Published online Dec 14, 2015. doi: 10.3748/wjg.v21.i46.13177
Peer-review started: May 28, 2015
First decision: June 20, 2015
Revised: June 27, 2015
Accepted: September 14, 2015
Article in press: September 15, 2015
Published online: December 14, 2015
Processing time: 196 Days and 8.6 Hours
AIM: To report a systematic review, establishing the available data to an unpublished 2a strength of evidence, better handling clinical practice.
METHODS: A systematic review was performed using MEDLINE, EMBASE, Cochrane, LILACS, Scopus and CINAHL databases. Information of the selected studies was extracted on characteristics of trial participants, inclusion and exclusion criteria, interventions (mainly, mucosal resection and submucosal dissection vs surgical approach) and outcomes (adverse events, different survival rates, mortality, recurrence and complete resection rates). To ascertain the validity of eligible studies, the risk of bias was measured using the Newcastle-Ottawa Quality Assessment Scale. The analysis of the absolute risk of the outcomes was performed using the software RevMan, by computing risk differences (RD) of dichotomous variables. Data on RD and 95%CIs for each outcome were calculated using the Mantel-Haenszel test and inconsistency was qualified and reported in χ2 and the Higgins method (I2). Sensitivity analysis was performed when heterogeneity was higher than 50%, a subsequent assay was done and other findings were compiled.
RESULTS: Eleven retrospective cohort studies were selected. The included records involved 2654 patients with early gastric cancer that filled the absolute or expanded indications for endoscopic resection. Three-year survival data were available for six studies (n = 1197). There were no risk differences (RD) after endoscopic and surgical treatment (RD = 0.01, 95%CI: -0.02-0.05, P = 0.51). Five-year survival data (n = 2310) showed no difference between the two groups (RD = 0.01, 95%CI: -0.01-0.03, P = 0.46). Recurrence data were analized in five studies (1331 patients) and there was no difference between the approaches (RD = 0.01, 95%CI: -0.00-0.02, P = 0.09). Adverse event data were identified in eight studies (n = 2439). A significant difference was detected (RD = -0.08, 95%CI: -0.10--0.05, P < 0.05), demonstrating better results with endoscopy. Mortality data were obtained in four studies (n = 1107). There was no difference between the groups (RD = -0.01, 95%CI: -0.02-0.00, P = 0.22).
CONCLUSION: Three-, 5-year survival, recurrence and mortality are similar for both groups. Considering complication, endoscopy is better and, analyzing complete resection data, it is worse than surgery.
Core tip: As clinical and oncological outcomes of endoscopic resection of early gastric cancer compared to surgery have not been reported in systematic reviews, this study adds an important value to scientific literature, as it establishes and unifies data regarding this comparison. There are only retrospective cohort studies on this topic (2b evidence-level according to Oxford Centre). This review brings the information to an unpublished 2a strength of evidence, better handling nowadays clinical practice.
-
Citation: Kondo A, de Moura EGH, Bernardo WM, Yagi OK, de Moura DTH, de Moura ETH, Bravo JGP, Yamazaki K, Sakai P. Endoscopy
vs surgery in the treatment of early gastric cancer: Systematic review. World J Gastroenterol 2015; 21(46): 13177-13187 - URL: https://www.wjgnet.com/1007-9327/full/v21/i46/13177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i46.13177
Almost one million new cases of stomach cancer were estimated to have occurred in 2012 (952000 cases, 6.8% of the total), making it the fifth most common malignancy in the world, after cancers of the lung, breast, colorectum and prostate[1]. Despite advances in diagnosis and treatment, prognosis of gastric cancer remains poor, usually attributed to diagnosis at a late stage.
Early gastric cancer (EGC) was defined by the Japanese Society of Gastroenterological Endoscopy in 1962 as adenocarcinoma involving mucosa or submucosa irrespective of nodal status[2]. While radical gastrectomy can achieve adequate oncological clearance with wide resection margins and lymphadenectomy, it poses significant perioperative morbidities and compromises long-term gastrointestinal function as well as quality of life[3]. With the low risk of lymph node metastasis, methods of endoscopic treatment were pioneered for EGC[4].
According to current guidelines, absolute indication for endoscopic treatment of EGC is defined as moderately or well-differentiated intramucosal adenocarcinoma that is elevated and smaller than 2 cm in diameter or is depressed and smaller than 1 cm without ulceration[5]. The absolute (standard) indication criteria, however, is so strict that unnecessary surgeries are likely performed[6]. Therefore, expanded criteria for endoscopic resection was suggested[5] and many reports have shown good results. Gotoda et al[6] proposed this criteria: (1) mucosal cancer without ulcer findings, irrespective of tumor size; (2) mucosal cancer with an ulcer ≤ 3 cm in diameter; and (3) minimal (≤ 500 μm from the muscularis mucosa) submucosal invasive cancer ≤ 3 cm in size.
This study adds an important value to scientific literature as it establishes, unifies and, accordingly, set the available published data regarding the best way to treat ECG.
Clinical and oncological outcomes of endoscopic resection in patients with EGC, compared to surgery, have not been reported in systematic reviews.
This systematic review and meta-analyses was developed to address the short- and long-term outcomes of endoscopic resection compared to surgery in the treatment of EGC.
This study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses)[7] recommendations. The review was registered on PROSPERO international database (http://www.crd.york.ac.uk/prospero/)[8] under number CRD42014015127.
Types of studies: Clinical trials and/or observational studies of adequate quality; Types of participants: Patients with EGC; Types of intervention: Endoscopic treatment and surgery; Types of outcome measures: Basically, procedure-related adverse event, short- and long-term survival rates.
Studies were identified searching electronic databases and scanning reference lists of the selected articles. No limits were initially applied for language. This search was applied for Medline and EMBASE (considering all years), Cochrane and LILACS (via BVS), Scopus and CINAHL (via EBSCO). The last manual search was run on January, 20th, 2015 and automatic updates were evaluated for new studies monthly until April, 2015 for Medline.
The following search strategy, stratified by the components Population (P), Intervention (I) and Comparison (C), based on MeSH terms, was first used for MEDLINE, named MAINMEDLINE for abbreviation purposes: P: “{[Gastric cancer (MeSH)] OR [(Adenocarcinoma OR Carcinoma OR Early Detection of Cancer OR Carcinoma in Situ OR Polyps) AND (Stomach OR Gastric)] OR [(Gastric Mucosa OR Early Diagnosis) AND (Gastric Cancer)]} AND I: [Endoscopy (MeSH) OR Endoscopy, Digestive System (MeSH) OR Endoscopy, Gastrointestinal (MeSH)] AND C: [Gastrectomy (MeSH) OR Digestive System Surgical Procedures (MeSH) OR Surgery (Subheading) OR General Surgery (MeSH) OR Minimally Invasive Surgical Procedures (MeSH)]”.
Aiming high quality studies, MAINMEDLINE was filtered twice (#1 and #2): #1 “(MAINMEDLINE AND random*)”; and #2 “(MAINMEDLINE AND therapy/broad [filter]). The totality of articles was obtained mixing the investigation in the Pubmed Advanced Search Builder: “(#1 OR #2).” The Medline search strategies were peer reviewed (Kondo A and Bernardo WM).
The EMBASE search was: “[(gastric cancer AND endoscopy AND surgery) AND (“clinical trial” OR “controlled study” OR “major clinical study” OR “prospective study” OR “retrospective study”)]”. For Cochrane, LILACS, Scopus and CINAHL, the search was: “(gastric cancer AND endoscopy AND surgery)”.
Eligibility assessment and the selection of screened records were performed independently in an unblinded standardized manner by the reviewers. Disagreements were resolved by consensus.
The method of data extraction consisted of filling out information sheets. A Scottish Intercollegiate Guidelines Network (SIGN)[9] based checklist was used. Relevant data were extracted from each included study using a standardized extraction form. Extracted data were double checked by the reviewers. Disagreements were resolved by discussion.
Information was extracted on: (1) characteristics of trial participants (including age and pattern of different types of EGC), trials’ inclusion and exclusion criteria, length of follow-up; (2) interventions (considering different modalities in endoscopic treatment, mainly mucosal resection and submucosal dissection; vs surgical approach: tailored gastrectomy and respective lymphadenectomy, if necessary, according to tumor stage); and (3) outcomes (adverse events, different survival rates, mortality, recurrence and complete resection rates).
Overall 3- and 5-year survival rates included patients alive in these analyzed follow-up periods, despite the presence or absence of cancer.
Treatment-related morbidity and adverse events include any adverse procedure-related implication.
Complete resection was considered the total removal of gastric cancer, using surgical or endoscopic approach, with no residual viable cell on the local procedure lay.
Recurrence was characterized by the reappearance of gastric cancer after its treatment, and after a period of no clinical or imagenological detection. It was considered both local and distant recurrence for analysis. Metachronous gastric cancer was not considered in this set.
Mortality data are based on procedure-related death.
Risk of bias in individual studies: To ascertain the validity of eligible studies, the reviewers measured the risk of bias using the Newcastle-Ottawa Quality Assessment Scale[10] for cohort studies and the Scottish Intercollegiate Guidelines Network checklist[9]. The critical evaluation of the included trials should reveal a score ≥ 6, total of 9 highest possible score. The levels of evidence according to the Oxford Centre for Evidence-based Medicine[11] (2009 classification) were obtained.
Summary measures: The analysis of the absolute risk of survival rates, adverse event, complete resection, recurrence and mortality rates was performed. Also, data on absolute risk reduction (ARR) or increase (ARI) and number needed to treat (NNT) or harm (NNH) were analyzed on main outcomes, with 95%CI.
Planned methods of analysis: The analysis was performed using the software Review Manager (RevMan) 5.3[12], by computing risk differences (RD) of dichotomous variables using fixed-effects model and providing the respective forest and funnel plots. Data on RD and 95%CIs for each outcome were calculated using the Mantel-Haenszel test and inconsistency (heterogeneity) was qualified and reported in χ2 and the Higgins method, termed I2. Data on ARR or ARI and NNT or NNH were obtained using the Critically Appraised Topic (CAT)[13] software. The statistical review of the study was performed by a biomedical statistician (Bernardo WM).
Risk of bias across studies: Risks of publication bias for outcomes across studies were plotted (funnel plots) and identified (outliers detection), along with I2 quantitative analysis.
Additional analyses: The cut-off value for heterogeneity of 50% was assumed to be adequate for this meta-analysis. Trying to find out if the results from this meta-analysis are adequate and reliable for medical practice and not arbitrary or based in unclear data, sensitivity analysis was performed when heterogeneity (I2) was higher than 50%. A subsequent assay was done, excluding the outliers, and other findings were compiled. When outliers were not detected, true heterogeneity was presumed, i.e., publication bias was excluded.
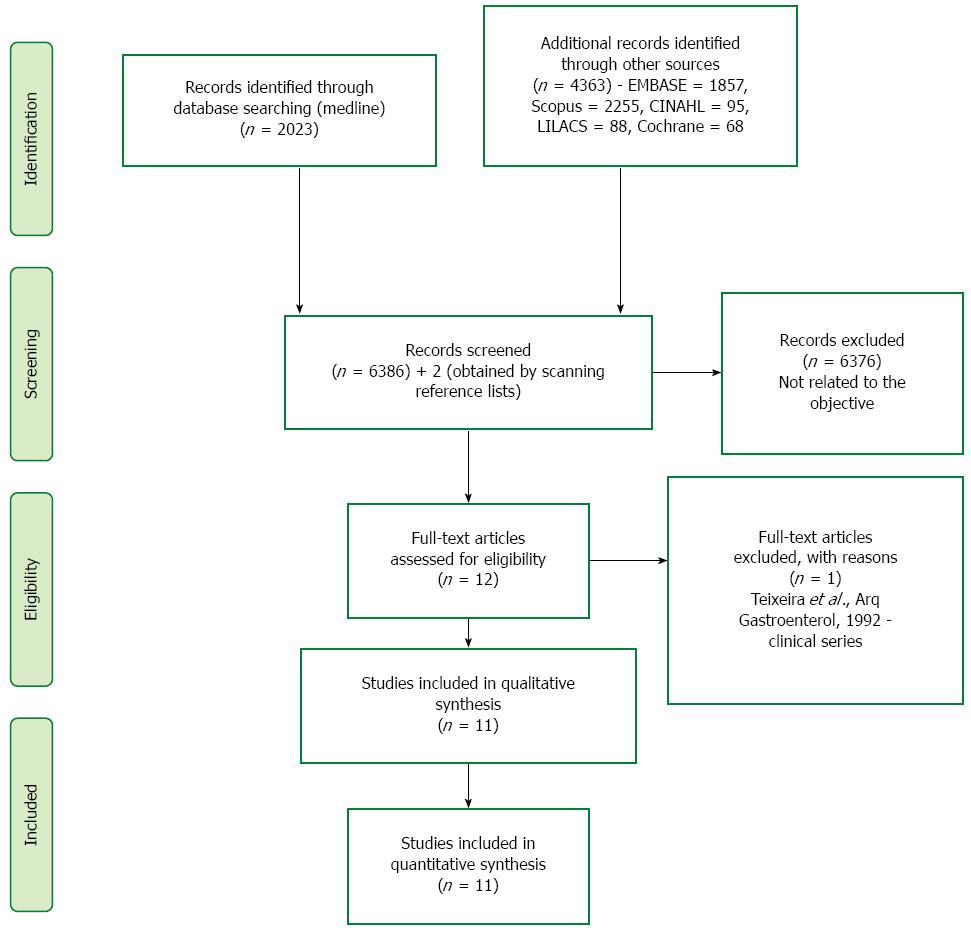
Six thousand, three hundred and eighty six (6386) studies were screened and the articles assessed for eligibility were selected after the title and abstract were read. Six thousand, three hundred and seventy six (6376) articles were excluded because they did not contemplate the following comparison. Ten studies compare endoscopic treatment vs surgery in the treatment of EGC. Scanning the reference lists of the selected articles, two more studies were found (Fukase et al[14] and Etoh et al[15]). These articles were not identified in the initial search strategy for two reasons. The study published by Fukase in 1994 did not have its journal (Digestive Endoscopy) indexed until then. The article written by Etoh in 2005 would be found if the Medline filter “[epidemiologic methods]”, not used, was applied. A clinical series study was excluded (Teixeira et al, Arq Gastroenterol, 1992). Eleven studies were included in quantitative and qualitative synthesis. An adapted PRISMA flow diagram illustrates the study selection process (Figure 1)[7].
Methods: The eleven studies selected were retrospective cohorts (RC). Ten were published in English and the other one was an article written in Japanese (translated by Yagi OK). No randomized controlled trials were found.
Participants: The included records involved 2654 patients. The inclusion criteria entailed adults with EGC that filled the absolute (AI) or expanded indication (EI) for endoscopic resection. As the number of studies was relatively small, all follow-up periods, in months, were adopted. The studies published by Fukase and Nishida et al[16] considers the Kaplan Meier curve.
Intervention: Different endoscopic treatment modalities were analyzed, mainly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Mucosectomy with ligation device (EMR-L) and high-frequency cauterization (HFC) was also verified.
Comparison: Tailored modalities of surgical procedures (mainly, gastrectomy ± lymphadenectomy) were compared to endoscopic approach.
Outcomes: The primary outcomes assessed were the short- and long-term survival rates (SR), e.g., 3-, 5- and 10-year. The secondary and additional outcomes were: adverse event (AER), complete resection (CRR), recurrence (RR) and mortality rates (MR). A summary of the characteristics of the included studies is shown in Table 1[14-24].
| Ref. | Study design | No. of patients | Inclusion criteria | EA | SA | Follow-up (median) | Outcomes |
| EA + SA | |||||||
| Nishida et al[16] | RC | 109 | AI | EMR, laser | Not specified | (2-3 yr) | 3-5 yr SR + CRR |
| Fukase et al[14] | RC | 175 | AI | EMR, HFC, laser | Not specified | 6 mo (5-10 yr) | 5-10 yr SR + CRR |
| Kim et al[17] | RC | 55 | AI | EMR-L | Gastr + lymphadenect | 35.3 mo + 29.3 mo | 3 yr SR + CRR + RR |
| Etoh et al[15] | RC | 93 | AI | EMR | Gastrectomy | 57 mo | 3-5 yr SR + CRR + AER + MR |
| Choi et al[18] | RC | 551 | AI | EMR | Gastrectomy | 81 mo + 88 mo | 3-5-10 yr SR + RR + AER + MR |
| Chiu et al[19] | RC | 114 | AI | ESD | Gastr + lymphadenect | 27 mo + 77.6 mo | 3 yr SR + AER + MR |
| Fukunaga et al[20] | RC | 287 | AI or EI | ESD | Not specified | 45.3 mo + 67.2 mo | 5 yr SR + AER + RR |
| Park et al[21] | RC | 225 | AI or EI | ESD | Gastr + lymphadenect | 17.6 mo + 24.2 mo | 5 yr SR + RR + AER + MR |
| Kim et al[22] | RC | 213 | AI or EI | ESD | Gastr + lymphadenect | 76.7 mo + 65.5 mo | 3 yr + 5 yr + CRR + AER + RR |
| Choi et al[23] | RC | 375 | AI | EMR, ESD | Gastr + lymphadenect | 76.4 mo | 5 yr SR + RR + AER |
| Kim et al[24] | RC | 457 | EI | EMR, ESD | Gastr + lymphadenect | 58.6 mo | 5 yr SR + RR + AER |
Using a standard approach with defined criteria, risk of bias was assessed to define methodological quality and the levels of evidence were compiled, as shown in Table 2[14-24].
| Ref. | Representativeness of exposed cohort and selection of the non exposed cohort (max. 2 points) | Ascertainment of exposure (max. 1 point) | Demonstration that outcome of interest was not present at start of study (max. 1 point) | Comparability of cohorts on the basis of the design or analysis (max. 2 points) | Assessment of outcome (max. 1 point) | Length and adequacy of follow up (max. 2 points) | Score and levels of evidence |
| Nishida et al[16] | 2 | 1 | 1 | 1 | 1 | 1 | 7 - 2b - acceptable (+) |
| Fukase et al[14] | 2 | 1 | 1 | 1 | 1 | 1 | 7 - 2b - acceptable (+) |
| Kim et al[17] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
| Etoh et al[15] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
| Choi et al[18] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
| Chiu et al[19] | 2 | 1 | 1 | 1 | 1 | 2 | 8 - 2b - acceptable (+) |
| Fukunaga et al[20] | 2 | 1 | 1 | 1 | 1 | 2 | 8 - 2b - acceptable (+) |
| Park et al[21] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
| Kim et al[22] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
| Choi et al[23] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
| Kim et al[24] | 2 | 1 | 1 | 2 | 1 | 2 | 9 - 2b - acceptable (+) |
Two studies obtained score 7 according to the Newcastle-Ottawa Quality Assessment Scale[10] for cohort studies. Other two scored 8 points and seven studies reached 9 points.
Aiming to increase confidence in the strength of association between exposure and outcomes, other parameters were used. According to the Scottish Intercollegiate Guidelines Network checklist[9], all studies were considered acceptable, i.e., having some flaws and an associated risk of bias (possibility of conclusion change in the light of further studies). The SIGN classification considers three category levels: 0 (low quality), + (acceptable) and ++ (high quality). The included studies were classified as having level of evidence 2b (2009 Oxford Centre for Evidence-based Medicine)[11].
The results of individual studies are show in absolute numbers in the next forest plots.
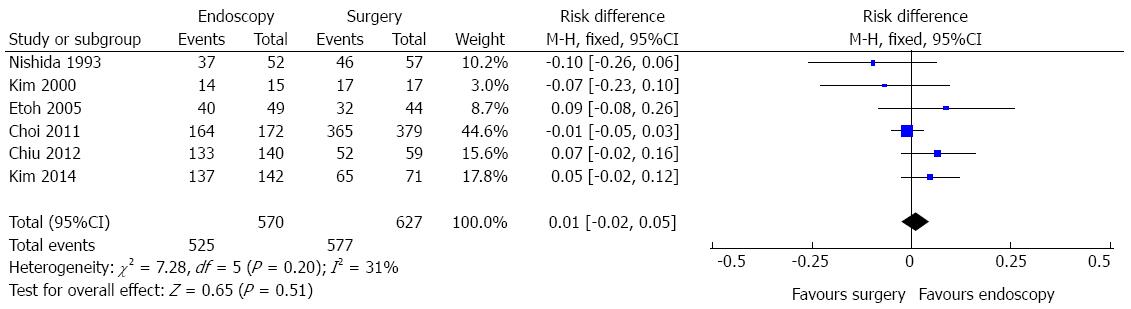
Three-year survival data were available for six studies, reporting information of 1197 patients, as shown in Figure 2. In the pooled analysis, there were no differences in 3-year survival data after endoscopic and surgical treatment of EGC [risk difference (RD) = 0.01, 95%CI: -0.02-0.05)]. Heterogeneity was low (I2 = 31%).
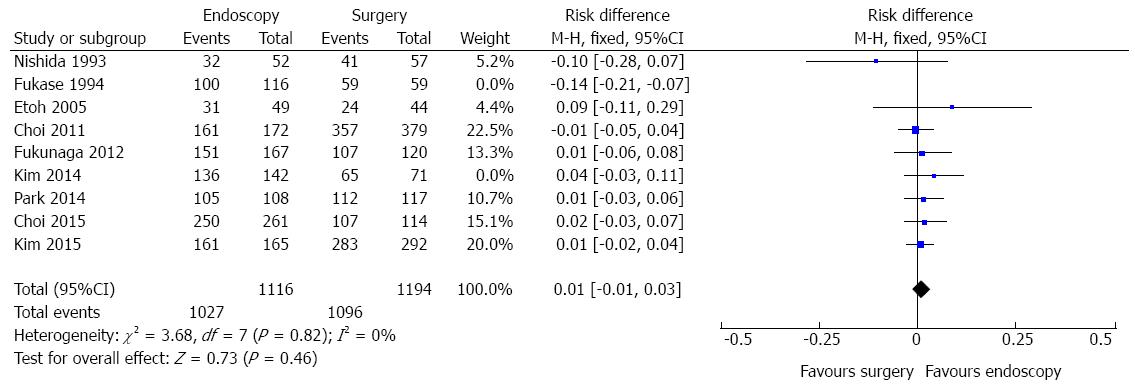
Five-year survival data were identified in nine studies. However, significant heterogeneity was initially detected (χ2 = 20.87 and I2 = 62%). Sensitivity analysis, through a funnel plot, identified one study (outlier) differing from the others (Fukase, 1994).
Exclusion of this study decreased the statistical heterogeneity to 0% (Figure 3) and did not affect the finding of no evidence of a difference in 5-year survival rate between endoscopic and surgical treatment of EGC (RD = 0.01, 95%CI: -0.01-0.03). Information of 2310 patients was obtained.
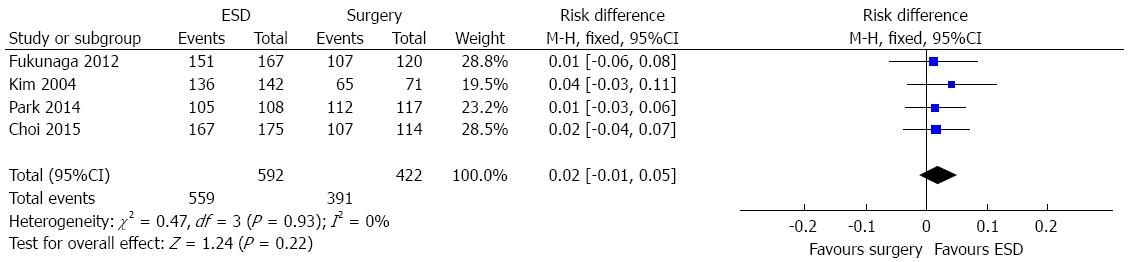
In a subgroup analysis, considering the available six studies comparing ESD vs surgery, four cohorts (1014 patients) evaluated the five-year survival. There was no difference between endoscopic and surgical treatment of EGC (RD = 0.02, 95%CI: -0.01-0.05), as shown in Figure 4.
Adverse events include any harmful procedure-related implications, such as anastomotic leakage, stenosis, perforation, hemorrhage, infection, mechanical ileus, herniation or, even, clinical illness decompensation (acute renal failure, pulmonary infection, myocardial infarction and so on).
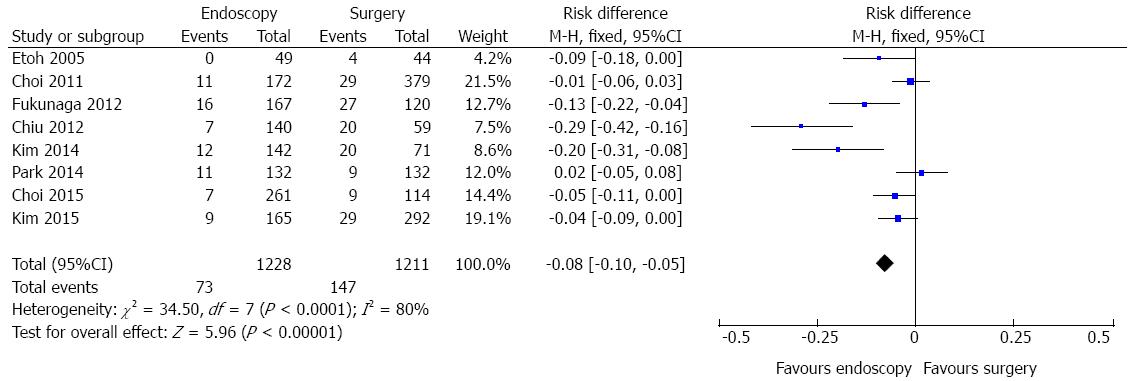
Adverse event data were identified in eight studies (Figure 5). A high heterogeneity was initially detected (χ2 = 34.50 and I2 = 80%), a funnel plot was drawn and two outliers detected (Chiu, 2012 and Kim, 2014). As, excluding these studies, heterogeneity persisted high (χ2 = 9.95 and I2 = 50%) and there was no change in diamond trend, they were kept for meta-analysis. The same finding was obtained if the random effects model was applied. Data analysis of 2439 patients showed significant difference (RD = -0.08, 95%CI: -0.10--0.05), demonstrating better results with endoscopic approach. The pooled NNT (1/[RD]) was 12.
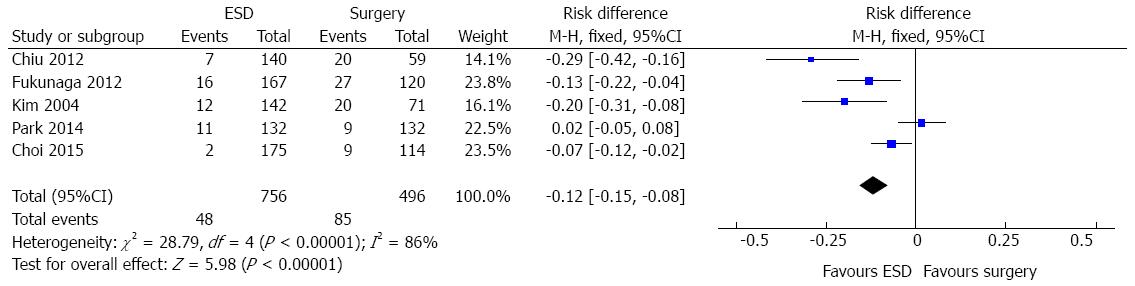
In the ESD subgroup analysis, five studies (1252 patients) evaluated the adverse event data. A high heterogeneity was initially detected (χ2 = 28.79 and I2 = 86%), a funnel plot was drawn and two outliers detected (Park, 2014 and Choi, 2015). As, excluding these studies, heterogeneity persisted high and there was no change in diamond trend, they were kept for meta-analysis. Data analysis showed significant difference [RD = -0.12, 95%CI: -0.15-(-0.08)], favouring endoscopic approach (Figure 6).
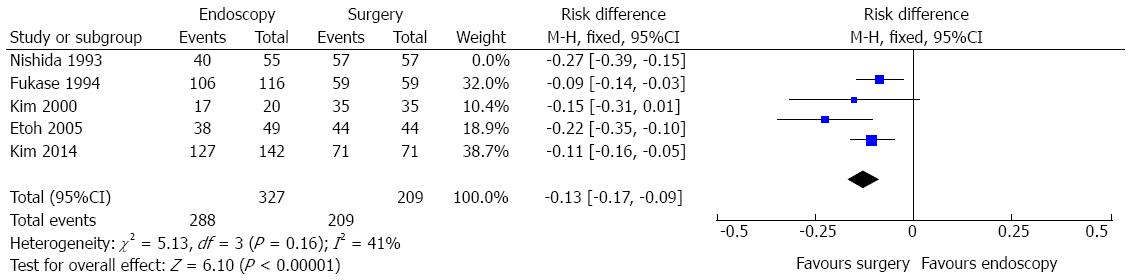
Complete resection data comparing endoscopic and surgical treatment of EGC were seen in five studies. The heterogeneity was represented by χ2 = 13.59 and I2 = 71%. The funnel plot analysis showed an outlier (Nishida, 1993). Excluding this study, heterogeneity dropped (I2 = 41%%) and there was no change in diamond trend. Data analysis, thus, was made considering a total amount of 536 patients and four studies. It showed significant difference in complete resection rates [RD = -0.13, 95%CI: -0.17-(-0.09)], exhibiting improved results in the surgical group (Figure 7). The pooled NNH (1/[RD]) was 7.
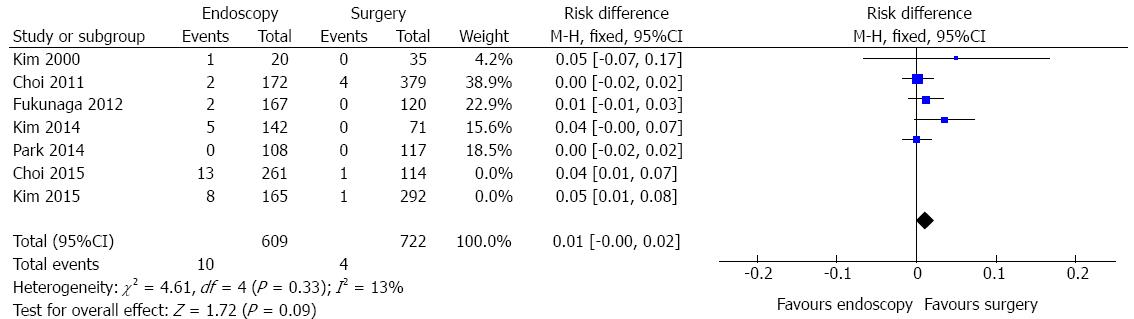
Recurrence data were identified in seven studies and the heterogeneity was represented by χ2 = 16.51 and I2 = 64%. The funnel plot analysis showed two outliers (Choi, 2015 and Kim, 2015). Excluding these studies, heterogeneity dropped (I2 = 13%) and there was a change in diamond trend. A total of 1331 patients were analyzed (Figure 8). There was no difference in terms of recurrence between endoscopic and surgical treatment of EGC (RD = 0.01, 95%CI: -0.00-0.02).
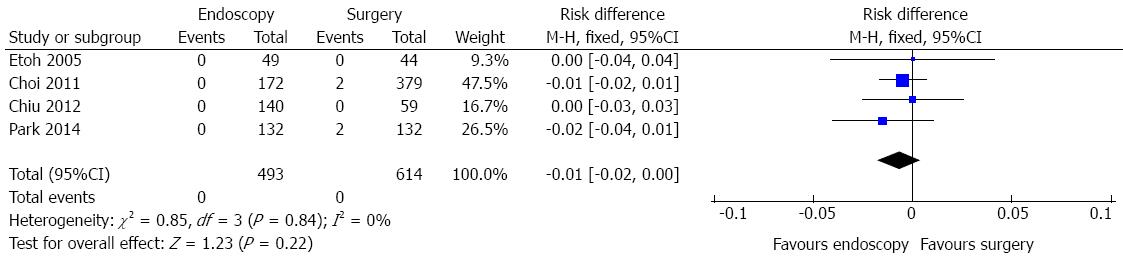
Mortality data include peritonitis due to perforation at the operation site, small bowel strangulation, splenic artery bleeding and anastomosis site leakage.
Mortality data were obtained in four studies and a total of 1107 patients were analyzed (Figure 9). No heterogeneity was detected (χ2 = 0.85 and I2 = 0%).In the pooled analysis, there was no evidence of a difference in terms of mortality between endoscopic and surgical treatment of EGC (RD = -0.01, 95%CI: -0.02-0.00).
The reported analysis combines data across studies in order to verify treatment effects with more precision than is possible in a single study. The greater advantage is that it increases the level of evidence of single retrospective cohort studies from 2b to 2a (systematic reviews of 2b studies) according to Oxford Centre[11].
Although gastrectomy (associated or not with lymphadenectomy) is the conventional oncologic surgical procedure to treat early gastric cancer, endoscopic approach has become an interesting and less invasive alternative for a select group of patients[5,6].
Basically, the two main endoscopic approaches consist of tumor resection, whether it is EMR or ESD. Many studies have demonstrated that endoscopic treatment of EGC is safe, even for elderly patients[25-29]. Nowadays, specially after 2004, EMR was replaced with ESD, which has higher rates of en bloc and complete endoscopic and pathological resection[30]. Even disregarding the oldest studies, published in the early 1990s by Nishida et al[16] and Fukase et al[14], when endoscopic resection was not completely and well-established, the three-, five-year survival rates and complete resection data did not significantly changed.
This systematic review fails to compare, on a subgroup analysis, the complete resection rates of ESD vs surgery. Although it is known that gastric tumor R0 resection is nearly 100% with surgical approach, no study regarding this comparison was found. According to Kim et al[22] and Choi et al[23], the complete resection rates, considering ESD, may reach 97.7% in some selected cases. These high total resection rates resulted, as seen previously in Figure 8, in lower recurrence rates, similar to surgery group. It is important to highlight that, even in situations of positive resection margins, an additional endoscopic treatment is feasible.
Although the expanded criteria proposed by Gotoda[6] was based on surgical specimens analysis, usually clinicians choose treatment modality based on limited information, such as endoscopic gross finding and histopathology of biopsy specimens[21]. It is known that, in some cases, a high rate of histologic discrepancy between endoscopic forceps biopsy and endoscopic resection specimens may be present[31]. An important limitation of the available studies is that the obtainable data includes retrospective final pathology after endoscopic resection and surgery, rather than the criteria of pretreatment evaluation. Nevertheless, all data considered only patients with EGC, adding, on the other hand, more strength to the evidence. However, as previously cited, recent studies have implied that long-term outcomes of ESD for non-differentiated EGC are great[32,33].
Some recent studies indicate comparable results between absolute and expanded indications, and considering all variables involved in treatment decision, a less harmful position should be first considered and, if possible, adopted. Thus, a cautious and careful approach on patients filling the expanded criteria ought to be chosen, once a limited number of studies, reporting equivalent outcomes to surgery, are published. An unexpected inadequate endoscopic resection or recurrence identification would be inconveniences related to this practice. Thus, well-designed prospective randomized clinical trials are needed to evaluate the outcomes of endoscopic resection, considering the expanded indications.
The decision of which treatment modality is the best comprises a complex interaction of factors, such as patient clinical condition and age, tumor characteristics, physician expertise (surgeon, pathologist and endoscopist) and hospital infrastructure.
Overall, the evidence of survival rates seems to be enough to determine the comparative effectiveness of endoscopic resections over surgical procedures, once no statistically significant difference was seen.
Analyzing the nine studies contemplating 5-year survival, only one study (Fukase et al[14], 1994) was discrepant from the others. This study was removed from pooled analysis (outlier). In a subgroup analysis, considering the available six studies comparing ESD vs surgery (1014 patients), no difference was verified.
Some other reports have shown that endoscopic treatment for EGC can achieve survival rates similar to those seen in patients that undergo surgical procedures, irrespective the criteria adopted. However, well-designed prospective randomized controlled trials should be performed to assign this statement properly.
Acceptable evidence was appointed regarding adverse event rates. Data analysis of 2439 patients showed significant difference favouring endoscopic treatment. The pooled NNT was 12. Considering that the selected studies were done in specialized centers, with high gastrointestinal surgical volume and excellence endoscopic units, reproducibility must be interpreted with caution. In the ESD vs surgery subgroup analysis (1252 patients), significant difference favouring endoscopic approach was also detected.
Data on complete resection rates confirm the advantages of surgical procedures in obtaining free resection margins, as they have wider boundaries. There was a significant difference favouring gastrectomy group (RD = 13%, P < 0.0001, NNH = 7). Although significantly more efficient in R0 resection, surgery incorporates an inherent risk of adverse events, even in experienced hands. It is reported a lower complete resection rates of EGC performing EMR[16,17]. More than one session was needed in some studies, although authors emphasize the possibility of sequential endoscopic resection procedures or, even, rescue surgery. Nowadays, enlarged dissections, such ESD, improve these results. This sequential approach seems to have no effect in survival rates, as evidenced.
Data on recurrence demonstrated no difference between endoscopic and surgical treatment after a complete tumor removal, i.e., R0 resection (RD = 1%, P = 0.09), irrespective the number of endoscopic sessions needed to achieve this objective. As mentioned above, endoscopic retreatment or salvage surgery may be the choice according to tumor restaging.
Mortality data showed no evidence of a difference in the four meta-analyzed studies (RD = 1%, P = 0.22).
Important to remember is that statistical significance of the effect does not always suggest clinical relevance, or likewise, a non-significant result may not mean the ineffectiveness of a treatment. All data should be considered in the context of different patients and settings.
Nowadays, unnecessary surgeries seem to be performed in some early cancers and elderly patients, what could improve morbidity and mortality rates. Recent studies report a beneficial impact, favouring prognosis, in selected patients when considering age and comorbidities. Although not addressed in this review, endoscopic treatment shows better perioperative outcomes in terms of procedure time, costs and hospital stay[18,19,21]. Thus, endoscopic treatment has its role increased in recent times due to technology development, indication expansion, tumor oncological aspects, patient clinical condition, early diagnosis and physician enthusiasm.
Despite the treatment guidelines, it is equally, or even more, important that these patients be treated in reference centers, used to a high-volume routine and host of specialized and meshed professionals, whatever the approach. Generalization to nonspecialized institutions requires discretion, aiming to improve outcomes.
Publication bias regards to a problematic tendency of researchers to handle the reporting of results that are positive and good, leading to a misleading bias in the overall published literature[34]. It was detected on funnel plot analysis for the outcomes of 5-year survival, complete resection and recurrence data.
A limitation within this meta-analysis is the presence of heterogeneity detected within some outcomes and low number of studies in this topic. Although sensitivity analysis was done, some degree of heterogeneity is inevitable and it may undermine the quality and legitimacy of the results obtained. The categorization of values for I2 considers adjectives of low, moderate and high to 25%, 50%, and 75%, respectively[35]. Quantification of heterogeneity is only one component of a wider investigation of variability across studies and the observed degree of inconsistency may have clinical implications.
The retrospective nature of the selected studies is also a limitation compared to prospectively and randomized collected database. The choice of endoscopic resection or surgery was not based on randomization. Although some studies used propensity-score matching to minimize selection biases, hidden ones may remain with the influence of unmeasured confounders.
In conclusion, addressing the outcomes of endoscopic resection compared to surgery in the treatment of early gastric cancer, this systematic review concludes that 3-, 5-year survival, recurrence and mortality rates are similar.
Considering procedure-related adverse event rates, endoscopic approach achieves significantly better results and, analyzing complete resection data, surgical procedures are preferred.
The authors thank Mrs. Marta Regina Rodrigues (departamental librarian), who helped with the selected studies’ aquisition.
Early gastric cancer (EGC) was defined as adenocarcinoma involving mucosa or submucosa irrespective of nodal status. While radical gastrectomy can achieve adequate oncological clearance, it poses significant perioperative morbidities. Thus, methods of endoscopic treatment were pioneered for EGC. This study adds an important value to scientific literature as it establishes, unifies and, accordingly, set the available published data regarding the best way to treat ECG.
Clinical and oncological outcomes of endoscopic resection in patients with EGC, compared to surgery, have not been reported in systematic reviews.
This systematic review and meta-analyses was developed to address the short- and long-term outcomes of endoscopic resection compared to surgery in the treatment of EGC. According to current guidelines, absolute indication for endoscopic treatment of EGC is so strict that unnecessary surgeries are likely performed. Therefore, expanded criteria for endoscopic resection was suggested. This study emphasizes the major points related to this topic, aiming to improve results in clinical practice.
This review suggests that, despite treatment guidelines, it is important that patients be treated in reference centers, used to a high-volume routine and host of specialized and meshed professionals, whatever the approach. Generalization to nonspecialized institutions requires discretion. The retrospective nature of the selected studies is a limitation. Prospective randomized controlled database is needed.
EGC was defined as adenocarcinoma involving mucosa or submucosa irrespective of nodal status. It can be treated by surgery (even conventional, laparoscopic or robotic approach) or endoscopy (resection procedures such as mucosectomy or endoscopic submucosal dissection).
In this systematic review, the authors have presented the clinical and oncological outcomes of endoscopic resection in patients with EGC, compared to surgery, bringing the information to an unpublished 2a strength of evidence, better handling nowadays clinical practice.
P- Reviewer: Hussain A, Vieth M S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | International Association of Cancer Registry (IACR): GLOBOCAN. 2012;. |
| 2. | Murakami T. Early cancer of the stomach. World J Surg. 1979;3:685-692. [PubMed] |
| 3. | Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, Di Leo A, Gaudio M, Nanni O, Carli A. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001;31:495-499. [PubMed] |
| 4. | Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579. [PubMed] |
| 5. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 6. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [PubMed] |
| 7. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7642] [Article Influence: 477.6] [Reference Citation Analysis (1)] |
| 8. | PROSPERO Centre for Reviews and Dissemination, University of York. “Guidance notes for registering a systematic review with PROSPERO″. Available from: http://www.crd.york.ac.uk/PROSPERO/. |
| 9. | SIGN Scottish Intercollegiate Guidelines Network, Healthcare Improvement Scotland, Edinburgh. Available from: http://www.sign.ac.uk/methodology/checklists.html. |
| 10. | Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 11. | CEBM Centre for Evidence-Based Medicine. Levels of Evidence (March 2009). Available from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. |
| 12. | (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2014; Available from: http://tech.cochrane.org/revman. |
| 13. | Centre for Evidence-Based Medicine, Headington, Oxford. Critically Appraised Topics (CAT). Available from: http://www.cebm.net/catmaker-ebm-calculators/. |
| 14. | Fukase K, Matsuda T, Suzuki M, Toda H, Okuyama Y, Sakai J, Saito H, Sato S, Mito S. Evaluation of the efficacy of endoscopic treatment for gastric cancer considered in terms of long-term prognosis: a comparison with surgical treatment. Dig Endosc. 1994;6:241-247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Etoh T, Katai H, Fukagawa T, Sano T, Oda I, Gotoda T, Yoshimura K, Sasako M. Treatment of early gastric cancer in the elderly patient: results of EMR and gastrectomy at a national referral center in Japan. Gastrointest Endosc. 2005;62:868-871. [PubMed] |
| 16. | Nishida T, Haruma K, Tanaka S, Inoue K, Teshima H, Yoshihara M, Tari A, Sumii K, Kajiyama G. [Comparison of endoscopic therapy and conventional surgery for the treatment of early gastric cancer in elderly patients]. Nihon Ronen Igakkai Zasshi. 1993;30:376-381. [PubMed] |
| 17. | Kim HS, Lee DK, Baik SK, Kim JM, Kwon SO, Kim DS, Cho MY. Endoscopic mucosal resection with a ligation device for early gastric cancer and precancerous lesions: comparison of its therapeutic efficacy with surgical resection. Yonsei Med J. 2000;41:577-583. [PubMed] |
| 18. | Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim do H, Lee JH, Kim MY, Kim BS, Oh ST. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Chiu PW, Teoh AY, To KF, Wong SK, Liu SY, Lam CC, Yung MY, Chan FK, Lau JY, Ng EK. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc. 2012;26:3584-3591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Fukunaga S, Machida H, Tominaga K, Tanaka H, Muguruma K, Ohira M, Nagami Y, Sugimori S, Okazaki H, Tanigawa T. Short- and long-term prognosis of patients with early gastric cancer: comparative analysis between endoscopic submucosal dissection and surgical operation. Gastrointest Endosc. 2012;75:Abst 234-235. [DOI] [Full Text] |
| 21. | Park CH, Lee H, Kim DW, Chung H, Park JC, Shin SK, Hyung WJ, Lee SK, Lee YC, Noh SH. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: a propensity-matched analysis. Gastrointest Endosc. 2014;80:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Kim DY, Hong SJ, Cho GS, Jeong GA, Kim HK, Han JP, Lee YN, Ko BM, Lee MS. Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: a retrospective cohort study. Gut Liver. 2014;8:519-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, Ryu KW, Nam BH, Kook MC, Kim YW. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333-341.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Kim YI, Kim YW, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Ryu KW, Kook MC. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy. 2015;47:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Tokioka S, Umegaki E, Murano M, Takeuchi N, Takeuchi T, Kawakami K, Yoda Y, Kojima Y, Higuchi K. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J Gastroenterol Hepatol. 2012;27 Suppl 3:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Hirasaki S, Tanimizu M, Nasu J, Shinji T, Koide N. Treatment of elderly patients with early gastric cancer by endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med. 2005;44:1033-1038. [PubMed] |
| 27. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Yahagi N, Omata M. Technical feasibility of endoscopic submucosal dissection for gastric neoplasms in the elderly Japanese population. J Gastroenterol Hepatol. 2007;22:311-314. [PubMed] |
| 28. | Toyokawa T, Fujita I, Morikawa T, Okamoto A, Miyasaka R, Watanabe K, Horii J, Gobaru M, Terao M, Murakami T. Clinical outcomes of ESD for early gastric neoplasms in elderly patients. Eur J Clin Invest. 2011;41:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Isomoto H, Ohnita K, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Nakao K, Kohno S. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur J Gastroenterol Hepatol. 2010;22:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Lee CK, Chung IK, Lee SH, Kim SP, Lee SH, Lee TH, Kim HS, Park SH, Kim SJ, Lee JH. Is endoscopic forceps biopsy enough for a definitive diagnosis of gastric epithelial neoplasia? J Gastroenterol Hepatol. 2010;25:1507-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, Hing C, Kwok CS, Pang C, Harvey I. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14:iii, ix-xi, 1-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |