Published online Nov 28, 2015. doi: 10.3748/wjg.v21.i44.12660
Peer-review started: April 26, 2015
First decision: June 2, 2015
Revised: June 30, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: November 28, 2015
Processing time: 216 Days and 16.9 Hours
AIM: To analyze therapeutic changes in Crohn’s disease (CD) patients following video capsule endoscopy (VCE) and to assess the usefulness of Lewis score and the Patency Capsule.
METHODS: Patency Capsule was performed in every patient that had indication for VCE, and those with negative patency did not undergo VCE. Patients with established CD that underwent VCE between January 2011 and February 2014 were selected for this study; those with suspected CD were excluded, independent of VCE results, since our purpose was to address differences in therapeutic regimen in CD patients before and after VCE. Patients with inconclusive VCE were also excluded. Patients had to be free of non-steroidal anti-inflammatories for at least 1 mo. Those patients who met these criteria were allocated into one of three groups: Staging group (asymptomatic CD patients that underwent VCE for staging of CD), Flare group (patients with active CD), or Post-op group (CD patients evaluated for post-operative recurrence). Lewis score was calculated for every VCE procedure. Statistical analysis was performed to address the impact of VCE findings on the therapeutic management of CD patients and to evaluate the utility of the Lewis score.
RESULTS: From a total of 542 VCEs, 135 were performed in patients with CD. Patency capsule excluded nearly 25% of the patients who were supposed to undergo VCE. No videocapsule retention during VCE was reported. From these 135 patients, 29 were excluded because CD diagnosis was not established at the time of VCE. Therefore, a total of 106 patients were included in the final analysis. From these, the majority were in the Staging group (n = 73, 69%), and the remaining were in the Flare (n = 23, 22%) or Post-op (n = 10, 9%) group. Median time between diagnosis and VCE was 5.5 years. Overall, VCE determined changes in the treatment of 40% of patients: only 21% remained free of immunosuppressors after VCE compared to 44% before VCE (P < 0.001). The differences in therapy before and after VCE achieved statistical significance in the Staging and Flare groups. In addition, patients were significantly different when stratified regarding time since diagnosis to the date of VCE. A higher Lewis score was associated with therapeutic modifications (P < 0.0001); where a score higher than 1354 was related to 90% probability of changing therapy [area under the receiver operative characteristic (AUROC) 0.80 (95%CI: 0.69-0.88)].
CONCLUSION: VCE significantly changed the therapeutic management of CD patients, even in those with long-term disease. Systematic use of Patency capsule allowed for no videocapsule retention.
Core tip: Our work analyzed the therapeutic management of patients with Crohn’s disease (CD) and concluded that a very significant proportion of patients modify their therapeutic regimens after performing video capsule endoscopy (VCE), even in those with long-term disease or those without symptoms. This finding highlights the importance of this procedure in the management of CD. The systematic use of Patency capsule is controversial; however, we showed in our study that after excluding patients with negative patency, who did not undergo VCE, none of the patients had video capsule retention during VCE, highlighting the importance of Patency capsule in this setting.
- Citation: Santos-Antunes J, Cardoso H, Lopes S, Marques M, Nunes AC, Macedo G. Capsule enteroscopy is useful for the therapeutic management of Crohn’s disease. World J Gastroenterol 2015; 21(44): 12660-12666
- URL: https://www.wjgnet.com/1007-9327/full/v21/i44/12660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i44.12660
Crohn’s disease (CD) is a chronic inflammatory disease associated with mucosal and transmural inflammation of the bowel wall, and its diagnosis relies on the combination of clinical, endoscopic, radiologic and histopathological features.
Regarding endoscopic assessment, ileocolonoscopy is the first procedure for the establishment of the diagnosis. However, evaluation of the entire small bowel is mandatory, since it can change the therapeutic approach used and overall prognosis[1]. In this setting, international guidelines[1] regard cross-sectional studies, such as entero-computed tomography (CT) scan or magnetic resonance imaging (MRI), as first line tools, since they can evaluate extra-luminal features and characterize intra-abdominal adverse events related to CD, such as abscesses or fistulas. Video capsule endoscopy (VCE) is considered a second-line tool in those CD patients with atypical symptoms, in which imaging was negative[1].
Nevertheless, VCE is often used as a first-line study in the suspicion of CD, after ileocolonoscopy[2,3]. Its efficacy in detecting lesions in the upper small bowel seems higher than entero-CT or MRI, with similar accuracy for distal lesions[4-6].
Since the role of VCE in established CD is not completely defined, namely whether VCE is useful for treatment guidance, a few studies tried to evaluate its impact on determining treatment guidance and analyzing the therapeutic changes attributed to VCE[7-11]. However, some of these previous studies included a very low number of patients or had very short disease duration at the time of VCE, thereby compromising the interpretation of the results. Evaluation of possible changes in management includes searching for changes in inflammatory bowel disease (IBD) specific modification strategies, further radiologic or endoscopic studies, or even surgical interventions.
In this study, our main goal was to analyze the changes in the therapeutic regimen of patients with long-term CD after undergoing VCE. Additionally, we studied the impact of Lewis score in this setting and the number of videocapsule retentions with the systematic use of Patency capsule.
All patients with established CD that underwent VCE since January 2011 to February 2014 were included in the study. Patients were assigned to one of three groups. The first group (Staging group) included patients with clinical remission who underwent VCE to assess disease extent or small bowel re-evaluation. The second group (Flare group) included patients who were undergoing re-evaluation because of a flare and had clinical deterioration or raised inflammatory markers. The third group (Post-op group) included patients who were being evaluated for post-operative recurrence.
Patients with suspected CD in which VCE did not confirm the diagnosis or those with VCE considered inconclusive were excluded from the study. Also, patients with suspected CD in which VCE confirmed the diagnosis were also excluded, since our main goal was to evaluate changes in therapeutic regimens for CD before and after VCE. Patients had to be free of non-steroidal anti-inflammatories for at least 1 mo.
All the VCEs were performed after confirming small bowel patency using Agile Patency capsules (Given®, Imaging Ltd. Yoqneam, Israel), which were read 30 h after ingestion. VCEs were performed using PillCam® SB2 or SB3 capsules (Given®, Imaging Ltd.). On the previous day, patients were asked to follow a liquid diet and to perform a bowel preparation. On the day of the procedure, patients were on a clear-liquid diet for 6 h after swallowing the capsule. RAPID® Real-Time Viewer was performed in all patients after 2 h of ingestion, and domperidone 10 mg was prescribed if the capsule remained in the stomach. A new evaluation was performed 1 h later, and if the capsule was still retained in the stomach, a new dose of domperidone 10 mg was administered. If the medication failed, an upper endoscopy was performed to place the device in the duodenum.
All the exams were read by two experienced gastroenterologists using RAPID Reader®. Lewis score was calculated in order to assess the severity of the disease in all procedures, being classified as normal or clinical insignificant if lower than 135 points, mild disease between 135 and 790, and moderate/severe disease above 790, as described elsewhere[12].
In addition to demographic, clinical, and analytical data, medical therapy at the time of VCE and therapy modifications due to VCE were recorded. In order to simplify the results, the “Anti-tumor necrosis factor (TNF) group” included patients taking anti-TNF (infliximab or adalimumab) in monotherapy or combination therapy, and the “Immunosuppression group” included those under azathioprine (AZA) either in monotherapy or combination with 5-amynosalicilates (5-ASA); the remaining patients were under monotherapy with 5-ASA or had no therapy.
Changes in CD treatment in the Staging group and Post-op group were only attributable to VCE findings, since these patients were in clinical and analytical remission. Patients in the Flare group had clinical or analytical active disease, but statistical analysis was conducted to conclude if VCE findings were associated with changes in therapeutic regimen, independent of the flare itself.
Statistical analysis was performed using SPSS software version 22 (SPSS Inc., Chicago, IL, United States). Continuous variables were analyzed using T-student tests or Mann-Whitney test when normal distribution was not verified. Categorical variables were analyzed using Pearson’s Chi-square, Fisher’s exact tests or McNemar test as appropriate. Logistic regression was performed in order to assess variables independently associated with changes in therapeutic regimen. A P value below 0.05 was considered statistically significant.
Among the 542 VCEs performed during the analyzed period, 135 were performed in patients with CD, after positive patency was confirmed by Patency capsule (Patency capsule excluded nearly 25% of the patients who were supposed to perform VCE). From these 135 patients, 29 were excluded because they did not have established diagnosis of CD at the time of VCE. In total, 106 patients were included for the final analysis.
Most of the procedures were performed in patients within the Staging group (n = 73, 69%), with the remaining patients in the Flare (n = 23, 22%) and Post-op (n = 10, 9%) groups. Baseline characteristics are shown in Tables 1 and 2. Fifty-six percent were female, with mean age of 40 ± 13 years. Most patients (81%) had an inflammatory phenotype; 70% had isolated ileal disease. After VCE analysis, upper tract involvement was identified in 49 (46%) patients.
| Population characteristics (n= 106) | Value |
| Male gender | 47 (44) |
| Age - mean | 40 ± 13 yr |
| Median time between diagnosis and VCE | 5.5 (IQR 2-10) yr |
| Montreal classification | |
| Age at diagnosis | |
| A1: Below 17 | 7 (7) |
| A2: 17-40 | 84 (79) |
| A3: Above 40 | 15 (14) |
| Behavior | |
| B1: Non-stenosing/non-penetrating | 86 (81) |
| B2: Stenosing | 12 (11) |
| B3: Penetrating | 8 (8) |
| Location | |
| L1: Terminal ileum | 74 (70) |
| L2: Colonic | 10 (9) |
| L3: Ileocolonic | 22 (21) |
| + L4: Upper disease | 38 (36) |
| Treatment before VCE | |
| Anti-TNF | 21 (20) |
| Immunosuppressors | 38 (36) |
| Aminosalicylates only | 40 (38) |
| No treatment | 7 (6) |
| Variable | Univariate analysis | Multivariate analysis |
| Age | P = 0.477 | - |
| Male gender | P = 0.517 | - |
| Smoking | P = 0.771 | - |
| C-reactive protein | P = 0.188 | - |
| Disease time duration | P = 0.073 | - |
| Age at diagnosis | P = 0.097 | - |
| Ileal vs colonic vs ileocolonic disease | P = 0.009 | P = 0.367 |
| Disease behaviour | P = 0.564 | - |
The median time between the diagnosis of CD and VCE was 5.5 [interquartil range (IQR) 2-10] years. Regarding disease activity (Lewis score), 51 (48%) had normal or clinical insignificant lesions (25% of the total procedures were normal), 14 (13%) had mild disease, and 41 (39%) had moderate to severe disease.
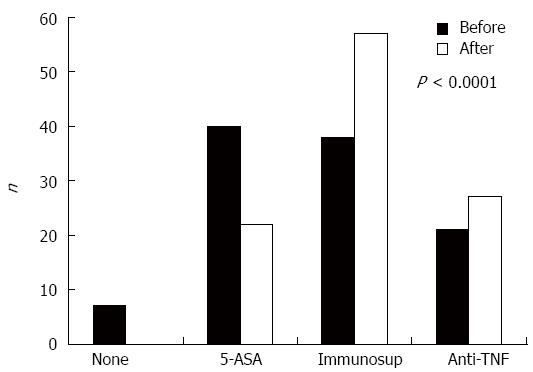
Overall, VCE results guided changes in the treatment of 40% of the patients. At the time of VCE, 38% were under 5-ASA, 36% were under immunosuppressors, 20% were under Anti-TNF, and 6% had no treatment. After VCE, no patient remained without therapy; and the percentage of patients under 5-ASA decreased to almost half and those under AZA and anti-TNF rose significantly (P < 0.0001, Figure 1). Similarly, these results were significant when stratifying patients based on time between diagnosis and VCE (less than 1 year and more than 1, 5, and 10 years of the disease) (data not shown). Overall, only 21% of the patients remained free of immunosuppressors after VCE compared to 44% before VCE (P < 0.001).
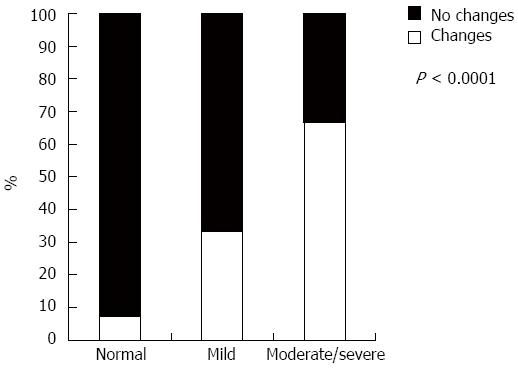
When analyzing Lewis score, only 7% with normal or almost normal VCE changed therapy, whereas 67% changed therapy when VCE demonstrated moderate to severe disease (P < 0.0001, Figure 2). Those patients who changed therapy clearly had higher median Lewis score values (1446 vs 552, P = 0.006). Patients with a Lewis score higher than 1354 had a 90% probability of changing their medication [AUROC 0.80 (95%CI: 0.69-0.88)].
We found differences in the median Lewis score among the different groups. Patients in the Flare group had higher Lewis score values than the Staging (1648 vs 816, P = 0.040) and Post-Op (1648 vs 327, P = 0.035) groups. No significant differences were found between Staging and Post-op groups.
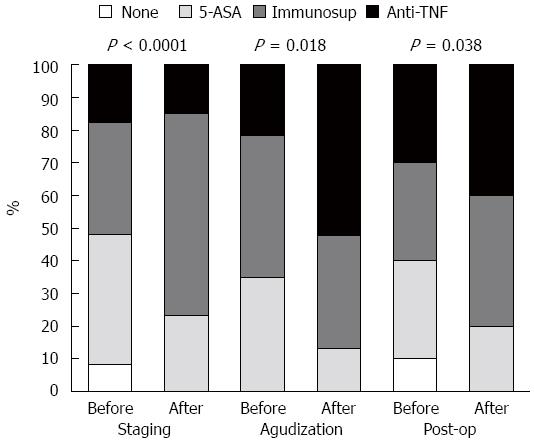
Regarding the indication for VCE, the percentage of patients under AZA was duplicated in the Staging group (P < 0.0001) after VCE, while in the Flare group, the number of patients doubled under anti-TNF (P = 0.032). In the Post-op group, there was an increase in the number of those taking AZA or anti-TNF, but it was not statistically different (P = 0.133, Figure 3).
When performing multivariate analysis, we found that the factors age, C-Reactive protein levels, smoking habits, or duration of the disease were not linked with changes in therapeutic regimen after VCE.
VCE was not retained in any of the patients. Furthermore, no clinical symptoms or other adverse events were reported in the patients where Patency capsule demonstrated negative patency.
VCE is a valuable tool in the assessment of the small bowel[13-19], but its importance in the evaluation and follow-up of CD patients is not well established[20,21]. An indicator to determine the impact of this method on CD is modification of the therapeutic approach after VCE. Some studies address this issue, but there were some limitations.
A recent study[8] found that the number of patients with CD under anti-TNF or immunosuppressants rose after VCE. However, most of the patients underwent VCE in the first year of the disease; and, consequently, 48 of 50 patients were only on 5-ASA or steroids before VCE (only one was under AZA and one under anti-TNF), which makes this difference expectable. An advantage of our study is the inclusion of patients with long-term disease (median time 5.5 years) and patients whose CD is adequately managed, namely anti-TNF and immunosuppressants. Our results showed that VCE was decisive for therapeutic changes even in patients with more than 10 years of CD evolution, highlighting the importance of VCE in this pathology. Similar results were found in a previous study of 71 patients with CD that included subjects with long-term disease[10]. The treatment modification rate was even higher in a study performed in a pediatric population with CD[22].
VCE promoted changes in therapy in every group, although in the Post-op group a statistically significant difference was not achieved, probably due to the small amount of patients (n = 10). In fact, post-op evaluation is emerging as a potential indicator for VCE[23,24]. It is considered to have the same sensibility, specificity, and negative and positive predictive values[2] as colonoscopy. In addition to the latter being a more accessible procedure, VCE was previously shown to find more endoscopic recurrences than colonoscopy[25], with the advantage of allowing for proximal small bowel evaluation[26].
