Published online Nov 28, 2015. doi: 10.3748/wjg.v21.i44.12696
Peer-review started: March 29, 2015
First decision: June 19, 2015
Revised: July 8, 2015
Accepted: September 13, 2015
Article in press: September 14, 2015
Published online: November 28, 2015
Processing time: 244 Days and 12.4 Hours
AIM: To review applications of confocal laser endomicroscopy (CLE) in pancreatobiliary lesions and studies that assessed training and interpretation of images.
METHODS: A computerized literature search was performed using OVID MEDLINE, EMBASE, Cochrane library, and the ISI Web of Knowledge from 1980 to October 2014. We also searched abstracts from major meetings that included the Digestive Disease Week, Canadian Digestive Disease Week and the United European Gastroenterology Week using a combination of controlled vocabulary and text words related to pCLE, confocal, endomicroscopy, probe-based confocal laser endomicroscopy, and bile duct to identify reports of trials. In addition, recursive searches and cross-referencing was performed, and manual searches of articles identified after the initial search was also completed. We included fully published articles and those in abstract form. Given the relatively recent introduction of CLE we included randomized trials and cohort studies.
RESULTS: In the evaluation of indeterminate pancreatobiliary strictures CLE with ERCP compared to ERCP alone can increase the detection of cancerous strictures with a sensitivity of (98% vs 45%) and has a negative predictive value (97% vs 69%), but decreased the specificity (67% vs 100%) and the positive predictive value (71% vs 100%) when compared to index pathology. Modifications in the classification systems in indeterminate biliary strictures have increased the specificity of pCLE from 67% to 73%. In pancreatic cystic lesions there is a need to develop similar systems to interpret and characterize lesions based on CLE images obtained. The presence of superficial vascular network predicts serous cystadenomas accurately. Also training in acquiring and interpretation of images is feasible in those without any prior knowledge in CLE in a relatively simple manner and computer-aided diagnosis software is a promising innovation.
CONCLUSION: The role of pCLE in the evaluation of pancreatobiliary disorders might be better suited for those with an intermediate and low probability.
Core tip: Current endoscopic evaluation of biliary and pancreatic duct strictures and pancreatic lesions using standard methods are suboptimal. Confocal laser endomicroscopy (CLE) is starting to establish a role in such cases with multiple studies suggesting that image interpretation is not as difficult as initially perceived. Furthermore the diagnostic discriminatory value of images obtained by CLE could decrease the need for repeated and invasive investigations, as the case in the serous cystadenomas. Although classification systems have been developed and improved with regards to the performance of CLE in biliary strictures they are still evolving for pancreatic lesions and require further validation.
- Citation: Almadi MA, Neumann H. Probe based confocal laser endomicroscopy of the pancreatobiliary system. World J Gastroenterol 2015; 21(44): 12696-12708
- URL: https://www.wjgnet.com/1007-9327/full/v21/i44/12696.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i44.12696
Despite the technological developments in the field of imaging as well as available options for endoscopic evaluation whether through endoscopic retrograde cholangiopancreatography (ERCP), cholangioscopy, or endoscopic ultrasound (EUS) the diagnostic yield of these tests is still suboptimal with regards to pancreatobiliary disorders, mainly biliary and pancreatic duct strictures as well as pancreatic cystic or solid lesions. The applications of confocal laser endomicroscopy (CLE) have expanded beyond luminal applications to direct tissue imaging including the pancreas[1] and the liver[2]. Thus, CLE has permitted real time in-vivo histological evaluation of areas of suspected neoplasia. With this new technology it has become possible to detect neoplasia and subsequently acquiring targeted biopsies as well as the confirmation of non-diseased tissue and the decreased need of random biopsies[3].
In CLE a low-power laser light is focused on a single point in a microscopic field of view. The light emanating from that point is focused through a pinhole to a detector and thus the point of illumination and the pinhole are focused onto the same point and are said to be ‘‘confocal’’ with each other[4], this process decreases the effect of scattered light thus permitting a higher spatial resolution. The beam focused spot traverses a line rapidly from left to right, and is swept top to bottom that would cover the area of interest and then the detected signal is digitized, resulting in the construction of a two-dimensional grey-scale image (Supplement figure 1).
As the experience with CLE is relatively new we sought to systematically review the literature for the available evidence with regards to the benefits of CLE in patients with pancreatobiliary disorders not overlooking abstracts at major congresses.
A computerized literature search was performed using OVID MEDLINE, EMBASE, Cochrane library, and the ISI Web of Knowledge from 1980 to October 2014. We also searched abstracts from major meetings that included the Digestive Disease Week, Canadian Digestive Disease Week and the United European Gastroenterology Week. We used a combination of controlled vocabulary and text words related to pCLE, confocal, endomicroscopy, probe-based confocal laser endomicroscopy, and bile duct to identify reports of trials (Appendix 1). In addition, recursive searches and cross-referencing were performed, and manual searches of articles identified after the initial search was also completed.
We included all clinical studies, both fully published and those to date having appeared only in abstract form that assessed the use of probe based CLE (pCLE) whether it was used through a needle (nCLE), for pancreatic lesions, or through a cholangioscope or catheter for the evaluation of pancreatobiliary disorders. We included all adult human studies published in any language.
To enhance the image quality of CLE certain contrast agents are used. Intravenous fluorescein sodium in a concentration of 10% is the most widely used contrast agent in CLE, a portion of it is bound to albumin while the remainder diffuses into the capillaries and causes enhancement of the extracellular matrix of tissue[5]. The use of fluorescein has been found safe with only mild adverse events in 1.4% of 2272 procedures performed[3], these included nausea/vomiting, transient hypotension without shock, injection site erythema, diffuse rash and mild epigastric pain. Fluorescein is usually administered about 2 to 3 min prior to acquiring images[6].
Confocal miniprobes used in pancreatobiliary disorders: The CholangioFlex miniprobe (Mauna Kea Technologies, Paris, France) is usually used for confocal imaging of the pancreatobiliarysystem. The probe requires a working channel of at least 1.2 mm and has a working length of 4 m. Lateral resolution of the probe is 3.5 μm with a field of view of 325 μm.
For cystic or mass lesions, the nCLE system (Mauna Kea Technologies, Paris, France) is used. The miniprobe is passed through a 19-gauge needle and has a lateral resolution of 3.5 μm and a field of view of 300 μm.
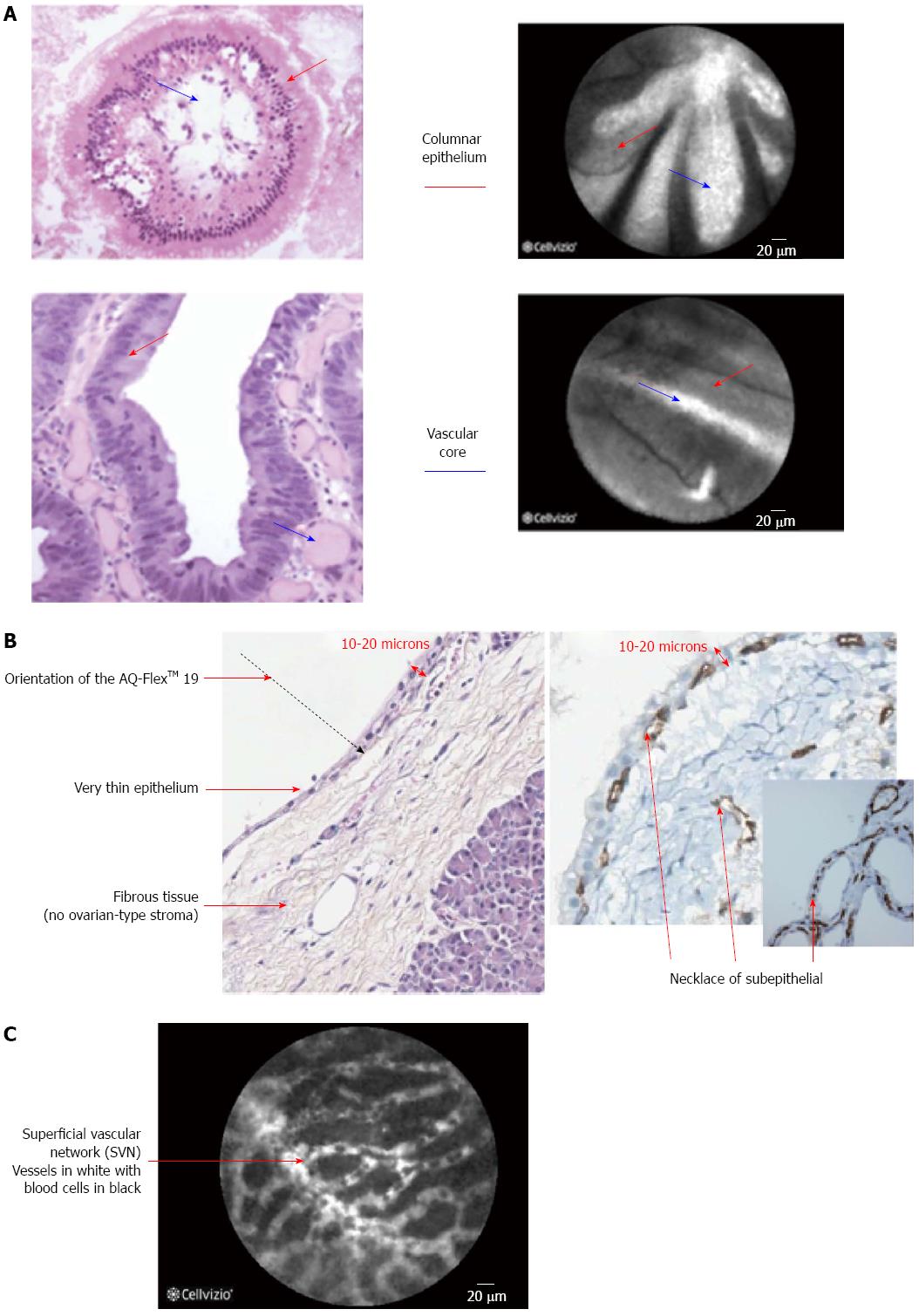
The initial evaluation of pancreatic cells in ex-vivo animal studies suggested that microvessel structures and connective tissue structure were better visualized by CLE when compared to conventional histology[7] and CLE images have also been correlated very well with histology in animal models[8]. The findings on CLE and there interpretation are displayed in (Table 1) (Figure 1), of note during the imaging of the biliary system due to the depth of penetration of the CLE probe the normal mucosa of the CBD, that is very thin, cannot be seen on CLE images that are obtained[9]. One of the key features of neoplastic lesions is disorganized angiogenesis[10].
| Findings on CLE | Interpretation |
| Normal | |
| Reticular pattern seen in normal tissue and smaller than blood vessels[6] | Lymphatics |
| Thick white bands[52] | Angiogenesis |
| Reticular arrangement of dark-gray bands on a light-gray background and normal vessels (thin and regular) and no visible glands[9] | Normal common bile duct chorion |
| Black clumps[69] | Areas of decreased fluorescein uptake |
| Large white streaks/bands[69] | Dilated, tortuous blood vessels |
| Loss of mucosal architecture[6,69] | Fibrosis |
| Multiple thin white bands[15] | Vascular congestion |
| Thin dark bands forming a reticular pattern (diameter < 20 μm) | Submucosal collagen network |
| Thin white bands (diameter < 20 μm) | Small caliber blood vessels |
| Light grey background | Lymphatic sinuses |
| Abnormal | |
| Thick collagen bundles | Desmoplasia (growth of fibrous and connective tissue) |
| Thick white bands (diameter > 20 μm) | Malignant bile duct stricture |
| Multiple white bands | Inflammatory bile duct stricture |
| Dark clumps and epithelium | Malignancy |
| Type of pancreatic lesion | Characteristics on nCLE |
| Exocrine adenocarcinomas | Dark cells aggregates with pseudo-glandular aspects |
| Straight hyperdense elements more or less thick corresponding to tumoral fibrosis | |
| Tumors with acini cells and neuroendocrine tumors | Very dense network of small vessels on a dark background |
| Serous cystadenoma | Highly dense and dynamic network of blood capillaries, present in the superficial layer of the cyst wall, superficial vascular network, (pathognomonic) |
| Intraductal papillary mucinous neoplasm | Papillae characterized by two epithelial borders surrounding a bright vascular flow |
| Mucinous cystadenoma neoplasm | An epithelial border lined the cyst wall, with or without deep blood vessels, and without papillary organization |
The Miami classification was an attempt to identify as well as standardize the interpretation of finding on pCLE of the biliary system in cases on indeterminate biliary strictures[11,12]. The highest sensitivity was found when there was the combination of thick white bands or dark clumps or epithelial structures, with a sensitivity of 94% and specificity of 46%[11]. While the highest specificity was found when there was a combination of white bands or thick white bands or fluorescein leakage or dark clumps, with a sensitivity of 61% and a specificity of 100%[11].
The interobserver agreement on the findings of pCLE was found to be poor to fair when evaluating videos sent to 6 observers in 5 centers even when the Miami classification was used to standardize image interpretation[13].
A refinement of the Miami classification[12,14], was recently made and was coined the Paris classification[15]. The aim of the Paris classification was to decrease the number of false positive results when evaluating indeterminate strictures of the biliary system as in inflammatory strictures. Sixty cases from a prospective registry were reviewed and four criteria for benign inflammatory strictures were described: vascular congestion, dark granular patterns with scales, increased inter-glandular space, and thickened reticular structure[15]. In a validation study for the Paris classification it was found to increase the specificity to 73% compared to 67% when using the Miami criteria[16], a similar finding was obtained in a second study[17].
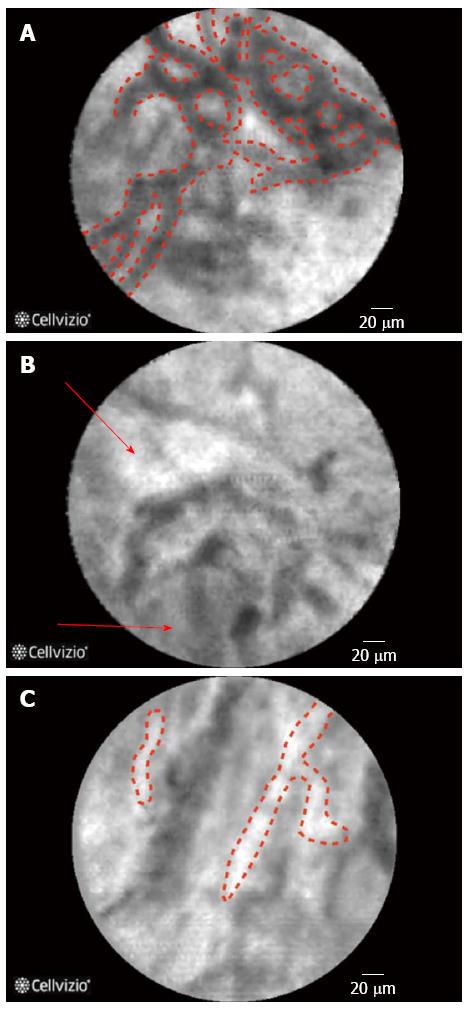
Giovannini et al[18] attempted to identify CLE findings for EUS nCLE in patients in pancreatic lesions whether they were cystic or solid as well as lymph nodes in the celiac area and mediastinum. Although the number of cases in that series was small (11 cases in total), nonetheless the authors found that in benign intraductal papillary mucinous neoplasms (IPMNs) there were finger-like projections corresponding to the villous changes seen in IPMN (Figure 2) while in pancreatic adenocarcinomas, there was vascular leakage with irregular vessels and leakage of fluorescein into the tumor as well as large dark clumps that corresponds to humps of malignant cells[18]. The in vivo nCLE study in the pancreas with endosonography of cystic tumors (INSPECT) group had also attempted to develop image criteria and a classification of nCLE findings in pancreatic cysts[19]. All these proposed criteria will require further validation in future studies.
In one recent study by Meining et al[20] the investigators that performed the procedures had no prior experience with the use or interpretation of pCLE, except for one. Each endoscopist had to complete a standard training module of 20 pCLE videos and then had to complete a review of another 20 pCLE video cases and provide a correct diagnosis of benign versus malignant in each video with a minimum score of 90%[20].
Furthermore, when comparing the first 45 cases of the same cohort to the remainder of cases there was an increase in the specificity of the diagnosis reached by the endoscopists from 55% to 71%, but this did not reach statistical significance[20]. Ease of obtaining pCLE video sequences and image interpretation slightly improved but the ease of tissue sampling was the same[20]. Although these results are encouraging it should be noted that the endoscopists were not blinded to the pre-procedure evaluation and these results might be biased but at the same time they do represent real-life experience.
A study involving gastroenterology fellows with no prior experience with pCLE underwent training with a set of ten video images that were repeated till the trainees were comfortable then they were asked to interpret 20 videos per week over three weeks, at the last session the accuracy by all beginners was 83% with an interobserver agreement of 0.63 which is substantial[21]. This study is encouraging with regards to the interpretation of images obtained but does not address the point of the technical skills required for obtaining these images. Another study involving five practicing gastroenterologists and the majority with less than 10 cases of experience with pCLE demonstrated that when receiving formal training by an expert (experience with more than 50 procedures) with educational videos pre- and post-training improved the diagnostic accuracy as well as the interobserver agreement[22].
A computer-aided diagnosis software (Smart Atlas; Mauna Kea Technologies, Paris, France) that assist endoscopists interpreting pCLE sequences and automatically discriminates between benign and malignant strictures[23] as well as cystic lesions of the pancreas[24]. Preliminary results of the performance characteristics of this software are encouraging and the results of prospective trials evaluating the new technique are highly anticipated.
nCLE of solid and cystic pancreatic lesions: A number of studies reported the use of nCLE in-vivo for pancreatic lesions[25-27]. A feasibility study on the use of nCLE through a 19-gauge needle inserted into pancreatic lesions, mainly cysts, was technically successful in the majority of the cases (17/18 cases) and the image quality was good to very good in 10/18 cases. Two patients in the study cohort developed pancreatitis[1]. To overcome this an investigational prototype pCLE (AQFlex) has been developed that is compatible with a 19-gauge FNA needle The probe was used through a transgastric as well as a transduodenal approach for a total of 66 patients with cystic lesions in the pancreas[28]. In this series the 2 patients had pancreatitis while one developed transient abdominal pain, and three developed intracystic bleeding without need for any intervention[28]. Also in the DETECT study the use of nCLE alone had a sensitivity of 75% in differentiating mucinous from non-mucinous cysts while when combined with cystoscopy it had a sensitivity of 100%[29]. Although the procedure is safe and feasible, standard criteria for interpretation of obtained images are still to be established[27], some characteristics of pancreatic lesions are displayed in Table 1. Preliminary data from the Clinical evaluation Of nCLE in The lymph nodes Along with masses and Cystic Tumors of the pancreas (CONTACT) demonstrated that the normal pancreatic tissue had an image resembling “coffee beans”, histologically corresponding to acini. Exocrine adenocarcinomas had two signs on nCLE; (1) dark cells aggregates with pseudo-glandular aspects; and (2) straight hyperdense elements more or less thick corresponding to tumoral fibrosis. Both these signs were absent in tumors with acinarcells and neuroendocrine tumors, instead these tumors demonstrated a very dense network of small vessels on a dark background[30]. The same study described findings in cystic lesions of the pancreas[31,32]. One particular finding is that serous cystadenomas have a superficial vascular network, which can be highlighted by nCLE (Figure 2). The specificity and positive predictive value (PPV) of this sign was found in a preliminary series to be 100%. This is of importance as its presence could avoid unnecessary surgery[33,34].
The first case report of the use of pCLE in the pancreatic duct was by Meining et al[35] in 2009. Evaluation of pancreatic duct strictures involves insertion of the pCLE through a cholangioscope[35]. A case series of 5 patients who had pancreatic duct strictures underwent pCLE during ERCP and the findings were compared to cytology and when available histology. pCLE was able to identify main duct intraductal papillary mucinous neoplasm with severe dysplasia and adenocarcinoma as well as benign strictures accurately[36]. A second series similarly identified IPMN with dysplasia but in this series two pancreatic duct strictures had positive Miami classification criteria although on histology they were proven to be inflammatory[37]. Another case series of 18 patients with indeterminate pancreatic duct strictures demonstrate almost perfect agreement between cyto/histopathology and pCLE (kappa coefficient = 0.8, P≤ 0.001)[38].
In a pilot study by Nakai et al[39] in vivo detection of epidermal growth factor receptor (EGF-R) and survivin were possible using a EUS-guided fine needle imaging technique that incorporated nCLE after injection of FITC-labeled specific antibodies against EGF-R and survivin. The EGF-R antibodies were localized to ductal-lining cells and many acinar cells while survivin was confined to acinar cells[39].
Bakhru et al[40] assessed the interobserver agreement for five variables for 12 pCLE videos these variables were the presence of an epithelial outer border with irregular thickness, dark epithelium without discernable individual cells, heterogeneously distributed elongated crypts, reduced number of goblet cells, neovascularization, and the final diagnosis. The study included 6 gastroenterologists from five centers and the interobserver agreement was poor to slight for all variables except for the presence of an epithelial outer border with irregular thickness that was fair[40]. The results did not differ much when the gastroenterologists were stratified by their level of experience[40].
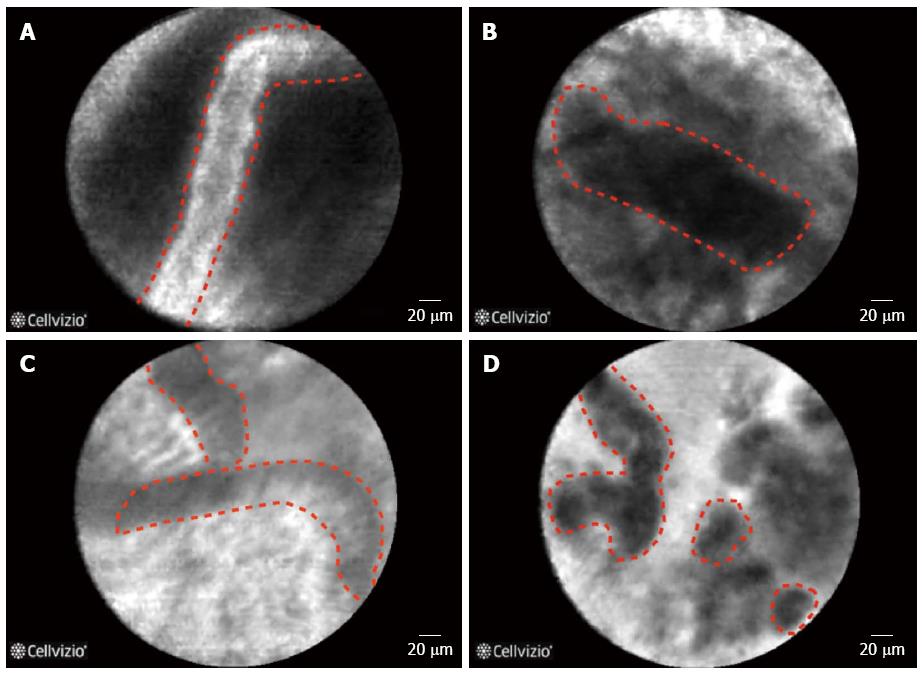
The evaluation of biliary strictures, whether in the context of primary sclerosing cholangitis (PSC) or not, can be a challenge for clinicians, and the current approaches suffer from a low sensitivity. The application of pCLE for the differentiation between neoplastic and non-neoplastic strictures have yielded very promising results[41,42] (Figure 3). The advantage of pCLE is its higher sensitivity in the detection of neoplastic lesions and an earlier detection of disease at a potentially resectable stage[15]. Furthermore, the more confident the diagnosis the lesser the need for repeated investigations and thus reducing the costs as well as morbidity associated with repeated manipulation of the biliary system[15]. The performance characteristics of CLE in pancreatobiliary strictures are displayed in Table 2 as well as in specific organs in supplement Table 1.
| Criterion | Author and the description of the examined area | Sensitivity | Specificity | PPV | NPV |
| Meining et al[41,53] | |||||
| Malignant biliary strictures | Loss of reticular pattern of epithelial bands of less than 20 μm | 83% | 88% | NR | NR |
| Irregular epithelial lining, villi, or gland-like structures | |||||
| Tortuous, dilated, and saccular vessels with inconsistent branching | |||||
| Presence of “black areas” of more than 60 to 80 μm (focally decreased uptake of fluorescein) | |||||
| Meining et al[20] | |||||
| Suggestive ofmalignancy | Thick, dark bands (> 40 μm) | 98% | 67% | 71% | 97% |
| Thick, white bands (> 20 μm) | |||||
| Dark clumps | |||||
| Epithelium visualized (villi, glands) | |||||
| Fluorescein leakage | |||||
| Suggestive ofbenign strictures | Thin, dark (branching) bands | ||||
| Thin, white bands | |||||
| Meining et al[14] | |||||
| Normal bile ducts | Reticular network of thin dark branching bands (< 20 μm) | 97% | 33% | 80% | 80% |
| Light gray background | |||||
| Vessels < 20 μm | |||||
| Malignant biliary strictures | Thick white bands (> 20 μm) | ||||
| Thick dark bands (> 40 μm), | |||||
| Dark clumps | |||||
| Epithelial structures | |||||
| Normal vessels (thin and regular) | |||||
| No visible glands | |||||
| Giovannini et al[18] | |||||
| Benign IPMN | Finger-like projections corresponding to villous changes of intestinal IPMN type | NR | NR | NR | NR |
| Pancreatic adenocarcinoma | Vascular leakage with irregular vessels with leakage of fluorescein into the tumor | ||||
| Large dark clumps which correspond to humps of malignant cells | |||||
| Benign Lymph nodes | Diffuse small cells into a homogeneous stroma with normal vascularization | ||||
| Malignant lymph nodes | Glandular structures with dark cells, large dark clumps and neo-vascularization with huge leakage of fluorescein | ||||
| Filoche et al[56] | |||||
| Benign inflammatory strictures of the common bile duct | Vascular congestion but still regular | NR | NR | NR | NR |
| Roughness aspect | |||||
| Increased inter-glandular space | |||||
| Thickened reticular structure | |||||
| Caillol et al[15] | |||||
| Inflammatory stenosis | Multiple thin white bands (Vascular congestion) | 96.3% | 63.6% | 68.4% | 95.5% |
| Roughness aspect | |||||
| Increased inter-glandular space | |||||
| Thickened reticular structure |
Different probes have been used for pCLE of the biliary system, GastroFlex (UHD; Mauna Kea Technologies, Paris, France) miniprobe[43] and CholangioFlex probes. The drawback of the GastroFlex probe is its larger diameter and thus it might be difficult to negotiate through strictures although a case series demonstrated successful insertion of this probe in 10/11 patients with various indications for pCLE with one patient developing pancreatitis after the procedure. Although the GastroFlex probe has a higher lateral resolution when compared to the CholangioFlex probe (1 μm vs 3.5 μm)[44] there is no clear advantage of the use of one over the other[45].
The pCLE probe has been delivered successfully through various cholangioscopes as well as catheters[20,46]. When using a cholangioscope pCLE had a sensitivity of 96% (95%CI: 84% to 100%) and a specificity of 76% (95%CI: 53% to 91%), while when using a catheter the sensitivity was 100% (95%CI: 83% to 100%) and the specificity was 62% (95%CI: 45% to 78%)[46] but there was no statistical difference in the accuracy between these delivery techniques[46] but the operators confidence about the diagnosis was much higher when using cholangioscopy when compared to a catheter based approach for pCLE of biliary strictures (43.2% vs 9.8%, respectively)[46]. In a randomized trial for comparison between catheter-guided (Fluoroscopy only) pCLE and cholangioscopy-guided pCLE the accuracy of cholangioscopy-guided pCLE was 82% compared to 78% for catheter-guided pCLE. Of note, the sample size of the study was small[47].
The addition of pCLE with ERCP in the evaluation of indeterminate pancreatobilary strictures can increase the detection of cancerous strictures[9,48] with a sensitivity of (98% vs 45%) and a negative predictive value (NPV) (97% vs 69%), although it decreased the specificity (67% vs 100%) and the PPV (71% vs 100%) when compared to index pathology[48]. Similar findings were found in other studies[6,37,42,49-54].
Although conventionally the use of pCLE for the evaluation of biliary strictures is through a side viewing duodenoscope, a case series showed pCLE through direct peroral cholangioscopy in 22 out of 24 patients with biliary strictures[54]. An interesting approach for this case series is that they classified patients based on the pre pCLE evaluation for the probability of a malignant etiology for biliary stricture into a range from very unlikely to certainly based on the clinical evaluation as well as imaging[54], pCLE was found to be complementary to peroral cholangioscopy and ERCP in cases where a malignant etiology was suspected and did not effect the management decision but it might be sufficient for confirmation of a malignant etiology when tissue acquisition is not required[54].
The use of pCLE in hilar strictures has also been proven to be of use in a series of 19 patients with the correct identification of all cases with neoplasia but one false positive case was reported[55].
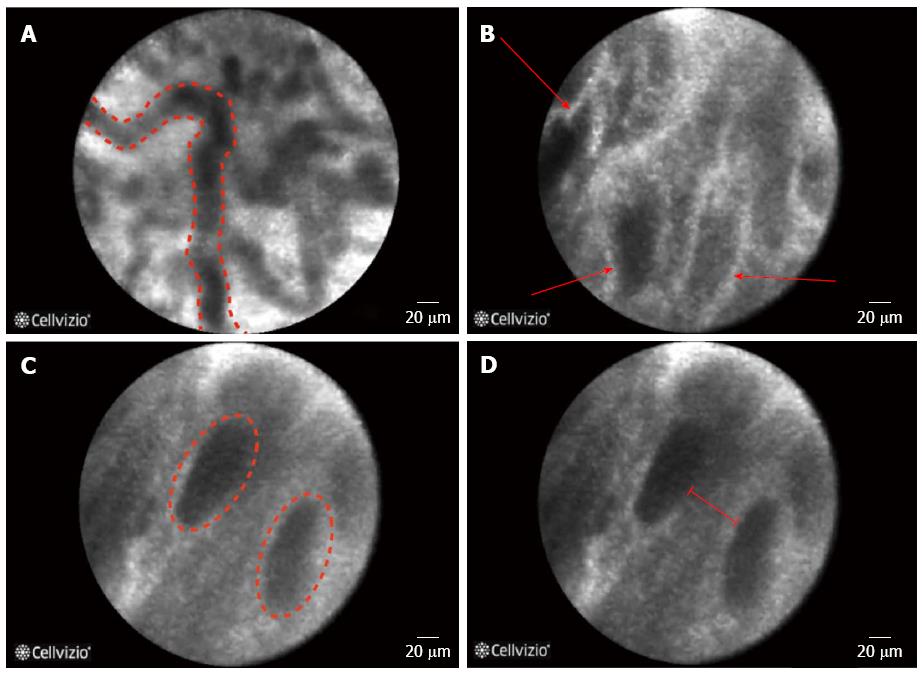
Filoche et al[56] identified four characteristics on biliary pCLE that were associated with benign inflammatory strictures; vascular congestion, roughness aspect, increased inter-glandular space and thickened reticular structure (Figure 4). In this study the authors sought to explain the false positive cases in 60 cases that were enrolled in a registry and found that pCLE diagnosis was either influenced by the ERCP impression or the presence of less than 3 malignant Miami classification criteria[56].
Giovannini et al[57] evaluated the effect of biliary stenting in 54 patients with indeterminate biliary stenosis and found that biliary stenting decreased the accuracy of pCLE when using the Miami criteria, similar findings were replicated where a decrease in the sensitivity from 88% to 75% and specificity from 83% to 71% was found in those who had cholangitis or a stent inserted prior to pCLE imaging[58]. Although this requires validation in other series but it might be prudent to perform pCLE prior to biliary stenting in cases with biliary strictures of unknown etiology. Also, of note, in the study by Caillol et al[15] they noted that stricture dilation could induce fluorescein leakage thus giving the impression of a malignant stricture while it was subsequently found to be benign.
A recent consensus report by 16 physicians validated seven statements with regards to the use of pCLE in biliary strictures; (1) CLE can be used to evaluate biliary strictures, and the probe can be delivered via a catheter or a cholangioscope; (2) CLE is more accurate than ERCP with brush cytology and/or forceps biopsy in determining malignant or benign strictures, using established criteria; (3) The accuracy of CLE in indeterminate biliary strictures may be decreased by prior presence of plastic stent; (4) The NPV of CLE is very high; (5) The use of CLE can assist clinical decision-making such as excluding malignancy; (6) CLE should be cited as a valuable tool for an increased diagnostic yield in official guidelines; and (7) The «black bands» that can be seen in pCLE images have been shown to be collagen fibrils that predictably increase in pathologic tissue[59].
A preliminary analysis of the multicenter multinational FOCUS trial demonstrated that the clinical impression of physicians and pCLE during workup of biliary strictures outperform tissue sampling where the combination of brush cytology and biopsy would have missed 5 malignant strictures out of 36 patients[60]. While the addition of histology/cytology to pCLE resulted in a marginal increase in sensitivity (from 89% to 93%) but did not change the specificity (79%) compared to the addition of pCLE alone[61].
The work up of patients with PSC who develop strictures can be a challenge. The management will depend on the etiology of these strictures whether they are neoplastic or just inflammatory. In a series of 15 patients with 19 strictures[62], both extra and intrahepatic, were evaluated by ERCP and pCLE. Due to the inflammatory nature of PSC the authors used a scoring system based on the Miami classification when there were 2 of 5 malignant criteria the lesion was classified as “suspicious” and 1 criterion as “reactive” and the finding of a reticular pattern was deemed as “benign”[62] the finding on pCLE were compared to ERCP, cholangioscopy, histology/cytology, liver explants, fluorescence in-situ hybridization (FISH), or 12 mo of follow-up. Visualization was successful in 95% of the procedures, pCLE was found to have a sensitivity of 100% (95%CI: 40% to 100%), specificity of 50% (95%CI: 9% to 90%), PPV of 67% (95%CI: 24.5% to 94%) and a NPV of 100% (95%CI: 20% to 100%)[63]. The authors suggested the high negative predictive value of pCLE could guide in the interval of surveillance in patients with dominant biliary strictures[62].
The role of pCLE in the evaluation of pancreatobiliary disorders might not be of high value in cases where the pretest probability of a malignant etiology is high but might be better suited for those with an intermediate and low probability. Furthermore it might guide tissue sampling to increase the yield when evaluating pancreatobiliary disorders. It does not seem, at least for now, that pCLE will eliminate the need for tissue acquisition and a histological diagnosis but it does supplement the evaluation of these patients. The field is evolving and as more centers acquire this technology and studies are conducted[50,63-68] the definite role of pCLE will be better defined.
The diagnostic criteria might need to be refined to be applicable to clinical practices outside centers of expert endoscopists and might need to be tailored to specific underlying disease like PSC and patients with chronic pancreatitis where discriminating normal from abnormal histology might be more challenging.
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project number RGP-VPP-279. Also we would like to thank Mauna Kea Technologies for providing images from their e-learning website: http://www.cellvizio.net.
Confocal laser endomicroscopy (CLE) is used for real time in-vivo histological evaluation of areas of suspected neoplasia allowing targeted biopsies. The applications of CLE in the pancreatobiliary system are relatively new and the added value of this image enhancing technology is evolving.
When evaluating indeterminate strictures of the biliary system, a refinement of the Miami classification (the Paris classification) has decreased the number of false positive results. While in the case of pancreatic lesions a classification system is still lacking but some of the imaging features appear to be very specific and might alter the management of how the authors survey patients with pancreatic cystic lesions. Furthermore, computer-aided diagnosis softwares that assist in interpreting CLE sequences are a promising development.
In this systematic review the authors attempted to review the most recent literature which describes potential applications of CLE in the area of diagnostic evaluation of lesions that are particularly difficult to reach using our current imaging and endoscopic armamentarium. There has been a great deal of advancements in the field but there still remains more to be achieved specifically in the fields of training as well as dissemination of this technology.
Probe based CLE is useful in cases of indeterminate biliary strictures where the pretest probability of malignant etiology are intermediate or low while it might not be the case when the pretest probability is high. As for needle based CLE in solid as well as cystic pancreatic lesions the role is still evolving but is very promising.
Immediate endoscopic optical histology without samples in accessible tissue surfaces is a new tool providing important advances in lesion recognition. CLE is used in assessing histological structure without samples in endoscopy using specially designed scopes but can also be used as thin probes which are able to reach and assess tissue structure in ducts, cysts and accessible tumors. The present paper is an extensive and complete, up-to-date review of the literature available since 2001; nevertheless most the papers were published from 2007 to 2015. This review describes some important aspects of CLE image evaluation, including specificity, sensitivity and positive predictive values, and also the learning curves in image interpretation which all are important for introducing a new device in clinical practice. The paper is long but is a well-designed systematic review and includes one part devoted to technical aspects, another on image interpretation and a third on its application in different localizations.
P- Reviewer: Aktas S, Bordas JM S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011;74:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Mennone A, Nathanson MH. Needle-based confocal laser endomicroscopy to assess liver histology in vivo. Gastrointest Endosc. 2011;73:338-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Löhr M. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Kiesslich R, Goetz M, Neurath MF. Virtual histology. Best Pract Res Clin Gastroenterol. 2008;22:883-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Loeser CS, Robert ME, Mennone A, Nathanson MH, Jamidar P. Confocal endomicroscopic examination of malignant biliary strictures and histologic correlation with lymphatics. J Clin Gastroenterol. 2011;45:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Keck T, Campo-Ruiz V, Warshaw AL, Anderson RR, Fernández-del Castillo C, González S. Evaluation of morphology and microcirculation of the pancreas by ex vivo and in vivo reflectance confocal microscopy. Pancreatology. 2001;1:48-57. [PubMed] |
| 8. | Goetz M, Memadathil B, Biesterfeld S, Schneider C, Gregor S, Galle PR, Neurath MF, Kiesslich R. In vivo subsurface morphological and functional cellular and subcellular imaging of the gastrointestinal tract with confocal mini-microscopy. World J Gastroenterol. 2007;13:2160-2165. [PubMed] |
| 9. | Giovannini M, Bories E, Monges G, Pesenti C, Caillol F, Delpero JR. Results of a phase I-II study on intraductal confocal microscopy (IDCM) in patients with common bile duct (CBD) stenosis. Surg Endosc. 2011;25:2247-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Meining A, Wallace MB. Endoscopic imaging of angiogenesis in vivo. Gastroenterology. 2008;134:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Slivka A, Chen YK, Pleskow DK, Stevens PD, Shah RJ, Chuttani R, Meining A. Miami classification of probe-based confocal laser endomicroscopy (pCLE) findings for the characterization of indeterminate strictures: First steps towards automated patient segmentation? Gastroenterology. 2011;1:S10. |
| 12. | Wallace M, Lauwers GY, Chen Y, Dekker E, Fockens P, Sharma P, Meining A. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 2011;43:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Talreja JP, Sethi A, Jamidar PA, Singh SK, Kwon RS, Siddiqui UD, Sawhney M, Bakhru MR, Gaidhane M, Kline P. Interpretation of probe-based confocal laser endomicroscopy of indeterminate biliary strictures: is there any interobserver agreement? Dig Dis Sci. 2012;57:3299-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Meining A, Shah RJ, Slivka A, Pleskow D, Chuttani R, Stevens PD, Becker V, Chen YK. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy. 2012;44:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Caillol F, Filoche B, Gaidhane M, Kahaleh M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris Classification. Dig Dis Sci. 2013;58:1784-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Gan SI, Jamidar PA, Giovannini M, Cesaro P, Costamagna G, Kahaleh M, Slivka A. From Miami to Paris: Validation of refined probe-based confocal microscopy classification of indeterminate biliary strictures. Gastroenterology. 2014;1:S-879. |
| 17. | Sarah L, Berson A, Dhooge M, Esch A, Tabouret T, Chaussade S, Prat F. Accuracy of confocal laser endomicroscopy for the diagnosis of indeterminate biliary strictures: Comparison of the Miami and Paris classifications. Gastroenterology. 2014;1:S-9. [DOI] [Full Text] |
| 18. | Giovannini M, Caillol F, Poizat F, Bories E, Pesenti C, Monges G, Raoul JL. Feasibility of Intratumoral Confocal Microscopy under Endoscopic Ultrasound Guidance. Endosc Ultrasound. 2012;1:80-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Meining A, Lo SK, Jamil LH, Wallace MB, Aslanian HR, Siddiqui UD, Giovannini M, Chang KJ, Hwang JH, Saunders MD. In vivo needle-based confocal laser endomicroscopy (nCLE) study in the pancreas with endosonography of cystic tumors (INSPECT): Interim results from an international prospective multicentric study. Gastroenterology. 2011;1:S194-S195. [DOI] [Full Text] |
| 20. | Meining A, Chen YK, Pleskow DK, Stevens PD, Shah RJ, Chuttani R, Slivka A. Direct visualization using probe-based confocal laser endomicroscopy (pCLE) for assessment of indeterminate biliary and pancreatic strictures - A multi-center experience using cellvizio. Gastroenterology. 2011;1:S107. [DOI] [Full Text] |
| 21. | Pittayanon R, Rerknimitr R, Wisedopas N, Charoensuk K, Khemnark S, Norrasetwanich N, Thienchanachaiya P, Lakananurak N, Linlawan S, Thanapirom K. The learning curve on the images obtained by probe-based confocal laser endomicroscopy (PCLE) for the interpretation of malignant biliary stricture. Gastrointest Endosc. 2013;1:AB311-AB312. |
| 22. | Talreja JP, Turner BG, Gress FG, Ho S, Sarkaria S, Paddu N, Natov N, Bharmal S, Gaidhane M, Sethi A. Pre- and post-training session evaluation for interobserver agreement and diagnostic accuracy of probe-based confocal laser endomicroscopy for biliary strictures. Dig Endosc. 2014;26:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Tafreshi MK, Joshi V, Meining A, Lightdale CJ, Giovannini M, Dauguet J, Ayache N, Andre B. Smart atlas for supporting the interpretation of probe-based confocal laser endomicroscopy (PCLE) of biliary strictures: First classification results of a computer-aided diagnosis software based on image recognition. Gastrointest Endosc. 2014;1:AB250-AB251. [DOI] [Full Text] |
| 24. | Tafreshi MK, Napoleon B, Lemaistre AI, Giovannini M, Joshi V, Dauguet J, Ayache N, Andre B. Smart atlas for supporting the interpretation of needle-based confocal laser endomicroscopy (NCLE) of pancreatic cysts: First classification results of a computer-aided diagnosis software based on image recognition. Gastrointest Endosc. 2014;1:AB328. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Gomez V, Shahid MW, Krishna M, Asbun HJ, Wallace MB. Confocal Laser Endomicroscopy (CLE): Evaluation of pancreatic cystic lesions in vivo and ex vivo. Gastrointest Endosc. 2011;1:AB111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Aslanian HR, Chhieng DC, Cai G, Siddiqui UD. EUS guided confocal laser endomicroscopy of the pancreas. Gastrointest Endosc. 2010;71:AB101-AB102. [DOI] [Full Text] |
| 27. | Karstensen JG, Cârţână T, Klausen PH, Hassan H, Popescu CF, Săftoiu A, Vilmann P. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy: a pilot study for use in focal pancreatic masses. Pancreas. 2015;44:833-835. [PubMed] |
| 28. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Nakai Y, Iwashita T, Park do H, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [PubMed] [DOI] [Full Text] |
| 30. | Giovannini M, Caillol F, Lucidarme D, Pujol B, Poizat F, Monges GM, Filoche B, Napoleon B. Needle-based confocal laser endomicroscopy (NCLE) for the diagnosis of pancreatic masses: Preliminary criteria (contact study). Gastroenterology. 2014;1:S-575. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Mialhe-Morellon B, Giovannini M. In vivo characterization of pancreatic cystic tumors by needle-based confocal laser endomicroscopy (NCLE). Proposition of a comprehensive classification. Gastrointest Endosc. 2014;1:AB327-AB328. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Napoleon B, Lemaistre AI, Pujol B, Mialhe-Morellon B, Caillol F, Giovannini M. In vivo characterization of mucinous cystadenomas by needle-based confocal laser endomicroscopy (NCLE). Gastroenterology. 2014;1:S-485. [DOI] [Full Text] |
| 33. | Napoleon B, Pujol B, Lemaistre AI, Caillol F, Lucidarme D, Filoche B, Mialhe-Morellon B, Fumex F, Lepilliez V, Giovannini M. In vivo characterization of pancreatic serous cystadenomas by needle-based confocal laser endomicroscopy (NCLE). Intra and inter observer agreement-contact study. Gastroenterology. 2013;1:S797. |
| 34. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Meining A, Phillip V, Gaa J, Prinz C, Schmid RM. Pancreaticoscopy with miniprobe-based confocal laser-scanning microscopy of an intraductal papillary mucinous neoplasm (with video). Gastrointest Endosc. 2009;69:1178-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Turner B, Bezak K, Sarkaria S, Lieberman M, Chan C, Gaidhane M, Kahaleh M. Probe-based confocal laser endomicroscopy in the pancreatic duct provides direct visualization of ductal structures and aids in clinical management. Am J Gastroenterol. 2012;107:S107-S108. [DOI] [Full Text] |
| 37. | Loehr M, Bergquist A, Swahn F, Enochsson L, Noel R, Ghazi S, Albiin N, Marschall HU, Permert J, Arnelo U. Confocal laser scanning microscopy in patients with biliary strictures and pancreatic duct lesions - A prospective pilot study. Gastroenterology. 2011;1:S756. [DOI] [Full Text] |
| 38. | Kahaleh M, Turner BG, Bezak K, Sharaiha RZ, Sarkaria S, Lieberman M, Jamal-Kabani A, Millman JE, Sundararajan SV, Chan C. Probe-based confocal laser endomicroscopy in the pancreatic duct provides direct visualization of ductal structures and aids in clinical management. Dig Liver Dis. 2015;47:202-204. [PubMed] |
| 39. | Nakai Y, Shinoura S, Ahluwalia A, Tarnawski AS, Chang KJ. In vivo visualization of epidermal growth factor receptor and survivin expression in porcine pancreas using endoscopic ultrasound guided fine needle imaging with confocal laser-induced endomicroscopy. J Physiol Pharmacol. 2012;63:577-580. [PubMed] |
| 40. | Bakhru MR, Sethi A, Jamidar PA, Singh SK, Kwon RS, Siddiqui UD, Sawhney M, Talreja JP, Kline P, Malik U. Interobserver agreement for confocal imaging of ampullary lesions: a multicenter single-blinded study. J Clin Gastroenterol. 2013;47:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Meining A. Confocal endomicroscopy. Gastrointest Endosc Clin N Am. 2009;19:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Lohr M, Swahn F, Enochsson L, Ghazi S, Segersvard R, Arnelo U. Confocal laser endomicroscopy of the pancreatobiliary ducts. Pancreas. 2009;38:1023-1024. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Shieh FK, Drumm H, Nathanson MH, Jamidar PA. High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol. 2012;46:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Shahid MW, Krishna M, Asbun HJ, Raimondo M, Woodward TA, Wallace MB. Detection of neoplasia in pancreatic cysts using probe-based confocal laser endomicroscopy: A prospective ex-vivo study. Gastrointest Endosc. 2011;1:AB378-AB379. |
| 45. | Shieh FK, Nathanson MH, Drumm H, Jamidar PA. High-definition in-vivo probe-based confocal laser endomicroscopy of the common bile duct. Gastrointest Endosc. 2010;71:AB158-AB159. [DOI] [Full Text] |
| 46. | Slivka A, Chen YK, Pleskow DK, Stevens PD, Shah RJ, Chuttani R, Meining A. Real-time microscopic evaluation of indeterminate strictures: Comparison of cholangioscopy vs catheter delivery for probe-based confocal laser endomicroscopy (pCLE) - A multi-center experience using cellvizio. Gastrointest Endosc. 2011;1:AB146-AB147. [DOI] [Full Text] |
| 47. | Pratico C, Leblanc S, Vienne A, Duchmann JC, Mangialavori L, Chaussade S, Prat F. Diagnostic performance of catheter-based compared with cholangioscopy-based endomicroscopy of indeterminate biliary strictures. Gastrointest Endosc. 2013;1:AB323. |
| 48. | Meining A, Chen YK, Pleskow D, Stevens P, Shah RJ, Chuttani R, Michalek J, Slivka A. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc. 2011;74:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Wysocki JD, Newby C, Joshi V. Use of probe-based confocal laser endomicroscopy (pCLE) in the diagnosis of cholangiocarcinoma. J Clin Oncol. 2012;1. |
| 50. | Bertani H, Manta R, Manno M, Pigo F, Conigliaro R. PCLE in the imaging of common bile duct mucosa: New frontiers in the diagnosis of cholangiocarcinoma. Gastrointest Endosc. 2012;1:AB482. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Leblanc S, Vienne A, Duchmann JC, Gaudric M, Boyer J, Mangialavori L, Chaussade S, Prat F. Combined cholangioscopy (Spyglass) and probe-based confocal laser endomicroscopy (pCLE) in undetermined biliary stenosis: Preliminary results. Gastroenterology. 2011;1:S756. [DOI] [Full Text] |
| 52. | Shah RJ, Chennat JS, Cesaro P, Slivka A, Sejpal DV, Jamidar PA, Sethi A, Gan SI, Walter MH, Gaidhane M. Distinguishing benign from malignant dominant biliary strictures in patients with primary sclerosing cholangitis utilizing probe-based confocal laser endomicroscopy (PCLE): A multi-center, expert consensus review. Gastrointest Endosc. 2013;1:AB164. |
| 53. | Meining A, Frimberger E, Becker V, Von Delius S, Von Weyhern CH, Schmid RM, Prinz C. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol. 2008;6:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Mehraban N, Simien M, Linder JD, Tarnasky PR. Clinical utility of biliary confocal laser endomicroscopy. Gastrointest Endosc. 2012;1:AB377. [DOI] [Full Text] |
| 55. | Cesaro P, Tringali A, Caillol F, Monges GM, Familiari P, Perri V, Spada C, Giovannini M, Costamagna G. In-vivo miniprobe-based Confocal fluorescence Endomicroscopy (Cellvizio) for hilar biliary strictures: An experience of two centres. Dig Liver Dis. 2011;43:S145. [DOI] [Full Text] |
| 56. | Filoche B, Caillol F, Gaidhane M, Kahaleh M. Probe-based confocal laser endomicroscopy (PCLE) for indeterminate biliary strictures: Improved interpretation increases accuracy. Gastrointest Endosc. 2012;1:AB381-AB382. [DOI] [Full Text] |
| 57. | Giovannini M, Caillol F, Monges GM, Bories E, Pesenti C, Turrini O, Delpero JR. Probe Based Confocal Laser Endomicroscopy (pCLE) in the bile duct for indeterminate stenosis: Be careful with the biliary stenting!!! Gastrointest Endosc. 2012;1:AB392-AB393. [DOI] [Full Text] |
| 58. | Caillol F, Bories E, Poizat F, Pesenti C, Esterni B, Monges G, Giovannini M. Endomicroscopy in bile duct: Inflammation interferes with pCLE applied in the bile duct: A prospective study of 54 patients. United European Gastroenterol J. 2013;1:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Carr-Locke DL, Arsenescu R, Bertani H, Caillol F, Costamagna G, Gan SI, Giovannini M, Gress FG, Haluszka O, Ho KY. The role of confocal laser endomicroscopy in the management of patients with biliary strictures: A consensus report based on clinical evidence. Gastroenterology. 2014;1:S387-S388. [DOI] [Full Text] |
| 60. | Cesaro P, Slivka A, Gan S, Giovannini M, Caillol F, Kahaleh M, Jamidar P, Costamagna G. Impact of optical biopsy on management of patients with biliary strictures: Interim results of a multicenter study. Dig Liver Dis. 2014;46:S43. |
| 61. | Slivka A, Gan SI, Giovannini M, Jamidar PA, Costamagna G, Cesaro P, Kahaleh M. Accuracy of optical biopsy using probe based confocal laser endomicroscopy (PCLE) in patients with indeterminate biliary strictures: Interim results with modified criteria of a large multicentric study. Gastrointest Endosc. 2014;1:AB264. [DOI] [Full Text] |
| 62. | Heif M, Yen RD, Fukami N, Brauer BC, Wani S, Shah RJ. Probe-based confocal laser endomicroscopy for dominant stenoses in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2012;1:AB397. [DOI] [Full Text] |
| 63. | Chang S, Kang B, Liu X, Dai Y, Chen D. The combined influence of surface modification, size distribution, and interaction time on the cytotoxicity of CdTe quantum dots in PANC-1 cells. Acta Biochim Biophys Sin (Shanghai). 2012;44:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Kalaitzakis E. Cholangioscopy (Spy glass). Scand J Gastroenterol. 2012;47:S16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Donelli G, Vuotto C, Cardines R, Mastrantonio P. Biofilm-growing intestinal anaerobic bacteria. FEMS Immunol Med Microbiol. 2012;65:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 66. | Zhang F, Meng Y, Sun J, Soriano S, Yang X. Intrabiliary molecular MRI-monitored local agent delivery. Mol Imaging Biol. 2012;14:S321. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Li Z, Lu M, Chu J, Qiao X, Meng X, Sun B, Zhang W, Xue D. Early proteome analysis of rat pancreatic acinar AR42J cells treated with taurolithocholic acid 3-sulfate. Pancreatology. 2012;12:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Heif M, Yen RD, Shah RJ. ERCP with probe-based confocal laser endomicroscopy for the evaluation of dominant biliary stenoses in primary sclerosing cholangitis patients. Dig Dis Sci. 2013;58:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Chennat J, Konda VJ, Madrigal-Hoyos E, Fernandez-Sordo J, Xiao SY, Hart J, Waxman I. Biliary confocal laser endomicroscopy real-time detection of cholangiocarcinoma. Dig Dis Sci. 2011;56:3701-3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Pittayanon R, Kongkam P, Sampatanukul P, Aniwan S, Angsuwatcharakon P, Treeprasertsuk S, Kullavanijaya P, Rerknimitr R. EUS guided needle-based confocal LASER endomicroscopy (nCLE) to distinguish between benign and malignant lesions in solid pancreatic masses: Preliminary results of a prospective single-blind study. Gastrointest Endosc. 2014;1:AB190-AB191. [DOI] [Full Text] |
| 71. | Giovannini M, Caillol F, Bories E, Monges GM. Emid study: Final results of a prospective bicentric study assessing probe-based confocal laser endomicroscopy (PCLE). Impact in the management of biliary strictures. Gastrointest Endosc. 2013;1:AB126. |