Published online Dec 7, 2015. doi: 10.3748/wjg.v21.i45.12963
Peer-review started: March 30, 2015
First decision: June 2, 2015
Revised: June 30, 2015
Accepted: September 14, 2015
Article in press: September 14, 2015
Published online: December 7, 2015
Processing time: 252 Days and 6.6 Hours
We present the case of a 53-year-old woman with long-standing ulcerative colitis and severe, steroid-dependent disease course unresponsive to treatment with azathioprine, methotrexate, anti-TNF antibodies (infliximab, adalimumab) and tacrolimus, who refused colectomy as a therapeutic option. As the pro-inflammatory cytokine interleukin-6 (IL-6) had been identified as a crucial regulator in the immunopathogenesis of inflammatory bowel diseases, we treated the patient with biweekly intravenous infusions of an anti-IL-6R antibody (tocilizumab) for 12 wk. However, no clinical improvement of disease activity was noted. In fact, endoscopic, histological and endomicroscopic assessment demonstrated exacerbation of mucosal inflammation and ulcer formation upon anti-IL-6R therapy. Mechanistic studies revealed that tocilizumab treatment failed to suppress intestinal IL-6 production, impaired epithelial barrier function and induced production of pro-inflammatory cytokines such as TNF, IL-21 and IFN-γ. Inhibition of IL-6 by tocilizumab had no clinical benefit in this patient with intractable ulcerative colitis and even led to exacerbation of mucosal inflammation. Our findings suggest that anti-IL-6R antibody therapy may lead to aggravation of anti-TNF resistant ulcerative colitis. When targeting IL-6, the differential responsiveness of target cells has to be taken into account, as IL-6 on the one side promotes acute and chronic mucosal inflammation via soluble IL-6R signaling but on the other side also strongly contributes to epithelial cell survival via membrane bound IL-6R signaling.
Core tip: Interleukin (IL)-6 is regarded as a pro-inflammatory cytokine in the immunopathogenesis of inflammatory bowel diseases. Unexpectedly, this first reported case describes that anti-IL-6R antibody treatment led to aggravated inflammation in a severe ulcerative colitis patient. Mechanistic studies revealed that anti-IL-6R treatment failed to suppress intestinal IL-6 production, impaired epithelial barrier function and induced production of pro-inflammatory cytokines. Our case report demonstrates that differential responsiveness of target cells has to be taken into account in therapeutic approaches, as IL-6 promotes mucosal inflammation via soluble IL-6R signaling, but also strongly contributes to epithelial cell survival via mIL-6R signaling.
- Citation: Atreya R, Billmeier U, Rath T, Mudter J, Vieth M, Neumann H, Neurath MF. First case report of exacerbated ulcerative colitis after anti-interleukin-6R salvage therapy. World J Gastroenterol 2015; 21(45): 12963-12969
- URL: https://www.wjgnet.com/1007-9327/full/v21/i45/12963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i45.12963
Ulcerative colitis (UC) is defined as a chronic relapsing inflammatory bowel disease (IBD) that is pathologically characterized by intestinal inflammation and epithelial injury. Insights into the immunopathogenesis of UC have implicated that pro-inflammatory cytokines are critically involved in the induction and perpetuation of the inflammatory process[1]. Targeted anti-cytokine therapies are therefore considered as an attractive treatment option, which is best reflected by the advent of anti-TNF antibodies as an efficacious treatment option[2]. Nevertheless, in the pivotal clinical trials for anti-TNF agents in UC, the initial response rate was approximately 60%, with a considerable proportion of these patients losing response within one year[3]. Therefore alternative cytokine targeted approaches are being sought after.
Interleukin-6 (IL-6) has been implicated to play an important role in the immunopathogenesis of IBD[4]. In agreement with this concept, mucosal IL-6 expression has been found to be elevated in active IBD[5]. Furthermore, serum-levels of IL-6 correlated with clinical disease activity in UC patients[6]. As these observations provide strong evidence for a potential functional role of IL-6 in chronic intestinal inflammation, we decided to treat an UC patient refractory to conventional therapies with a humanized anti-IL-6 receptor (IL-6R) antibody.