In the Staging and Flare groups, there was a clear and significant modification in therapeutic regimens after VCE, with a decrease in the number of patients under 5-ASA and an increase in patients under AZA (Staging group) and anti-TNF (Flare group). Downgrading of therapy was observed in three patients (infliximab to AZA), all in the Staging group. Those were patients with long-term remission who were under combination therapy and, after performing a VCE for address the possibility of anti-TNF withdrawal, had a normal exam. Previous studies have determined the utility of VCE for the assessment of small bowel mucosal healing after immunomodulator or biologic therapy[27,28], eventually contributing to a downgrade in CD therapy.
It should be noted that none of our patients were being treated with steroids. This was due to the duration of time between the flare and the realization of VCE. Patients in the Flare group started steroids as indicated, but when they came to undergo VCE, the steroid cycle was already completed. This delay in VCE could have been a problem in our analysis since some patients may escalate therapy upon flare before VCE, which could contribute to some attenuation in the differences between therapy before or after the VCE. Statistical differences were still found despite this time lag, making changes in therapy regimens more attributable to VCE findings than to clinical or analytical flare.
The importance of the VCE per se in the changes in therapeutic regimens was highlighted by the regression analysis. As observed, no other factor presented at baseline was independently related to therapeutic modifications. Age, gender, smoking habits, duration of the disease, and inflammatory markers at the time of the VCE were not determinant for therapeutic decisions, making therapeutic changes attributable to the results of VCE. Previous studies had already shown a weak correlation between VCE results and inflammatory biomarkers, making VCE very useful even in the absence of raised C-reactive protein or fecal calprotectin[11].
Lewis score can be a valuable tool for therapeutic management[29]; as expected, higher scores were related with more frequent changes in medical therapy, since they represent active disease requiring a more aggressive treatment.
All the VCEs were performed after confirming small bowel patency using Agile Patency capsules. This device proved to be a very useful tool for patients with known stenosis[30], but its systematic use, as we perform in our institution, is not consensual. International guidelines[2] state that the risk of capsule retention is high in patients with known CD, and, therefore, patency capsule or cross-sectional studies must be performed before VCE to exclude significant stenosis. In patients with suspected CD, the risk appears to be much less significant, and its use is controversial. Since patients with CD can have inflammatory changes in small bowel mucosa, raising the risk of capsule retention, we performed Patency capsule in every patient in this setting. In our Department, nearly 25% of the patients with CD do not perform videocapsule due to negative patency as assessed by Patency capsules. Consequently, we did not experience any videocapsule retention during VCE.
The main limitations of our study were the small number of patients in the Post-Group, which precluded significant results (although there was a clear trend towards therapeutic modifications after VCE), and its retrospective nature. Since this work was not designed to compare patients with and without VCE, we did not assess the differences in the follow-up between them. However, it is well known in the literature that a suboptimal treatment of CD could predispose to a worse outcome. Therefore, we strongly believe that therapy escalation, even in patients with clinical remission but with small bowel lesions detected by VCE, is of paramount importance for a better long-term outcome of CD.
Overall, we concluded that VCE is a very powerful tool for evaluating CD in all groups of patients, including those with long-term disease under immunosuppressors and anti-TNF. It was decisive for treatment guidance, which ultimately can lead to an earlier introduction of immunosuppressors and anti-TNF therapy, consequently improving overall prognosis.
The role of video capsule endoscopy (VCE) in treatment guidance is not well established for Crohn’s disease (CD). Previous studies have attempted to address this subject, but the data are still scarce, especially in patients with long-term disease.
The authors’ results demonstrated the utility of VCE and Patency capsule for the management of CD, namely for the guidance of medical therapy.
This study included patients with long-term CD and show how VCE can affect medical therapy. In addition, the clinical utility of patency capsule is well documented.
Patency capsule allowed for no VCE retention. A large proportion of patients with CD changed therapeutic regimen after VCE.
Lewis score classification according to VCE findings: normal or clinical insignificant disease if lower than 135 points, mild disease between 135-790, and moderate/severe disease above 790 points.
The authors have reported the usefulness of VCE for the management of the therapeutic regimen in patients with CD. This manuscript is well-written.
P- Reviewer: Oka S S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 3. | Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 4. | Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (36)] |
| 6. | O’Donnell S, Qasim A, Ryan BM, O’Connor HJ, Breslin N, O Morain CA. The role of capsule endoscopy in small bowel Crohn’s disease. J Crohns Colitis. 2009;3:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lorenzo-Zúñiga V, de Vega VM, Domènech E, Cabré E, Mañosa M, Boix J. Impact of capsule endoscopy findings in the management of Crohn’s Disease. Dig Dis Sci. 2010;55:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis. 2014;8:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Long MD, Barnes E, Isaacs K, Morgan D, Herfarth HH. Impact of capsule endoscopy on management of inflammatory bowel disease: a single tertiary care center experience. Inflamm Bowel Dis. 2011;17:1855-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Dussault C, Gower-Rousseau C, Salleron J, Vernier-Massouille G, Branche J, Colombel JF, Maunoury V. Small bowel capsule endoscopy for management of Crohn’s disease: a retrospective tertiary care centre experience. Dig Liver Dis. 2013;45:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Kopylov U, Nemeth A, Koulaouzidis A, Makins R, Wild G, Afif W, Bitton A, Johansson GW, Bessissow T, Eliakim R. Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis. 2015;21:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 13. | Mustafa BF, Samaan M, Langmead L, Khasraw M. Small bowel video capsule endoscopy: an overview. Expert Rev Gastroenterol Hepatol. 2013;7:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Hudesman D, Mazurek J, Swaminath A. Capsule endoscopy in Crohn’s disease: are we seeing any better? World J Gastroenterol. 2014;20:13044-13051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Hall B, Holleran G, McNamara D. Current applications and potential future role of wireless capsule technology in Crohn’s disease. Scand J Gastroenterol. 2014;49:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kopylov U, Seidman EG. Role of capsule endoscopy in inflammatory bowel disease. World J Gastroenterol. 2014;20:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Doherty GA, Moss AC, Cheifetz AS. Capsule endoscopy for small-bowel evaluation in Crohn’s disease. Gastrointest Endosc. 2011;74:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-1248; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (36)] |
| 20. | Lucendo AJ, Guagnozzi D. Small bowel video capsule endoscopy in Crohn’s disease: What have we learned in the last ten years? World J Gastrointest Endosc. 2011;3:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Mehdizadeh S, Chen GC, Barkodar L, Enayati PJ, Pirouz S, Yadegari M, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Capsule endoscopy in patients with Crohn’s disease: diagnostic yield and safety. Gastrointest Endosc. 2010;71:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Min SB, Le-Carlson M, Singh N, Nylund CM, Gebbia J, Haas K, Lo S, Mann N, Melmed GY, Rabizadeh S. Video capsule endoscopy impacts decision making in pediatric IBD: a single tertiary care center experience. Inflamm Bowel Dis. 2013;19:2139-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Yamamoto T. Diagnosis and monitoring of postoperative recurrence in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2015;9:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Kono T, Hida N, Nogami K, Iimuro M, Ohda Y, Yokoyama Y, Kamikozuru K, Tozawa K, Kawai M, Ogawa T. Prospective postsurgical capsule endoscopy in patients with Crohn’s disease. World J Gastrointest Endosc. 2014;6:88-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Pons Beltrán V, Nos P, Bastida G, Beltrán B, Argüello L, Aguas M, Rubín A, Pertejo V, Sala T. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Bourreille A, Jarry M, D’Halluin PN, Ben-Soussan E, Maunoury V, Bulois P, Sacher-Huvelin S, Vahedy K, Lerebours E, Heresbach D. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: a prospective study. Gut. 2006;55:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Hall B, Holleran G, Chin JL, Smith S, Ryan B, Mahmud N, McNamara D. A prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (37)] |
| 28. | Hall BJ, Holleran GE, Smith SM, Mahmud N, McNamara DA. A prospective 12-week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. Eur J Gastroenterol Hepatol. 2014;26:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (37)] |
| 29. | Cotter J, Dias de Castro F, Magalhães J, Moreira MJ, Rosa B. Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy. 2015;47:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, Rubin DT, Manten HD, Scapa E, Morgan DR. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. 2008;67:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |