INTRODUCTION
Helicobacter pylori (H. pylori) is a host-specific, bacterial pathogen that can establish a chronic infection within the human gastric mucosa. Chronic infection causes a variety of gastroduodenal diseases ranging from superficial gastritis and peptic ulcers to gastric cancer and mucosa-associated lymphoid tissue lymphoma[1,2]. Over the past several decades, a large number of epidemiological studies have revealed that the effects of H. pylori infections may not be confined to the digestive tract. These infections can be associated with extra-digestive pathologies, especially infections characterized by persistent, low-grade, systemic inflammation[3,4].
The incidence of type 2 diabetes mellitus (T2DM) is rising globally, and T2DM is responsible for an estimated 3.8 million adult deaths worldwide[5]. The pathogenesis of T2DM is complex, and the risk factors are associated with lifestyle (e.g., diet, obesity, and physical activity), genetic background, and socioeconomic factors[6,7]. However, these factors provide only partial explanations, and recent evidence has indicated the pathological involvement of inflammation in T2DM. Thus, chronic infections may be another contributing factor[8]. Previous studies have observed a higher prevalence of H. pylori infection in diabetic subjects compared to non-diabetic subjects[9,10]. Other researchers, however, have proposed an insignificant or even opposite association between H. pylori infection and diabetes[11,12]. Therefore, the relationship between H. pylori infection and T2DM is unclear.
Mongolian gerbils have frequently been used to study the pathogenesis of H. pylori infection because these gerbils are susceptible to colonization and develop gastric diseases due to infection[13,14]. Furthermore, no studies have been performed regarding the link between H. pylori infection and diabetes in Mongolian gerbils. Thus, this study evaluated the effects of chronic H. pylori infection on glucose and lipid metabolism, serum levels of cytokines, insulin and insulin-like growth factor 1 (IGF-1) levels in the pancreas, and the apoptotic rate of islet β-cells in vivo.
MATERIALS AND METHODS
Animals and bacterial strains
A total of 40 male, 5- to 8-wk-old, specific-pathogen-free, Mongolian gerbils (30-50 g) were purchased from the Zhejiang Academy of Medical Sciences (Zhejiang, China). The animals were randomly allocated into two groups: a control group (n = 20) and an H. pylori group (n = 20). All animals were housed in air isolation cages (IVC-II; Suzhou Fengshi Animal Equipment Co., Jiangsu, China) (12/12 h light/dark cycle; room temperature, 20-22 °C; 55% relative humidity) with the same access to food and tap water. The control group was administered Brucella broth, and the H. pylori group was challenged intra-gastrically five times every other day with approximately 109/CFU H. pylori ATCC43504 (CagA+, VacA+). Each group was then divided into two subgroups that were sacrificed at 6 or 12 mo. The control and H. pylori subgroups each contained 10 Mongolian gerbils. All protocols for animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. H. pylori infection was produced in mice in compliance with the institutional guidelines and regulations with an effort to minimize the number of animals used and their suffering.
Biochemical assays
At 6 and 12 mo, the animals were sacrificed after anaesthetization, and blood samples were obtained from the abdominal aorta for biochemical analysis. Body weight, abdominal circumference and body length were measured, and the body mass index (BMI) [body weight (g)/length2 (cm2)] and Lee’s index [body weight (g) × 103/nose-to-anus length (cm)]1/3 were calculated. Blood was placed in a centrifuge tube at room temperature and allowed to clot to obtain the serum. Serum was separated by centrifugation at 3000 rpm for 10 min. Serum glucose (Glu), glycated hemoglobin (GHb), glycated hemoglobin A1c (HbA1c), triacylglycerol (TG), and total cholesterol (TC) were assayed using an automatic biochemistry analyzer (Hitachi 7600, Japan). The serum levels of inflammatory cytokines, including interleukin (IL)-1β, IL-2, IL-4, IL-10, IL-12, tumor necrosis factor-α (TNF-α) and interferon (IFN)-γ, were assayed using ELISA kits (BD Bioscience, United States).
Histopathological examinations
To determine whether bacterial colonization had occurred in the stomach, whole stomachs were stored in 10% formaldehyde in Ca2+ and Mg2+ free phosphate-buffered saline (PBS) overnight at 4 °C prior to paraffin embedding, and H. pylori infection was observed via Giemsa staining. The pancreases were stored in 10% formaldehyde for immunohistochemistry and terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assays. Paraffin sections of 4 μm were cut with a microtome and stored at room temperature.
Immunohistochemistry assay
The primary antibodies used in this study were guinea pig polyclonal to insulin and rabbit polyclonal to IGF-1 (Abcam, United Kingdom). The anti-insulin antibody was diluted 1:100, and the anti-IGF-1 antibody was diluted 1:1000.
The paraffin sections were mounted on slides, dewaxed in xylene and sequentially dehydrated in 100%, 95% and 85% ethanol. The sections were stained following the PV-6000 Polymer Detection System (Zhongshan Goldenbridge, China) staining protocol. The sections were then washed in PBS, and endogenous peroxidase was blocked using 3% H2O2. After the specimens were incubated with the primary antibody overnight at 4 °C, they were washed with PBS and incubated with polymer helper for 30 min and polyperoxidase-anti-mouse or rabbit IgG for 30 min. After the sections were washed with PBS, they were incubated with 3,3’-diaminobenzidin (DAB, Zhongshan Goldenbridge, China). The control sections were incubated with PBS instead of the primary antibodies (negative controls). The sections were counterstained with hematoxylin.
The stained sections were chosen, reviewed, and scored from five randomly selected high power fields (40 × objective lens) by two pathologists blinded to the histopathological data. Grading discrepancies were re-reviewed and discussed to obtain a final score. Epithelial cells with yellow or brown staining in the nucleus and/or cytoplasm were defined as positive for immunoreactivity. The percentages of immunoreactive cells from 100 cells in each field were averaged from five fields and scored as follows: 0 = 0%-5.0% immunoreactivity; 1 = 5.1%-25.0% immunoreactivity; 2 = 25.1%-50.0% immunoreactivity; 3 = 50.1%-75.0% immunoreactivity; and 4 = 75.1%-100% immunoreactivity. In addition, the immunostaining intensity was semi-quantitatively assessed (0 = negative, 1 = weak staining, 2 = moderate staining, and 3 = intense staining). The overall protein expression level was reported as a grade calculated from an integral score of ‘‘area × intensity’’ as follows: grade 1 = scores 0-2 (negative); grade 2 = scores 3-5 (weakly positive); grade 3 = scores 6-8 (moderately positive); and grade 4 = scores 9-12 (strongly positive)[15,16].
Apoptosis assay
Apoptosis was quantified using the TUNEL method with the DeadEnd™ colorimetric apoptosis detection system (Promega Corp., United States) according to the manufacturer’s protocol. The number of TUNEL-positive cells was counted in 20 randomly selected fields per section under a microscope at 200-fold magnification. Apoptosis was calculated as the percentage of apoptotic nuclei (dark brown nuclei) versus the total nuclei of multinucleated TRAP-positive cells. A dark brown DAB signal indicated positive staining, and shades of blue-green to greenish-tan indicated a non-reactive cell[17,18].
Statistical analysis
The data are presented as mean ± SEM or percentages. Student’s t-test (SPSS v.16.0 for Windows; SPSS, Inc., United States) and the chi-square test were used to detect statistical differences. P < 0.05 was considered significant.
RESULTS
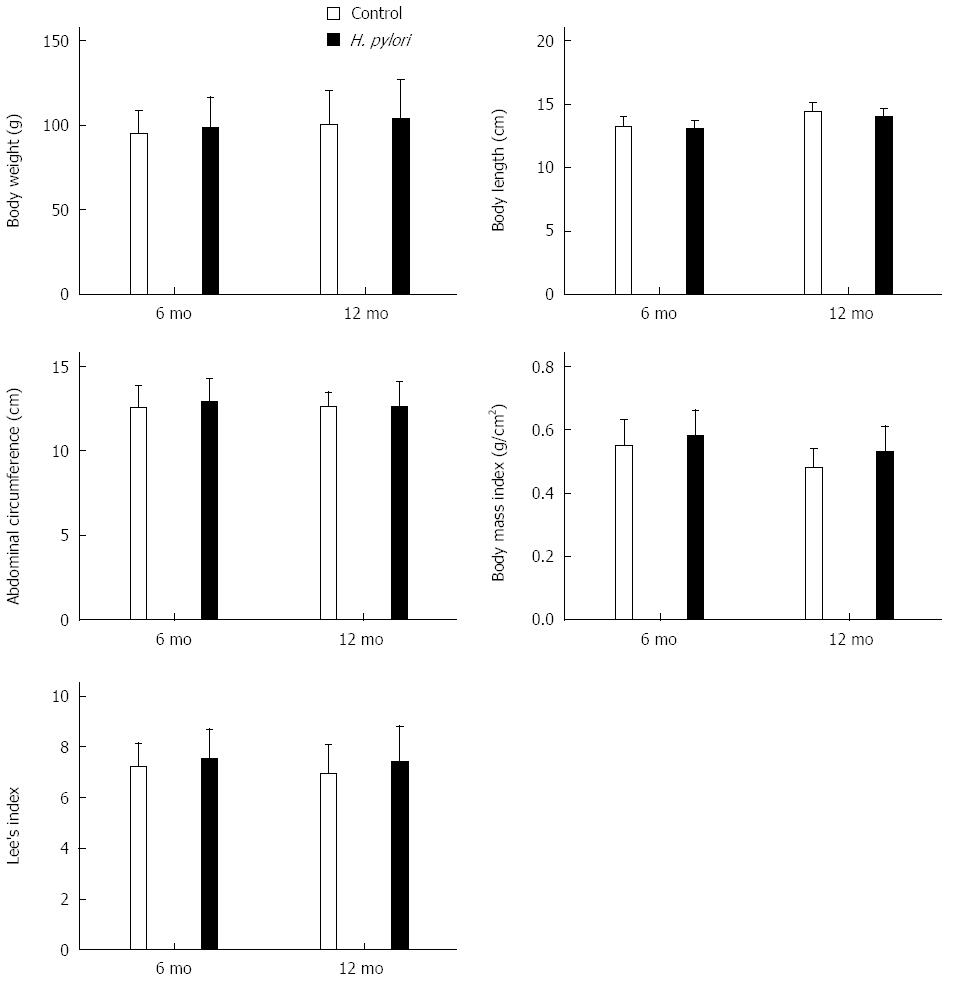
Effects of H. pylori infection on body weight, body length, abdominal circumference, BMI and Lee’s index in Mongolian gerbils
The Mongolian gerbils were successfully infected with H. pylori as confirmed by Giemsa staining. No animals challenged with Brucella broth alone had detectable evidence of H. pylori. Body weight, body length, abdominal circumference, BMI and Lee’s index were calculated at 6 and 12 mo after H. pylori infection in Mongolian gerbils (Figure 1). The body weight, abdominal circumference, BMI, and Lee’s index increased after H. pylori infection at each time point. However, these differences were not significant (P > 0.05) (Figure 1).
Figure 1 Effects of Helicobacter pylori infection on body indexes of Mongolian gerbils at different time points.
The body weight, body length, and abdominal circumference were measured, and body mass index and Lee’s index were calculated at 6 and 12 mo after Helicobacter pylori (H. pylori) infection. The data denote an upward trend of these parameters, although there were no significant differences (P > 0.05). Bars represent the mean ± SEM, n = 8-10 mice per group.
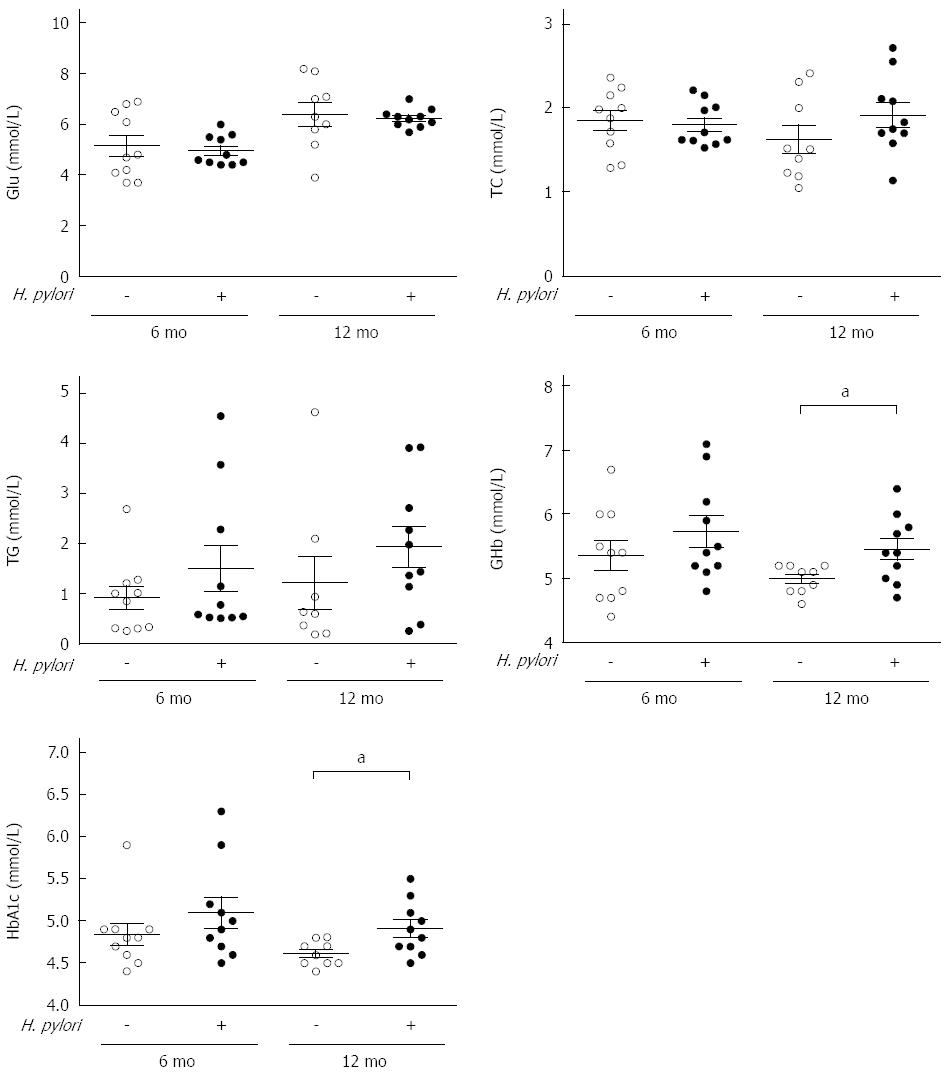
H. pylori infection and serum levels of biochemical indexes in Mongolian gerbils
At 6 and 12 mo after H. pylori infection, the animals were sacrificed after anesthetization. The serum levels of biochemical indexes, including fasting Glu, TG, TC, GHb and HbA1c, were assayed using an automatic biochemistry analyzer. The serum Glu, TG, and TC levels were not significantly different between the control and H. pylori groups (P > 0.05), although there was a slight increase in TG and TC in the infected groups compared to the control groups (Figure 2). HbA1c, the major fraction of GHb, is an efficient glucose monitoring index for patients with diabetes. At 12 mo post-infection, the levels of both GHb (5.45 ± 0.53 vs 4.98 ± 0.22, P < 0.05) and HbA1c (4.91 ± 0.61 vs 4.61 ± 0.15, P < 0.05) were significantly increased in the infected group compared to the control group (Figure 2).
Figure 2 Measurements of serum biochemical parameters of Mongolian gerbils after Helicobacter pylori infection at different time points.
Serum concentration of fasting glucose (Glu), triacylglycerol (TG), total cholesterol (TC), and glycated hemoglobin (GHb) and hemoglobin A1c (HbA1c) were measured at 6 and 12 mo after Helicobacter pylori (H. pylori) infection. Data are presented as mean ± SEM from eight to ten mice per group. aP < 0.05 between H. pylori infected mice and their controls at 12 mo concerning GHb and HbA1c.
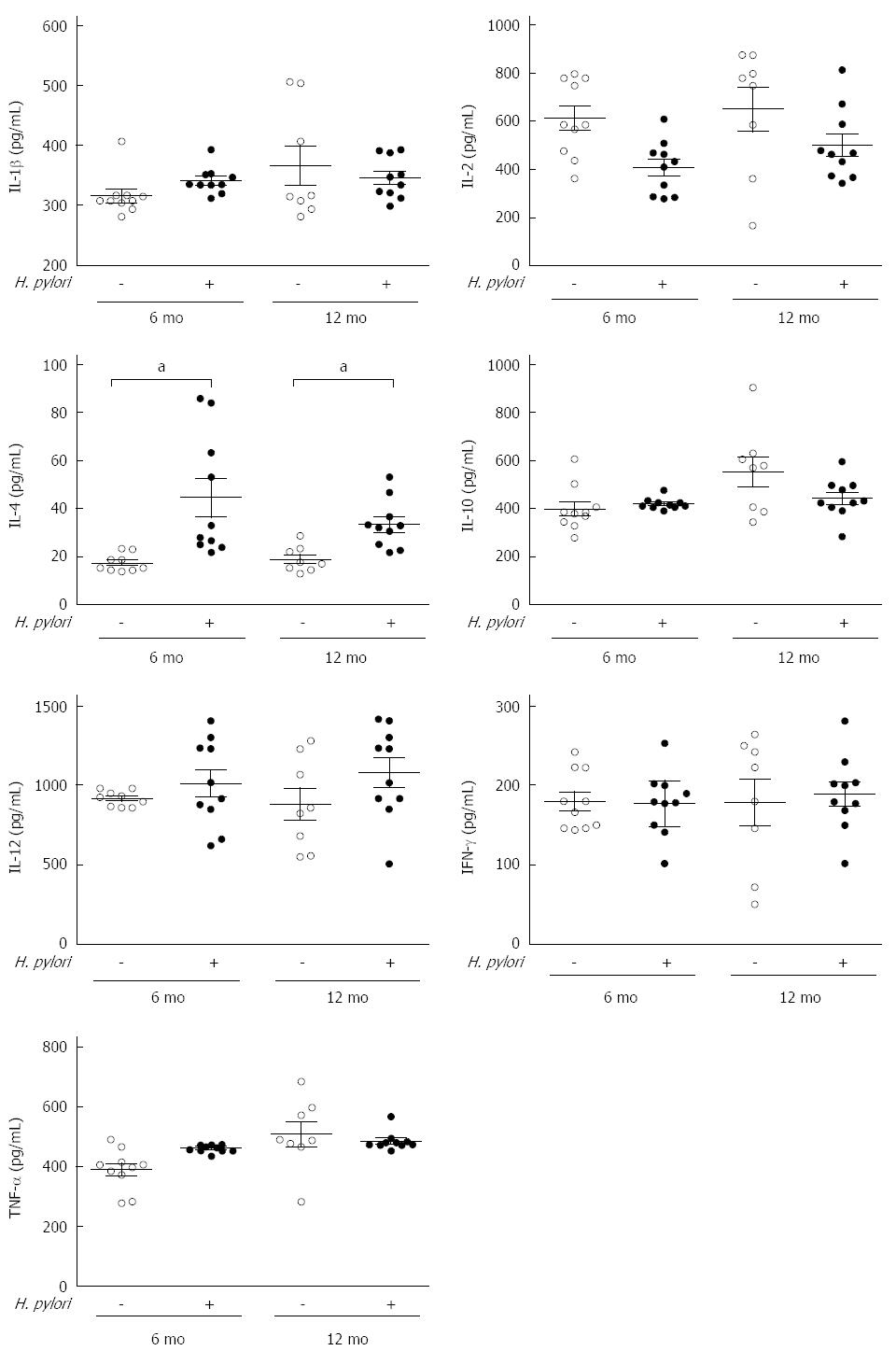
Effects of chronic H. pylori infection on the serum levels of inflammatory cytokines in Mongolian gerbils
It is commonly believed that the chronic inflammation induced by an H. pylori infection is a major link to T2DM. Thus, the serum levels of inflammatory cytokines, including IL-1β, IL-2, IL-4, IL-10, IL-12, TNF-α and IFN-γ, were assayed using ELISA kits. No significant differences were observed (P > 0.05), except for the serum level of IL-4, which was significantly increased in the H. pylori group compared to the control group at both 6 (44.36 ± 25.17 vs 17.38 ± 3.47, P < 0.05) and 12 mo (33.41 ± 10.00 vs 18.91 ± 5.31, P < 0.05) (Figure 3).
Figure 3 Effects of chronic Helicobacter pylori infection on the serum inflammatory cytokines in Mongolian gerbils at different time points.
The serum levels of cytokines including IL-1β, IL-2, IL-12, IL-4, IL-10, IFN-γ, and TNF-α were measured by ELISA at 6 and 12 mo after Helicobacter pylori (H. pylori) infection. Data are presented as mean ± SEM from eight to ten mice per group. Cytokines were not significant different except IL-4 (aP < 0.05 between H. pylori infected mice and their controls). IL: Interleukin; TNF: Tumor necrosis factor; IFN: Interferon.
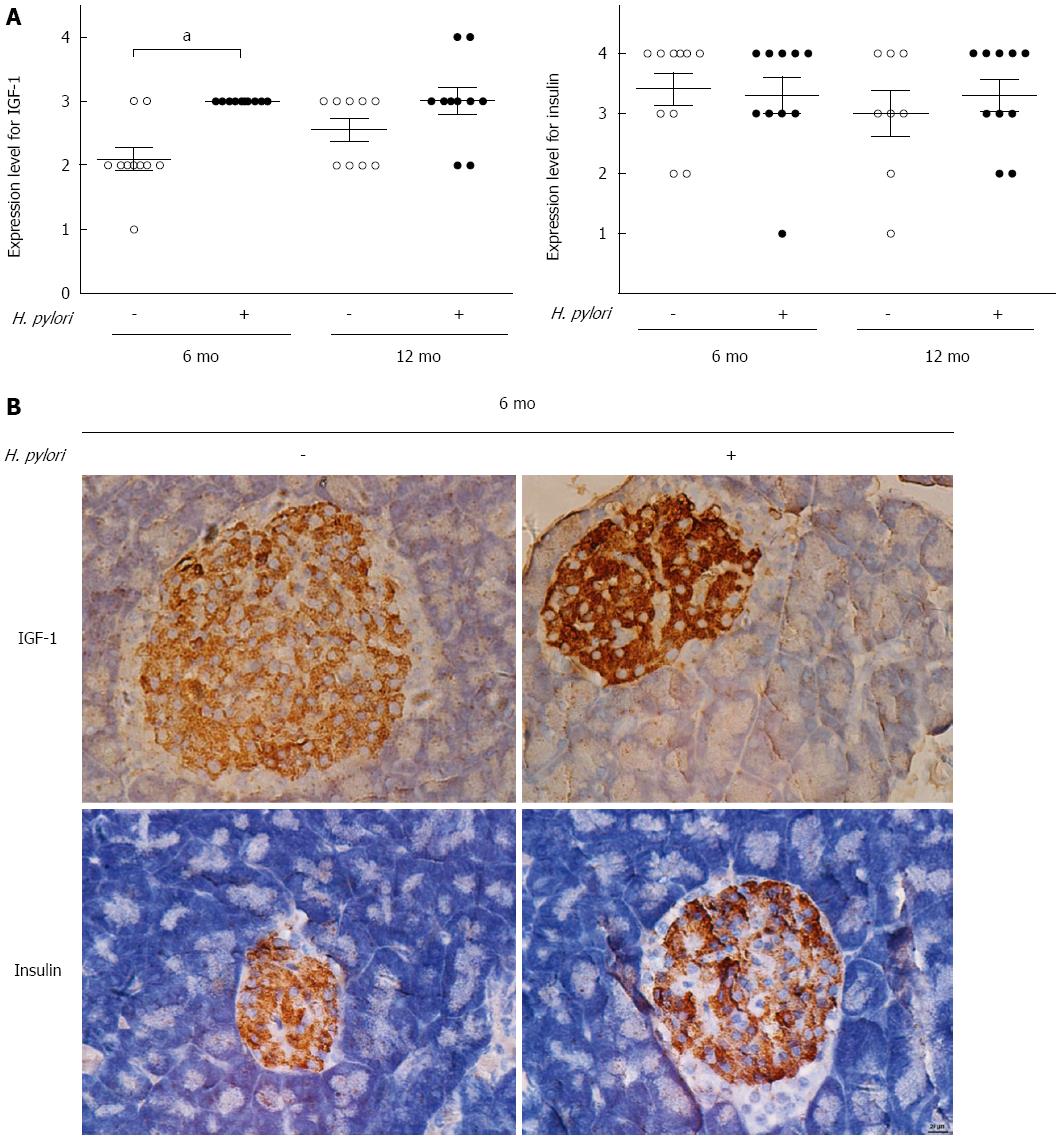
H. pylori infection and pancreatic insulin and IGF-1 in Mongolian gerbils
Insulin is the key hormone for blood glucose regulation. Generally, normoglycemia is maintained by a balanced interplay between insulin action and secretion. IGF-1 is structurally similar to insulin and is also involved in the physiological regulation of nutrient intake, metabolism and tissue growth. Thus, IGF-1 contributes to the complex balance between anabolism and consumption. To investigate the effects of H. pylori on the expression of insulin and IGF-1 in gerbil pancreatic tissues, immunohistochemical staining was used. Insulin expression did not differ between the H. pylori group and the controls at 6 or 12 mo (P > 0.05) (Figure 4). At 6 mo, the expression of IGF-1 was significantly increased in the H. pylori group compared to the control groups (P < 0.05). No significant difference was observed at 12 mo (P > 0.05) (Figure 4).
Figure 4 Effects of chronic Helicobacter pylori infection on the expression of insulin-like growth factor-1 and insulin in the pancreas of Mongolian gerbils.
A: Immunohistochemistry scores of insulin-like growth factor-1 (IGF-1) and insulin were determined in the control and Helicobacter pylori (H. pylori) groups at 6 and 12 mo (n = 8-10). Data are expressed as the mean ± SEM. aP < 0.05 between H. pylori infected mice at 6 mo and their controls concerning the expression of IGF-1; B: Representative immunohistochemical staining of IGF-1 and insulin expression in the pancreas of Mongolian gerbils at 6 mo after H. pylori infection (magnification × 400).
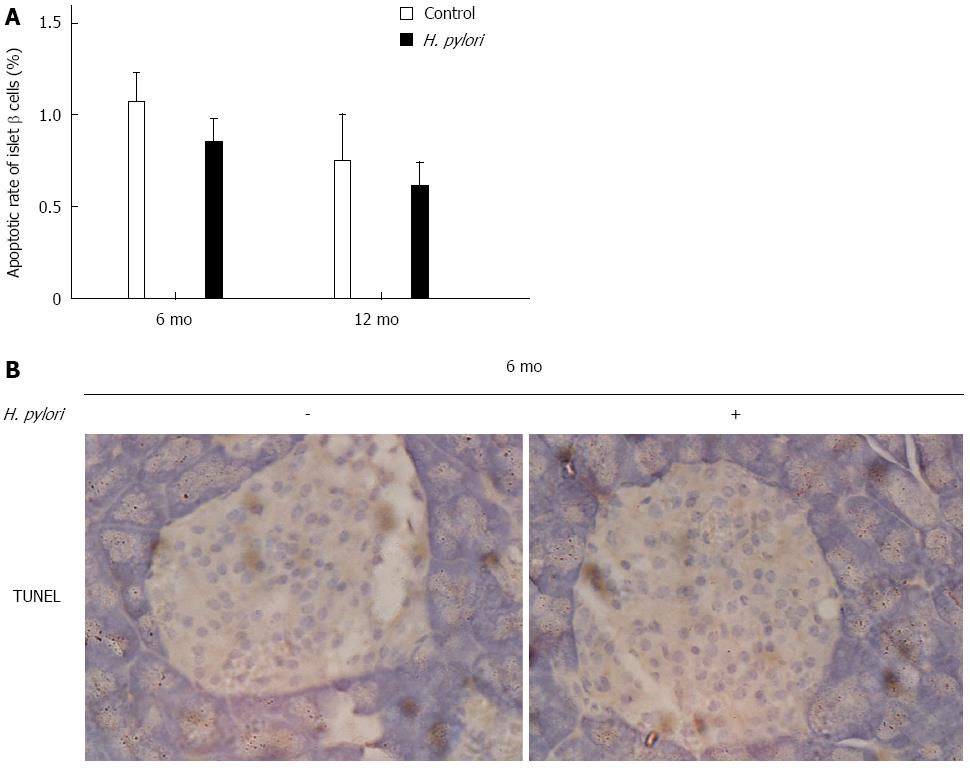
Effects of chronic H. pylori infections on apoptosis of islet β-cells in Mongolian gerbils
Islet β-cell dysfunction is a critical factor during the pathogenesis of T2DM. β-cell mass may be decreased during T2DM, and the underlying cause may be increased β-cell apoptosis[19]. TUNEL assay was used to quantify the apoptotic rate of islet β cells at 6 and 12 mo after H. pylori infection in Mongolian gerbils. There was no significant difference in β-cell apoptosis between the H. pylori infected and control groups (P > 0.05) (Figure 5).
Figure 5 Effects of chronic Helicobacter pylori infection on the apoptosis of islet β-cells in Mongolian gerbils.
A: Apoptotic rate of islet β-cells was detected by TUNEL assay in the control and Helicobacter pylori (H. pylori) groups at 6 and 12 mo. Data are expressed as the means ± SEM (n = 8-10 mice/group). P > 0.05 was observed between H. pylori infected mice and their controls; B: Representative images of islet β-cells in the pancreas of Mongolian gerbils at 6 mo after H. pylori infection by TUNEL staining (magnification × 400).
DISCUSSION
A growing body of epidemiological evidence supports a relationship between H. pylori infection and diabetes[9,10,20]. Simon et al[21] in 1989 first observed that the prevalence of H. pylori infection in patients with diabetes mellitus was significantly higher than that in asymptomatic controls. A recent meta-analysis also showed that the prevalence of H. pylori infection in patients with diabetes was higher than that of the control group[22]. Moreover, a recent prospective cohort study demonstrated that H. pylori infection leads to an increased rate of incident diabetes, which suggests a potential role for antibiotic and gastrointestinal treatments to prevent diabetes[23]. These studies suggested that H. pylori infection might cause diabetes. Furthermore, patients with diabetes are more prone to H. pylori infections than non-diabetic individuals. There are several reasons for this phenomenon. First, the immune system of diabetic patients is compromised, leading to an increased susceptibility to H. pylori infection[24]. In addition, altered glucose metabolism may produce chemical changes in the gastric mucosa and reduce acid secretion and gastrointestinal mobility (thus promoting H. pylori colonization)[25]. However, other studies have denied this association[11,12]. To better understand whether H. pylori infection plays a role in diabetes etiology, research regarding diabetes biomarkers is needed.
HbA1c is the major fraction of GHb and results from non-enzymatic glycosylation. HbA1c reflects the integrated blood glucose levels during the preceding 3-4 mo. Thus, HbA1c levels are predictive of both the prevalence and incidence of diabetes and are a more stable measurement than fasting blood glucose for the diagnoses of pre-diabetes and diabetes[26-28]. Two large national surveys reported that H. pylori seropositivity, especially CagA+ strains, was associated with higher mean HbA1c levels and that there was a synergistic effect of H. pylori and BMI on increased HbA1c levels. These findings support a role for H. pylori in impaired glucose intolerance[29]. Similar results were observed by Hsieh et al[9]. The authors observed that long-term H. pylori infection was significantly associated with high HbA1c levels and a higher T2DM prevalence in Taiwanese patients.
In this study, we examined the levels of HbA1c in H. pylori-infected Mongolian gerbils and found a significant increase in HbA1c at 12 mo. This result indicates an association between H. pylori colonization and diabetes in vivo. However, fasting glucose was not significantly different between the H. pylori group and the control group (which is not contradictory to the significantly increased HbA1c levels). A previous study also reported a significant association between H. pylori infection and elevated HbA1c without changes in fasting glucose levels in humans[9]. Fasting glucose, which is a less stable measurement than HbA1c, is susceptible to changes in daily activities, such as diet content and exercise. These fluctuations may confound evaluations of the association between chronic H. pylori infection and glucose regulation. Moreover, fasting blood glucose levels markedly increase due to β-cell dysfunction and insulin secretion deficiency. Our study showed that β-cell apoptosis and insulin expression in H. pylori-infected Mongolian gerbils were not different from those in the control group, which might contribute to the similarity in fasting glucose between the two groups. In contrast, another study demonstrated that gastric infection with some commensal strains of H. pylori ameliorates glucose homeostasis in mice and provides partial protection against some metabolic disorders[30]. The use of different bacterial and mice strains has produced inconsistent results, indicating that H. pylori may affect glucose metabolism in an individual/bacterial strain-dependent manner.
The mechanisms by which H. pylori infection increases HbA1c levels and potentiates the development of diabetes remain to be elucidated. Although insulin insensitivity is an early phenomenon, islet β-cell function declines gradually over time before the onset of clinical hyperglycemia and the occurrence of T2DM. IGF-1 is structurally similar to insulin and is also involved in the physiological regulation of nutrient intake, metabolism and tissue growth. Thus, IGF-1 contributes to the complex balance of anabolism and consumption. This balance can be disrupted during obesity and diabetes, and IGF-1 may be an important component[31]. It is commonly believed that pancreatic β-cell mass and function are crucial to whole body glucose regulation. Pancreatic islet cells produce IGF-1, which binds to IGF-1 receptors on β-cells[32]. IGF-1 was thought to be an important stimulus to pancreatic islet cell growth and to inhibit β-cell apoptosis[33,34]. The IGF-1 receptor and elements of the IGF-1 signal transduction pathway are expressed in pancreatic β-cells[35].
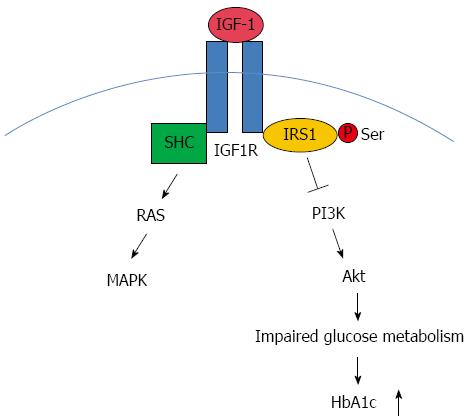
In our study, the expression of IGF-1 was significantly increased in H. pylori-infected Mongolian gerbils compared to the controls at 6 mo. No significant difference was observed at 12 mo. We assumed that there may be a time course accounting for the alteration in IGF-1 expression. At 6 mo, pancreatic IGF-1 probably increased to maintain normal HbA1c levels, and at 12 mo the HbA1c levels significantly increased due to the decompensation of pancreatic IGF-1. IGF-1, upon binding to its IGF-1 receptor, activates the intrinsic tyrosine kinase activity of the IGF-1 receptor[36]. The IGF-1 receptor then phosphorylates substrate proteins, including members of the IRS family, such as IRS1 and Shc, on selective tyrosine residues. Downstream of IGF-1 are two main pathways: phosphatidylinositol-3 kinase (PI3K)/Akt and MAPK[37]. IGF-1 acts via the MAPK pathway to mediate growth responses, and the PI3K pathway is thought to have predominantly metabolic effects, such as glucose uptake and Glut4 translocation. In addition to tyrosine phosphorylation, IRS proteins could undergo serine phosphorylation, which may attenuate signaling by decreasing normal tyrosine phosphorylation and promoting insulin resistance[38]. We propose that H. pylori infection-associated impaired glucose metabolism and HbA1c up-regulation may be mediated through the IGF-1 signaling pathway (Figure 6). However, insulin expression in the pancreas and the apoptotic rate of islet β-cells were not significantly different between the two groups, which suggests that the effects of H. pylori infection on glucose metabolism in Mongolian gerbils may not act through the injury of islet β-cells. It is well known that insulin resistance (IR) and abnormal insulin secretion are central to the development of T2DM, and IR is supposed to precede defects in insulin secretion[39]. A growing body of evidence has linked H. pylori infection to IR[40,41]. The limitation of our study is that we did not determine the insulin sensitivity of H. pylori-infected Mongolian gerbils at each time point. Therefore, it remains to be clarified whether H. pylori infection impairs glucose tolerance and subsequently breaks the balance of glucose metabolism.
Figure 6 Insulin-like growth factor-1 signaling with regard to Helicobacter pylori associated glucose dysregulation.
Insulin-like growth factor-1 (IGF-1), upon binding to its receptor IGF-1R, activates the intrinsic tyrosine kinase activity. IGF-1R then phosphorylates substrate proteins, including members of the IRS family such as IRS1 and Shc on selective tyrosine residues. Downstream to the receptors are two major pathways: the phosphatidylinositol-3 kinase (PI3K)/Akt pathway and MAPK pathway. IGF-1 acts via the MAPK pathway to mediate growth responses. The PI3K pathway is thought to have predominantly metabolic effects. We supposed that this pathway might play a part in Helicobacter pylori (H. pylori) associated abnormal glucose metabolism and the upregulation of HbA1c. SHC: Spontaneous human combustion.
Moreover, it is commonly believed that the chronic inflammation induced by H. pylori infection is the major link to T2DM. H. pylori infection, which leads to an increased production of lipopolysaccharides, may activate innate inflammatory processes[42]. Inflammatory processes correlate with elevated inflammatory cytokines, including C-reactive protein[43], IL-6 and TNF-α[44]. However, the inflammation hypothesis was not substantiated in our study. With the exception of IL-4, there were no significant differences in cytokines, including IL-1β, IL-2, IL-4, IL-10, IL-12, TNF-α and IFN-γ, between the H. pylori and the control groups. Interestingly, a similar phenomenon was reported by Jeon et al[23] who also found that serological evidence of H. pylori infection was associated with an increased rate of incident diabetes in an elderly Latino cohort, and the inflammatory cytokines did not appear to mediate the effect. An alternative hypothesis is that H. pylori-induced gastritis can affect the secretion of “gastric” hormones, including leptin and ghrelin[45], which might predispose patients to diabetes.
There are conflicting data regarding the effect of H. pylori infection on serum lipids[46,47] and body indices, including body weight and BMI[48,49]. In the present study, TC and TG concentrations were not significantly different between Mongolian gerbils with H. pylori infection and the controls. The H. pylori group had an increased body weight, BMI and Lee’s index compared to the control group. However, these differences were not significant. A possible explanation for this observation may be in the putative manipulation of systemic ghrelin levels, which is the pivotal hormone regulating food intake and appetite. There are limited and conflicting data regarding the effects of H. pylori eradication on glucose metabolism and insulin sensitivity. Zojaji et al[50] showed that H. pylori treatment can improve the mean HbA1c and metabolic abnormalities in patients with T2DM. Thus, it may be beneficial for patients at risk of developing diabetes to be checked for H. pylori infections. Gen et al[51] also demonstrated that successful H. pylori eradication significantly decreased fasting insulin and HOMA-IR levels. To date, no study has investigated the effects of H. pylori eradication on glucose metabolism in Mongolian gerbils.
In conclusion, this study identified a significant association between chronic H. pylori infection and high HbA1c levels in Mongolian gerbils. Insulin expression in the pancreas and the apoptotic rate of islet β-cells were not significantly different after H. pylori infection. Inflammatory cytokines (IL-1β, TNF-α and IFN-γ) did not appear to mediate this effect. Further studies are warranted to explore the impact of H. pylori infections on insulin resistance and the corresponding mediating factors. The treatment of H. pylori infection is important for the prevention of gastric cancer. However, whether H. pylori infection is a risk factor for T2DM remains to be determined.
COMMENTS
Background
Epidemiological evidence has revealed that the outcome of Helicobacter pylori (H. pylori) infection may not be confined to the digestive tract, and that the infection can be associated with extra-digestive pathologies such as type 2 diabetes (T2DM). The studies regarding the relationship between H. pylori infection and T2DM are inconsistent and most in human. Thus, the authors assessed the effects of chronic H. pylori infection on metabolic parameters of Mongolian gerbils.
Research frontiers
Previous studies have found a higher prevalence of H. pylori infection in diabetic patients and recently a prospective cohort study further demonstrated that H. pylori infection leads to an increased rate of incident diabetes. Consequently, the current hotspot is whether H. pylori infection plays a role in the development of T2DM.
Innovations and breakthroughs
Epidemiological studies suggest that chronic H. pylori infection is associated with T2DM although it is still controversial. And there are few studies on the relationship between H. pylori infection and diabetes in animal models. This study found that chronic H. pylori infection significantly increased the glycated hemoglobin and glycated hemoglobin A1c levels in Mongolian gerbils. Besides, the expression of insulin growth factor-1 was significantly increased after H. pylori infection, with elevated serum level of IL-4.
Applications
These data provide evidence that long-term H. pylori infection is significantly associated with high levels of HbA1c in Mongolian gerbils, which is consistent with observations in human. Evidence supporting the role of H. pylori in the development of T2DM would provide H. pylori eradication as an easy and convenient preventive measurement for diabetes.
Terminology
H. pylori is a host-specific bacterial pathogen that establishes a chronic infection in the human gastric mucosa. T2DM is now becoming a global epidemic with risk factors associated with lifestyle, genetic background and socioeconomic factors.
Peer-review
This is an interesting study regarding the possible connection between H. pylori infection, glucose metabolism, HbA1c and diabetes. The methodology of the study is right, the results are interesting and the detailed discussion covers the topic and the research question.