The patient, a 53-year-old woman, was diagnosed with ulcerative pancolitis at the age of 28 years by histopathological criteria. She initially responded to combined therapy with oral (3 g) and local (2 g) aminosalicylates and later systemic corticosteroids, but showed recurrent inflammatory episodes in the following years. The patient developed a steroid-dependent disease course with a requirement for steroid therapy ≥ 10 mg/d. Azathioprine 100 mg (2 mg/kg) therapy was initiated in 2005, upon which clinical response was achieved for 6 mo. No endoscopic examinations were performed at that time to assess endoscopic response to azathioprine therapy. Upon subsequent relapses that required repeated prednisolone treatment, azathioprine treatment was stopped and methotrexate therapy was initiated in 2008 outside our clinic, but had to be discontinued due to severe skin reactions. Azathioprine therapy was again started thereafter, as the patient reported more aggravated disease without azathioprine therapy. Therapy with the anti-TNF antibody infliximab was initiated in 2010 in addition to azathioprine therapy due to chronic active disease. After an initial response for over one year, even an intensified therapy with infliximab (10 mg/kg every four weeks) failed to ameliorate UC activity and the treatment was stopped thereafter. Anti-TNF antibody therapy with adalimumab (initially 160 mg and 80 mg, then 40 mg every two weeks) in addition to ongoing azathioprine therapy likewise failed to ameliorate colitis activity and was stopped after 3 mo. Therapy with the calcineurin-inhibitor tacrolimus was initiated thereafter, but had to be discontinued due to impairment of renal function in 2013.
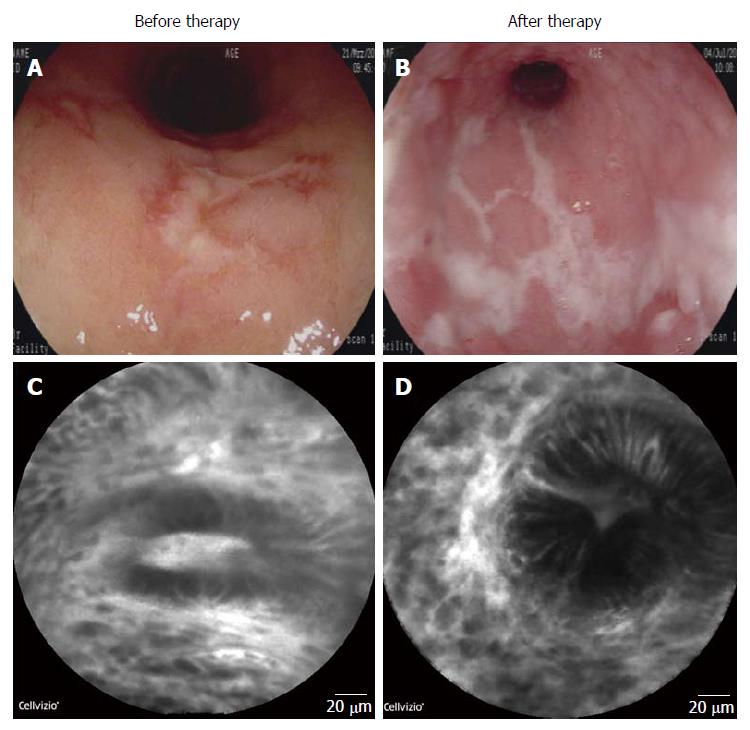
At this point the patient had up to 10 loose bowel movements per day with obvious blood. Blood count showed mild hypochromic anaemia (Hb 11.6 g/dL). C-reactive protein (CRP) levels were elevated (28.3 mg/L). The Truelove and Witts severity index indicated moderate disease. There was no tachycardia or pyrexia. Endoscopy revealed continuous colonic inflammation with enhanced granularity and isolated ulcerations (Figure 1A). The total Mayo score was 10, indicating severe disease. Endomicroscopic evaluation demonstrated dilated microvessels, leakage and disturbed crypt architecture as signs of mucosal inflammation (Figure 1C). Histopathological analysis of sigmoid biopsies by a pathologist resulted in a Riley histologic score[7] of 15 and a Geboes score of 0.3/1.3/2A.3/2B.3/3.2/4.3/5.4; both indicative of severe UC. An infection with cytomegalovirus (CMV) was repeatedly excluded and stool samples were always negative for infectious pathogens, including Clostridium difficile.
As the patient declined to undergo restorative proctocolectomy, no leucocytapheresis therapy was available nearby and the anti-adhesion molecule antibody vedolizumab had not been approved and was not available for therapy at that time, an anti-IL-6R antibody (tocilizumab) treatment was initiated with the understanding and appropriate prior informed consent of the patient. Azathioprine treatment was stopped beforehand.
Therapy with tocilizumab (Ro-Actemra, Hoffmann-La Roche, Switzerland) was intravenously administered (8 mg/kg) for 12 wk at biweekly intervals. Concomitant prednisolone therapy (10 mg/d) was unchanged during the treatment period. The therapy was well tolerated and no adverse events were recorded.
However, the patient showed no clinical improvement with persistence of 10 bloody stools per day. Blood count (Hb 12.5 g/dL) and CRP-level (1.2 mg/L) were normalised. Sigmoidoscopy up to 40 cm revealed augmented mucosal inflammation with progressive ulcer formation (Figure 1B). The total Mayo score remained at a score of 10. Endomicroscopy underlined increased signs of mucosal inflammation with enhanced leakage, impaired barrier function and disturbance of crypt architecture (Figure 1D). The Riley histologic score of sigmoid biopsies rose to 16 and the Geboes score was 0.2/1.3/2A.3/2B.3/3.2/4.3/5.4, both indicative of severe UC. As the patient refused surgical intervention and higher corticosteroid doses, she initially remained on prednisolone 10 mg/d. She later again needed intensified steroid treatment and was then put on therapy with the anti-adhesion molecule antibody vedolizumab. She showed partial clinical and endoscopic response to it. She still refused surgical intervention as a therapeutic option.
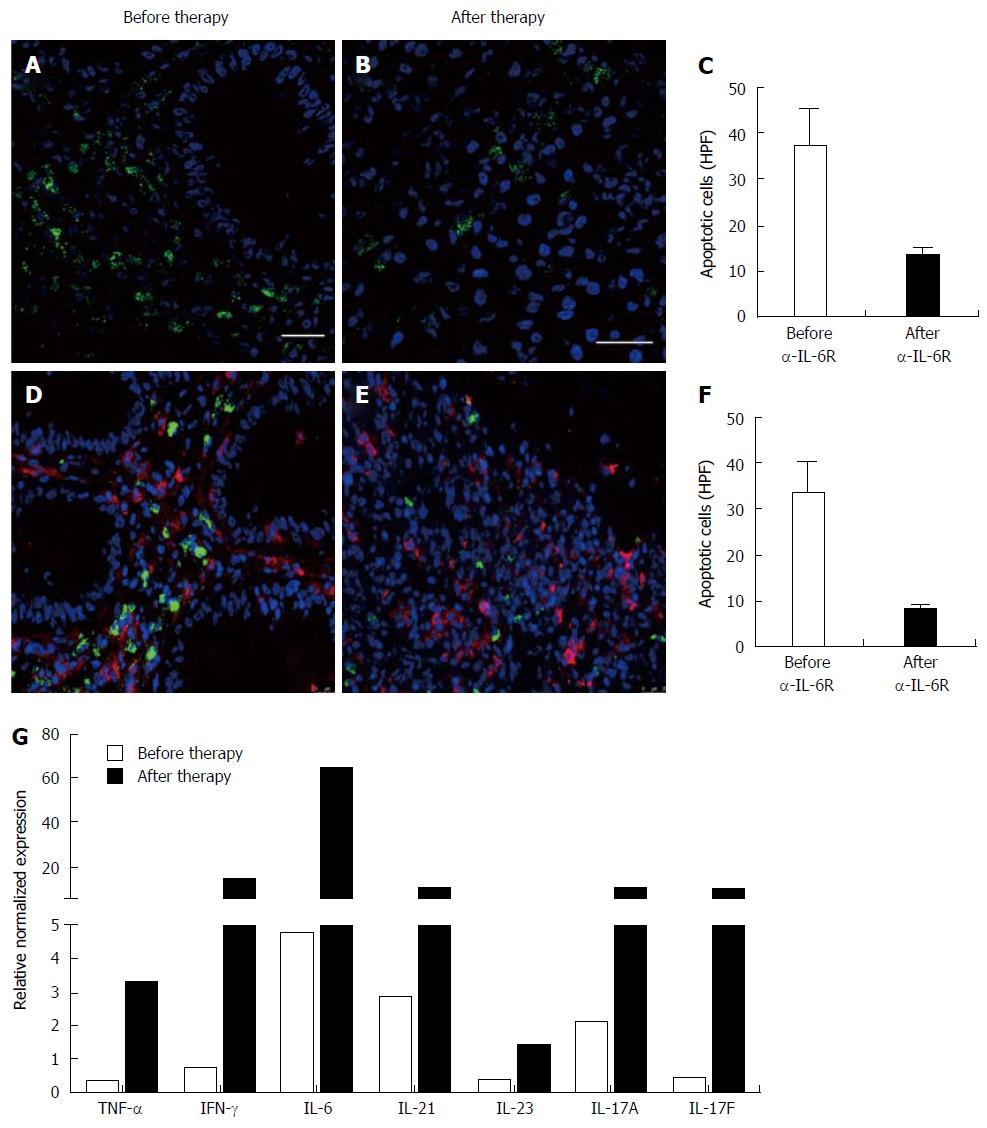
Immunohistochemistry revealed that tocilizumab treatment was not able to induce apoptosis in lamina propria mononuclear cells or in the subgroup of CD4+ mucosal T cells, and there was a significantly reduced amount of apoptotic cells upon treatment (Figure 2 A-F). Quantitative gene expression analysis showed that tocilizumab application was not able to suppress mucosal IL-6 levels. Instead there were even higher IL-6 levels after completion of therapy, suggesting that blockade of IL-6 signalling may induce compensatory IL-6 production. Other pro-inflammatory cytokines such as TNF, IFN-γ, IL-21 and IL-17A/F also showed higher expression levels after completion of therapy compared to levels before commencement of anti-IL-6R treatment (Figure 2G).
Altogether, clinical, endoscopic, endomicroscopic and histologic evaluation demonstrated that IL-6R inhibition was not able to exert a therapeutic effect in our patient. Instead, signs of aggravated mucosal inflammation and ulcer formation could be observed upon anti-IL-6R treatment.
Remarkable advances in our understanding of the immunpathogenesis of IBD have enabled the development of therapeutic agents directed at rational molecular targets. This approach is best exemplified by the selective blockade of pro-inflammatory cytokines by biological agents. The substantial therapeutic success of anti-TNF antibodies in the treatment of UC patients has validated this concept. Nevertheless, a relevant subgroup of patients does not respond to anti-TNF therapy, as they show little or no changes of clinical symptoms[3]. Selective targeting of other cytokines profoundly involved in the immunopathogenesis of IBD has therefore been the centre of attention for designing novel therapeutic strategies. Recently completed phase 2 trials with antibodies directed against interleukin-13 however did not reach their primary therapeutic endpoints in the treatment of UC patients[8,9]. Similarly, inhibition of IL-2R signaling with the monoclonal antibody basiliximab did not result in improvement of disease activity[10].
The cytokine IL-6 has been found to be a major regulator of T cell differentiation and activation and promotes a pro-inflammatory milieu in chronic inflammatory diseases. In UC, serum levels of IL-6, which is an important factor for the synthesis of acute phase proteins like CRP, correlate well with clinical disease activity and correspondingly mucosal IL-6 and soluble IL-6R (sIL-6R) production are increased in active disease[11]. It has also been shown that effective treatment of IBD with corticosteroids resulted in an inhibition of IL-6 production in the lamina propria, while patients who suffer from intractable disease exhibit augmented mucosal IL-6 levels[12]. Finally, application of a neutralizing anti-IL6R antibody caused significant suppression of mucosal inflammation in different experimental colitis models[4,13].
As the above data indicated a pivotal role of IL-6 in chronic intestinal inflammation and targeting of IL-6 signaling had already shown therapeutic efficacy in chronic and autoimmune diseases like rheumatoid arthritis[14], a pilot study on IL-6R blockade was initiated in patients with active Crohn’s disease. In this study, the anti-IL6R antibody tocilizumab or placebo was given as biweekly intravenous infusions over a treatment period of 12 wk. It was found that 80% of the tocilizumab treated patients had signs of clinical response, as compared with 31% in the placebo group. Moreover, 20% of the patients on this regimen went into remission as compared to 0% of the placebo group[15].
Based on these promising results, we initiated tocilizumab therapy in a patient with treatment-refractory UC. Unexpectedly, tocilizumab treatment did not ameliorate colitis activity and resulted in even aggravated mucosal inflammation and ulcer formation.
The complex of IL-6 and sIL-6R has been previously shown to activate gp130-positive lamina propria T cells lacking the membrane bound IL-6R in IBD. This so called trans-signalling process leads to STAT-3 activation and induction of anti-apoptotic genes like bcl-2 and bcl-xl within mucosal T cells. Augmented lamina propria T cell resistance against apoptosis then results in unrestrained accumulation of activated intestinal lymphocytes which perpetuate the inflammatory response[4]. In agreement with this anti-apoptotic role of IL-6, immunohistochemical stainings of colonic tissue samples from Crohn’s disease patients treated with tocilizumab showed that inhibition of IL-6 results in the induction of apoptosis in lamina propria mononuclear cells (LPMCs)[15]. However, in our UC patient tocilizumab application did not lead to the induction of apoptosis in LPMCs or mucosal CD4+ T cells, which is believed to be the central anti-inflammatory mechanism of action of anti-IL6R blockade in intestinal inflammation. Instead, we observed even a marked reduction of apoptosis in LPMCs after tocilizumab treatment compared to the staining prior to the initiation of therapy, suggesting that IL-6R blockade may predominantly cause pro-inflammatory effects in UC.
Apart from its pro-inflammatory role, IL-6 also contributes to the maintenance of epithelial cell homeostasis, as it is involved in epithelial repair and healing, resulting in mucosal reconstitution[16,17]. In contrast to IL-6, TNF has pro-inflammatory effects on the gut epithelium and may induce death of intestinal epithelial cells in IBD[18,19]. Thus, in contrast to TNF blockade, inhibition of IL-6 might have detrimental effects on the epithelial barrier function via blockade of the membrane bound IL-6R (mIL-6R) on intestinal epithelial cells. This adverse effect might be reflected by the reported heightened risk of gastrointestinal perforations upon tocilizumab treatment in rheumatoid arthritis patients[20]. Furthermore, one report showed the formation of multiple mucosal ulcers in the small and large intestine during tocilizumab treatment in rheumatoid arthritis[21]. Consistent with these reported clinical observations, tocilizumab treatment led to augmented mucosal ulcers in our UC patient, probably due to further impairment of epithelial barrier integrity with subsequent activation of mucosal immune cells resulting in pro-inflammatory cytokine production (TNF, IFN-γ, IL-21 or IL-17A/F) and progressive ulcer formation. The normalization of CRP levels under tocilizumab treatment do not correlate with the enhanced mucosal inflammation and might be explained by possible suppression of IL-6 levels in the blood, which may have resulted in diminished CRP blood levels. IL-6 blood levels were not measured in the patient. The discrepancy between persisting mucosal inflammation and normal CRP levels are in line with similar reports in UC patients[22].
The presented case report does not support the application of anti-IL6R antibodies in UC patients with anti-TNF refractory, severely progressed disease. When targeting IL-6, the differential responsiveness of target cells have to be taken into account, as IL-6 on the one side promotes acute and chronic mucosal inflammation via sIL-6R signaling but on the other side also strongly contributes to epithelial cell survival via mIL-6R signaling. Future therapeutic strategies in therapy refractory UC should aim to selectively target the pro-inflammatory function of IL-6, for instance by targeting sIL-6R signaling. Additionally, novel Janus kinase inhibitors (Jak) such as tofacitinib, that block the function of multiple Jak dependent pro-inflammatory cytokines simultaneously[23], should be explored in anti-TNF refractory UC.
In summary, this is the first reported case of IL-6 inhibition in a therapy-refractory severe ulcerative colitis patient. Although a potentially pro-inflammatory role of IL-6 has been suggested in disease pathogenesis[4], our anti-IL-6R antibody treatment led to aggravated mucosal inflammation and does therefore not advocate its application in severe UC patients.
A 53-year-old woman presented with treatment-refractory ulcerative colitis.
Clinical active, severe ulcerative colitis with diarrhoea and rectal bleeding.
Infectious colitis, Clostridium difficile colitis, CMV colitis.
HgB 11.6 g/dL, CRP 28.3 mg/L, kidney and liver function tests were within normal limits.
Endomicroscopy and endoscopy revealed continuous colonic inflammation with isolated ulcerations.
Histopathological analysis of sigmoid biopsies was indicative of severe ulcerative colitis.
The patient was treated with an anti-interleukin (IL)-6R antibody (tocilizumab).
This is the first reported case of IL-6 inhibition in severe, therapy-refractory ulcerative colitis.
The anti-IL-6R antibody tocilizumab has been approved for the treatment of rheumatoid arthritis but has until now not been used in severe ulcerative colitis.
This case report documents that anti-IL-6R antibody therapy had no clinical benefit in a patient with intractable ulcerative colitis and even led to exacerbation of mucosal inflammation.
This article is the first case report of anti-IL6R antibody treatment in a patient with severe, treatment-refractory ulcerative colitis.
P- Reviewer: Herculano R S- Editor: Yu J L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1971] [Article Influence: 179.2] [Reference Citation Analysis (1)] |
| 2. | Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Danese S, Colombel JF, Peyrin-Biroulet L, Rutgeerts P, Reinisch W. Review article: the role of anti-TNF in the management of ulcerative colitis -- past, present and future. Aliment Pharmacol Ther. 2013;37:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [PubMed] |
| 5. | Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. 1993;104:1285-1292. [PubMed] |
| 6. | Umehara Y, Kudo M, Nakaoka R, Kawasaki T, Shiomi M. Serum proinflammatory cytokines and adhesion molecules in ulcerative colitis. Hepatogastroenterology. 2006;53:879-882. [PubMed] |
| 7. | Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174-178. [PubMed] |
| 8. | Reinisch W, Panés J, Khurana S, Toth G, Hua F, Comer GM, Hinz M, Page K, O’Toole M, Moorehead TM. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Danese S, Rudziński J, Brandt W, Dupas JL, Peyrin-Biroulet L, Bouhnik Y, Kleczkowski D, Uebel P, Lukas M, Knutsson M. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut. 2015;64:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Sands BE, Sandborn WJ, Creed TJ, Dayan CM, Dhanda AD, Van Assche GA, Greguš M, Sood A, Choudhuri G, Stempien MJ. Basiliximab does not increase efficacy of corticosteroids in patients with steroid-refractory ulcerative colitis. Gastroenterology. 2012;143:356-364.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 443] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 12. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. [PubMed] |
| 13. | Yamamoto M, Yoshizaki K, Kishimoto T, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878-4882. [PubMed] |
| 14. | Nishimoto N, Yoshizaki K, Maeda K, Kuritani T, Deguchi H, Sato B, Imai N, Suemura M, Kakehi T, Takagi N. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol. 2003;30:1426-1435. [PubMed] |
| 15. | Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989-996; discussion 947. [PubMed] |
| 16. | Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089-1097. [PubMed] |
| 17. | Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut. 2010;59:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000-2004. [PubMed] |
| 19. | Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, Hildner K, Hoffman A, Kiesslich R, Rink AD. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14+ macrophages. Gastroenterology. 2011;141:2026-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Gout T, Ostör AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol. 2011;30:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Iwasa T, Nakamura K, Ogino H, Itaba S, Akiho H, Okamoto R, Iboshi Y, Aso A, Murao H, Kanayama K. Multiple ulcers in the small and large intestines occurred during tocilizumab therapy for rheumatoid arthritis. Endoscopy. 2011;43:70-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, Moum B. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |