mm undergo radical resection with lymph node dissection[3,18]. However, the proper method of removing rectal NETs 11-19 mm in size remains undetermined, and no controlled prospective trials have assessed treatment plans for these patients. We found that all three patients with tumors ≥ 20 mm in diameter, 6 (27.3%) of 22 with tumors 10-19 mm, and 3 (1.4%) of 222 with tumors < 10 mm presented with LNM. Although, surprisingly, 3 patients with tumors < 10 mm in diameter had LNM, two studies observed metastases to lymph nodes and distant organs in patients with rectal NETs ≤ 10 mm in size[6,19]. Thus, size of rectal NETs alone is insufficient to predict LNM and determine treatment plans.
The Surveillance, Epidemiology, and End Results registry database has shown that the incidence of rectal NETs has increased over the last 35 years[20]. Most rectal NETs are diagnosed incidentally, with the increase in incidence likely due to increases in screening sigmoidoscopy and colonoscopy[1]. Although size of rectal NETs incidentally diagnosed during lower endoscopy was the only factor associated with LNM, this study found that atypical features, especially surface changes, were strongly predictive of LNM. One of 3 patients with rectal NETs ≤ 10 mm and LNM had a semipedunculated lesion with surface erosion, while all 9 patients with tumors > 10 mm in size and LNM had lesions with one or more atypical features. The presence of atypical features can help determine treatment plans for patients with rectal NETs 11-19 mm in diameter. We suggest that rectal NTEs 11-19mm in diameter, which showed atypical features in endoscopic findings, should be performed the CT or EUS to evaluate the LNM.
In 2010, the World Health Organization (WHO) classified rectal NETs as malignant[21], with L-cell, glucagon-like peptide producing and pancreatic polypeptide/peptide YY (PPY/PYY) producing NETs defined as borderline malignant or of uncertain malignant potential. Although most rectal NETs are L-cell tumors, the L-cell phenotype was not associated with biologically favorable characteristics[10]. That study, with a population overlapping our study, recommended that clinical management of rectal NETs should depend on tumor size. Our analysis of the association between atypical features and immunohistochemical staining results found that L-cell phenotype and GLP1 were associated with atypical features. These findings suggested that increased tumor size may be associated with atypical features as well as non L-cell type. Prospective observational studies in large cohorts of patients are required to clarify these associations.
Although we analyzed a relatively large patient cohort, our study had the inherent limitations of a retrospective study. To minimize such biases, we did not include and analyze consecutive patients with rectal NETs. Second, we investigated LNM by radiologic imaging or pathologic reports. Most patients with rectal NETs underwent local excision, such as transanal excision, transanal endoscopic microsurgery, and endoscopic procedures, rather than radical resection. Although the LNM status of patients who underwent local excision was evaluated by abdominopelvic CT, CT was used only to evaluate lymph node status. To evaluate the lymph node status, we were using criteria that distinguished positive node which showed > 3 mm in diameter in perirectal area or > 1 cm in diameter in the pelvis[11,12]. These criteria showed about a sensitivity of 73% and a specificity of 58%. Thus, we have to consider a difference between CT finding and pathology. Third, we did not perform survival analysis. Median follow-up time of our study patients was 44 mo (range 0-78 mo), which, while longer than in other studies, was too short to assess distant metastases or tumor recurrence. Prospective long term follow-up studies are needed for survival analyses.
In conclusion, the present study, along with a previous study performed at our institution, suggests that rectal NETs ≤ 10 mm in diameter can be treated by local excision, whereas tumors ≥ 20 mm in diameter should be treated by radical resection with lymph node dissection. Atypical endoscopic features may help select the optimal treatment plans for patients with rectal NETs 11-19 mm in diameter.
Increases in rate of screening colonoscopy have resulted in increases in incidence and prevalence of rectal neuroendocrine tumors (NETs). The treatment plan of rectal NETs have been decided depends on tumor size. Previously, the authors had proposed the possible association between endoscopic atypical features and lymph node metastasis (LNM) in rectal NETs.
Tumor size, tumor shape, color and surface change were significantly related to LNM in univariate analysis. In multivariate analysis, tumor size and atypical surface change were independent risk factors for LNM [odd ratios with 95% confidence interval were 11.5 (2.5-52.9) and 27.4 (5.9-126.3), respectively]. The rate of atypical endoscopic feature was increased as tumor size increase (16.2% in less than 10 mm, 72.7% in 10-19 mm, 100% in 20 mm or over in diameter, P < 0.001).
In this study, the authors found atypical endoscopic features as well as tumor size were predictive factors for LNM in rectal NETs.
This study suggested that NETs which showed atypical endoscopic feature helpful for determining proper treatment.
The paper is an interesting analysis of the association between atypical features and LNM in rectal NETs. The atypical features of rectal NETs are important findings to determine the treatment plan during colonoscopy.
P- Reviewer: Boulay B, Osawa S S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Taghavi S, Jayarajan SN, Powers BD, Davey A, Willis AI. Examining rectal carcinoids in the era of screening colonoscopy: a surveillance, epidemiology, and end results analysis. Dis Colon Rectum. 2013;56:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 2. | Scherübl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy. 2009;41:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717-1751. [PubMed] |
| 4. | Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61:6-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 5. | Kulke MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, Choti MA, Clark OH, Doherty GM, Eason J, Emerson L. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10:724-764. [PubMed] |
| 6. | Shinohara T, Hotta K, Oyama T. Rectal carcinoid tumor, 6 mm in diameter, with lymph node metastases. Endoscopy. 2008;40 Suppl 2:E40-E41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Jetmore AB, Ray JE, Gathright JB, McMullen KM, Hicks TC, Timmcke AE. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum. 1992;35:717-725. [PubMed] |
| 8. | Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol. 1993;88:1949-1953. [PubMed] |
| 9. | Kim BN, Sohn DK, Hong CW, Han KS, Chang HJ, Jung KH, Lim SB, Choi HS, Jeong SY, Park JG. Atypical endoscopic features can be associated with metastasis in rectal carcinoid tumors. Surg Endosc. 2008;22:1992-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Lee SH, Kim BC, Chang HJ, Sohn DK, Han KS, Hong CW, Lee EJ, Lee JB, Lee DS, Lee IT. Rectal neuroendocrine and L-cell tumors: diagnostic dilemma and therapeutic strategy. Am J Surg Pathol. 2013;37:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Rifkin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology. 1989;170:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 208] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Balthazar EJ, Megibow AJ, Hulnick D, Naidich DP. Carcinoma of the colon: detection and preoperative staging by CT. AJR Am J Roentgenol. 1988;150:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 168] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Kasuga A, Chino A, Uragami N, Kishihara T, Igarashi M, Fujita R, Yamamoto N, Ueno M, Oya M, Muto T. Treatment strategy for rectal carcinoids: a clinicopathological analysis of 229 cases at a single cancer institution. J Gastroenterol Hepatol. 2012;27:1801-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Kim MS, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Clinical outcomes for rectal carcinoid tumors according to a new (AJCC 7th edition) TNM staging system: a single institutional analysis of 122 patients. J Surg Oncol. 2013;107:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Li AF, Hsu CY, Li A, Tai LC, Liang WY, Li WY, Tsay SH, Chen JY. A 35-year retrospective study of carcinoid tumors in Taiwan: differences in distribution with a high probability of associated second primary malignancies. Cancer. 2008;112:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Shields CJ, Tiret E, Winter DC. Carcinoid tumors of the rectum: a multi-institutional international collaboration. Ann Surg. 2010;252:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Ramage JK, Davies AH, Ardill J, Bax N, Caplin M, Grossman A, Hawkins R, McNicol AM, Reed N, Sutton R. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54 Suppl 4:iv1-i16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Yoon SN, Yu CS, Shin US, Kim CW, Lim SB, Kim JC. Clinicopathological characteristics of rectal carcinoids. Int J Colorectal Dis. 2010;25:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |