Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12439
Peer-review started: June 5, 2015
First decision: July 19, 2015
Revised: August 5, 2015
Accepted: September 14, 2015
Article in press: September 15, 2015
Published online: November 21, 2015
Processing time: 171 Days and 15.1 Hours
AIM: To evaluate combination transjugular intrahepatic portosystemic shunt (TIPS) and other interventions for hepatocellular carcinoma (HCC) and portal hypertension.
METHODS: Two hundred and sixty-one patients with HCC and portal hypertension underwent TIPS combined with other interventional treatments (transarterial chemoembolization/transarterial embolization, radiofrequency ablation, hepatic arterio-portal fistulas embolization, and splenic artery embolization) from January 1997 to January 2010 at Beijing Shijitan Hospital. Two hundred and nine patients (121 male and 88 female, aged 25-69 years, mean 48.3 ± 12.5 years) with complete clinical data were recruited. We evaluated the safety of the procedure (procedure-related death and serious complications), change of portal vein pressure before and after TIPS, symptom relief [e.g., ascites, hydrothorax, esophageal gastric-fundus variceal bleeding (EGVB)], cumulative rates of survival, and distributary channel restenosis. The characteristics of the patients surviving ≥ 5 and < 5 years were also analyzed.
RESULTS: The portosystemic pressure was decreased from 29.0 ± 4.1 mmHg before TIPS to 18.1 ± 2.9 mmHg after TIPS (t = 69.32, P < 0.05). Portosystemic pressure was decreased and portal hypertension symptoms were ameliorated. During the 5 year follow-up, the total recurrence rate of resistant ascites or hydrothorax was 7.2% (15/209); 36.8% (77/209) for EGVB; and 39.2% (82/209) for hepatic encephalopathy. The cumulative rates of distributary channel restenosis at 1, 2, 3, 4, and 5 years were 17.2% (36/209), 29.7% (62/209), 36.8% (77/209), 45.5% (95/209) and 58.4% (122/209), respectively. No procedure-related deaths and serious complications (e.g., abdominal bleeding, hepatic failure, and distant metastasis) occurred. Moreover, Child-Pugh score, portal vein tumor thrombosis, lesion diameter, hepatic arterio-portal fistulas, HCC diagnosed before or after TIPS, stent type, hepatic encephalopathy, and type of other interventional treatments were related to 5 year survival after comparing patient characteristics.
CONCLUSION: TIPS combined with other interventional treatments seems to be safe and efficacious in patients with HCC and portal hypertension.
Core tip: There are conflicting results about the safety and efficacy of transjugular intrahepatic portosystemic shunt (TIPS) combined with other interventional treatments for patients with hepatocellular carcinoma (HCC) and portal hypertension. We reviewed 209 patients with HCC and portal hypertension who underwent TIPS and other interventional treatments. Portosystemic pressure was decreased and portal hypertension symptoms were ameliorated, and no procedure-related deaths and serious complications occurred. The survival rates for TIPS in combination seem better than those reported for transarterial chemoembolization or radiofrequency ablation alone.
- Citation: Qiu B, Zhao MF, Yue ZD, Zhao HW, Wang L, Fan ZH, He FL, Dai S, Yao JN, Liu FQ. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol 2015; 21(43): 12439-12447
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12439.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12439
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer death worldwide[1], and the second most common malignancy in China[2], with an estimated 391250 new cases and 372750 deaths in 2012[3]. The mortality rate of HCC in China was 20.4 per 100000 according to the 2015 annual report from the American Society of Clinical Oncology (ASCO), and patient survival has increased significantly in recent years[4]. There is a variety of treatments for HCC depending on the nature of the tumor (size, stage, degree, and complications), and interventional treatments, including transarterial embolization/chemoembolization (TAE/TACE)[5,6] and radiofrequency ablation (RFA)[7], have become important for HCC recently. HCC often stems from hepatitis B cirrhosis and combines with portal hypertension[2], leading to esophageal gastric-fundus variceal bleeding (EGVB) and/or refractory ascites (or hydrothorax)[8,9]. Patients with HCC and portal hypertension often have no opportunity to receive radical surgery, liver transplantation, or even some interventional treatments. It is important to manage portal hypertension urgently in patients with HCC[10]. Transjugular intrahepatic portosystemic shunt (TIPS) is an expandable metal stent inserted via the jugular vein that creates a shunt from the portal vein to the systemic circulation via an artificial communication through the liver. TIPS is widely used as a treatment of portal hypertension and its complications[11-14] (such as EGVB, refractory ascites, hepatic hydrothorax, hepatorenal syndrome, and hepatopulmonary syndrome) and as a bridge to liver transplantation. Patients with portal hypertension have improvements in symptoms after TIPS, especially timely termination of acute EGVB and refractory ascites, which create opportunities for further treatment without affecting overall survival[15-17]. However, HCC and portal hypertension have been considered as relative contraindications for TIPS combined with other interventional treatments. There are conflicting results about the safety and efficacy of TIPS combined with other interventional treatments for patients with HCC and portal hypertension[18-26]. In this study, we reviewed and analyzed the data from 209 patients with HCC and portal hypertension who underwent TIPS and other interventional treatments from January 1997 to January 2010 at Beijing Shijitan Hospital.
Two hundred and sixty-one patients with HCC and portal hypertension underwent TIPS combined with other interventional treatments (TACE/TAE, RFA, hepatic arterio-portal fistulas embolization, and splenic artery embolization) from January 1997 to January 2010 at Beijing Shijitan Hospital. We recruited 209 patients (121 male and 88 female, aged 25-69 years, mean 48.3 ± 12.5 years) who had complete clinical data; the remaining the patients who lacked such data were excluded. Thirty-seven cases of HCC were diagnosed by pathological biopsy and 172 cases by ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), α-fetoprotein (AFP), and hepatic artery angiography. Hepatitis B cirrhosis was detected in 180 cases, hepatitis C cirrhosis in 12, overlapping hepatitis B and C cirrhosis in two, primary cholestasis cirrhosis in one, alcoholic liver cirrhosis in 13, and autoimmune liver cirrhosis in one. EGVB was seen in 182 cases, refractory ascites (and/or pleural effusion) in 39, and refractory ascites (and/or pleural effusion) combined with EGVB in 12. Preoperative splenectomy cutoff was undertaken in eight cases, hardening or ligation treatments in 41, and preoperative surgical excision of tumor in 35.
We evaluated the safety (procedure-related death and serious complications, such as abdominal bleeding, hepatic failure, and distant metastasis), efficacy (change of portal vein pressure before and after TIPS, symptom relief, including ascites, hydrothorax, EGVB, and distributary channel restenosis) of the procedure, and the cumulative rates of survival. We also retrospectively analyzed and compared the clinical characteristics of patients living ≥ 5 and < 5 years, including sex, age, Child-Pugh score before TIPS, portal vein tumor thrombosis (PVTT), tumor lesion, lesion diameter, hepatic arterio-portal fistulas, cancer diagnosed before and after TIPS, stents used, treatments received (RFA, TACE/TAE, and RFA+TACE/TAE), and complications (recurrence of ascites/bleeding, hepatic encephalopathy, and distributary channel function) that occurred during follow-up.
Indications: Acute or repeated variceal bleeding that failed conservative and endoscopic treatment; rebleeding after surgical shunting or laparosplenectomy; bleeding after preventive endoscopic/drug treatment; gastric or ectopic variceal bleeding; or refractory hepatic ascites/hydrothorax.
Relative contraindications: Serious dysfunction of blood coagulation and bleeding tendency; hepatic encephalopathy; serious infections; portal vein thrombosis; cavernous transformation of portal vein; or tumor too large to avoid during TIPS. The predicted survival of these patients was ≤ 3 mo.
Contraindications: Liver failure, severe cardiopulmonary dysfunction, multiple hepatic cysts, and refractory biliopancreatic obstruction.
The TIPS procedure was performed in the Interventional Radiology Suite under local anesthesia. The right jugular vein was punctured by RUPS-100 (Cook, Bloomington, United States) with a 10-F sheath. A 5-F multipurpose catheter was used to engage the hepatic vein (right usually) and the portal vein, perform portal vein angiography, and measure portal vein pressure before the shunting. A balloon catheter (6 or 8 mm in diameter) was used to expand the shunt along a guidewire, and the stents (7, 8, or 10 mm in diameter) were placed. Portal vein angiography and measurement of portal vein pressure were then conducted again.
Bare stents were used for 112 cases (Protégé stent, EV3 Company, Nathan Lane North, Plymouth, MN, United States; Smart stent, Cordis Company, Miami Lakes, Florida, United States). Covered stents were used for 97 cases (Fluency stent, Bard Company, Karlsruhe, Germany). One stent was used in 150 cases, and more than two stents were used in 59 cases. Stents with diameter 7, 8, and 10 mm were used for 3, 154, and 52 cases, respectively. The shunts were entered via the hepatic vein in 185 cases and high inferior vena cava in 24 cases. TIPS was combined with percutaneous liver biopsy and portal vein venography in 28 cases and indirect portal venography in 34 cases, providing that it was hard to puncture the portal vein, the risk of direct TIPS was high, or direct TIPS was failed. Varicose vein embolism was conducted in 199 cases.
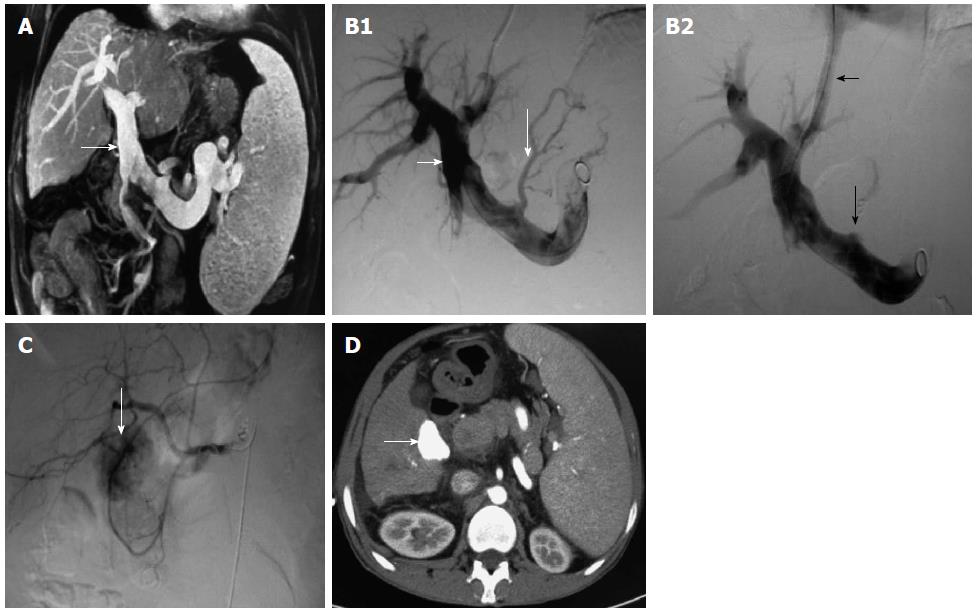
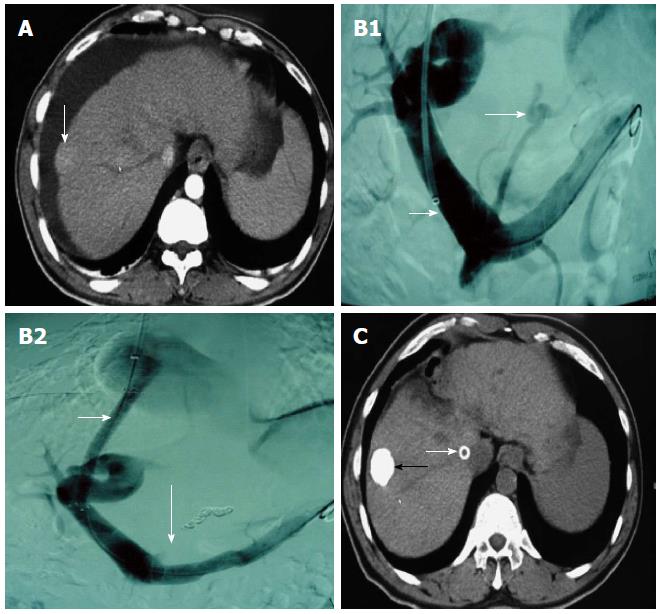
TACE or TAE was conducted before or after TIPS in 185 cases, from one to 11 times per patient. It was usually conducted once monthly for the first 3 mo, and the treatment intensity and interval were determined by the blood supply to the tumor lesion. RFA was conducted in 113 cases (mainly for lesions < 3 cm in diameter and lack of blood supply), which was conducted alone in 24 cases and combined with TACE/TAE in 89 cases several days before RFA. TACE or TAE was conducted alone in 96 cases, hepatic arterio-portal fistulas embolization was used in 22 cases, and transcatheter splenic arterial embolization was used in 14 cases (Figure 1 and Figure 2).
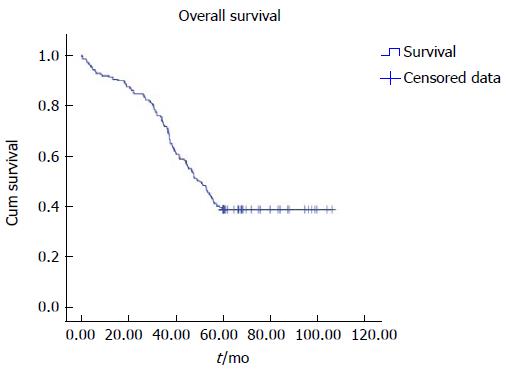
All cases were followed up until death or 5 years. We observed clinical symptoms, medical events, routine blood measurements, AFP, blood coagulation, liver and kidney functions, and blood ammonia and performed color ultrasound, barium meal, or gastroscope and CT/MRI. If the distributary channel was narrow, as shown by color ultrasound, or EGVB, resistant ascites, or hydrothorax occurred, we reviewed the distributary channel by radiography and measured the portal vein pressure at the same time. If the blood flow was normal, while portal pressure increased or the distributary channel was narrow or occluded under the radiography, we conducted balloon expansion and/or stent placement again. Then, if the original distributary channel was hard to reopen or the blood flow was insufficient, we conducted TIPS again to create a second channel. For the patients that needed sequential treatments for HCC after TIPS, we followed up or treated them once monthly in the first 3 mo, and then we followed up or treated them according to circumstances, usually once every 1-3 mo. For the patients who did not need sequential treatments for HCC, we followed up once every 3 mo. Follow-up results are shown in Table 1. Overall survival is shown in Table 2 and Figure 3.
| Items | Patients |
| Refractory ascites or hydrothorax | 15 (7.2) |
| EGVB | 77 (36.8) |
| Hepatic encephalopathy | 82 (39.2) |
| Distributary channel restenosis (yr) | |
| 1 | 36 (17.2) |
| 2 | 62 (29.7) |
| 3 | 77 (36.8) |
| 4 | 95 (45.5) |
| 5 | 122 (58.4) |
| Interventional treatments | |
| RFA | 113 (54.1) |
| Once | 34 (30.1) |
| Twice | 42 (37.2) |
| Thrice or more | 37 (32.7) |
| TACE/TAE | 185 (88.5) |
| Once | 31 (16.8) |
| Twice | 42 (22.7) |
| Thrice or more | 112 (60.5) |
| Fistula embolization | 22 (10.5) |
| Once | 6 (27.3) |
| Twice | 14 (63.6) |
| Thrice or more | 2 (9.1) |
| Splenic artery embolization | 14 (6.7) |
| Once | 7 (50.0) |
| Twice | 6 (42.9) |
| Thrice or more | 1 (7.1) |
| Interventional re-treatments | 102 (48.8) |
| Twice | 62 (60.8) |
| Thrice or more | 40 (39.2) |
| Balloon angioplasty | 9 (8.8) |
| Stent angioplasty | 93 (91.2) |
| Mean1 estimate | SE | 95%CI | Median estimate | SE | 95%CI | ||
| Lower | Upper | Lower | Upper | ||||
| 62.067 | 2.582 | 57.007 | 67.128 | 50.300 | 2.995 | 44.431 | 56.169 |
Statistical analysis was conducted using SPSS version 17.0 (Chicago, IL, United States). A t test was used for comparison of continuous measurement data, and χ2 test for comparison of data between patients living ≥ 5 and < 5 years. P < 0.05 was considered as statistically significant. The overall survival time was estimated using the Kaplan-Meier method. The median survival time was calculated using censored observations only.
The pre-TIPS portosystemic pressure was 29.0 ± 4.1 mmHg, which decreased to 18.1 ± 2.9 mmHg (t = 69.32, P < 0.05) after TIPS. The portal hypertension symptoms were relieved and improved; the rates of resistant ascites, hydrothorax, EGVB, hepatic encephalopathy, and distributary channel restenosis during follow-up were relatively impressive. Details including the interventional re-treatments for distributary channels and interventional treatments for tumor lesions are all presented in Table 1.
Clinical characteristics of patients living ≥ 5 and < 5 years were analyzed and compared: patients’ sex, mean age, lesion number, recurrence (ascites/bleeding), and distributary channel function did not differ significantly between the two groups (P > 0.05). Moreover, Child-Pugh score, with or without PVTT, lesion diameter, hepatic arterio-portal fistulas, cancer diagnosed before or after TIPS, stent type, hepatic encephalopathy, and interventional treatment differed significantly between the two groups (P < 0.05) (Table 3).
| Items | Total (n) | Patients living | t/χ2 | P value | |
| ≥5 yr | < 5 yr | ||||
| 209 | 81 (38.8) | 128 (61.2) | |||
| Sex | 0.367 | 0.545 | |||
| Male | 121 | 49 (60.5) | 72 (56.2) | ||
| Female | 88 | 32 (39.5) | 56 (43.8) | ||
| Age (mean ± SD, yr) | 49.2 ± 4 | 47.8 ± 8 | 2.868 | 0.488 | |
| Child-Pugh score | 6.888 | 0.032 (P < 0.05) | |||
| A | 83 | 38 (46.9) | 45 (35.2) | ||
| B | 72 | 30 (37.0) | 42 (32.8) | ||
| C | 54 | 13 (16.1) | 41 (32.0) | ||
| PVTT | 10.249 | 0.001 (P < 0.05) | |||
| With | 31 | 4 (4.9) | 27 (21.1) | ||
| Without | 178 | 77 (95.1) | 101 (78.9) | ||
| Lesion number | 0.263 | 0.608 | |||
| Single | 148 | 59 (72.8) | 89 (69.5) | ||
| Multiple | 61 | 22 (27.2) | 39 (30.5) | ||
| Lesion diameter | 18.351 | 0.0001 (P < 0.05) | |||
| ≤ 3 cm | 52 | 27 (33.3) | 25 (19.5) | ||
| > 3, ≤ 5 cm | 103 | 46 (56.8) | 57 (44.6) | ||
| > 5 cm | 54 | 8 (9.9) | 46 (35.9) | ||
| Hepatic arterio-portal fistulas | 6.537 | 0.011 (P < 0.05) | |||
| With | 22 | 3 (3.7) | 19 (14.8) | ||
| Without | 187 | 78 (96.3) | 109 (85.2) | ||
| Cancer diagnosed | 5.973 | 0.015 (P < 0.05) | |||
| Pre-TIPS | 142 | 47 (58.0) | 95 (74.2) | ||
| Post-TIPS | 67 | 34 (42.0) | 33 (25.8) | ||
| Stents | 4.446 | 0.035 (P < 0.05) | |||
| Bare stent | 112 | 36 (44.4) | 76 (59.4) | ||
| Covered stent | 97 | 45 (55.6) | 52 (40.6) | ||
| Recurrence (ascites/bleeding) | 3.624 | 0.057 | |||
| Yes | 92 | 29 (25.8) | 63 (49.2) | ||
| No | 117 | 52 (64.2) | 65 (50.8) | ||
| Hepatic encephalopathy | 3.887 | 0.049 (P < 0.05) | |||
| Yes | 82 | 25 (30.9) | 57 (44.5) | ||
| No | 127 | 56 (69.1) | 71 (55.5) | ||
| Interventional therapy | 7.556 | 0.023 (P < 0.05) | |||
| RFA | 24 | 13 (16.0) | 11 (8.6) | ||
| TACE/TAE | 96 | 28 (34.6) | 68 (53.1) | ||
| RFA + TACE/TAE | 89 | 40 (49.4) | 49 (38.3) | ||
| Distributary channel function | 3.274 | 0.070 | |||
| Restenosis | 122 | 41 (50.6) | 81 (63.3) | ||
| Unobstructed | 87 | 40 (49.4) | 47 (36.7) | ||
No procedure-related deaths or serious complications (e.g., abdominal bleeding, hepatic failure, and distant metastasis) occurred. The main cause of death during follow-up was gastrointestinal rebleeding (36 cases), which caused hemorrhagic shock, acute liver failure, and hepatic encephalopathy. Thirty-one cases died of liver failure or multiple organ failure; 29 of abdominal or lung infection; and 19 of tumor progression, which led to respiratory and circulatory failure. Other causes of death were hepatorenal syndrome and cardiovascular and cerebrovascular diseases.
Thus, the portal hypertension symptoms were ameliorated after TIPS and other interventional treatments with no procedure-related deaths and serious complications. Moreover, Child-Pugh score, PVTT, lesion diameter, hepatic arterio-portal fistulas, HCC diagnosed before or after TIPS, stent type, hepatic encephalopathy, and type of other interventional treatments were related to 5 year survival after comparing the characteristics of patients living ≥ 5 and < 5 years.
HCC tends to be associated with liver cirrhosis and portal hypertension, even gastrointestinal hemorrhage and/or refractory ascites[27]. In such cases, the priority is to manage the symptoms and complications of portal hypertension, rather than HCC itself. TIPS is an effective method for resolving the symptoms of portal hypertension[10]. After TIPS, the portal vein blood flow to the liver is reduced, and the hepatic artery blood flow is increased to compensate for the reduction[28,29], based on the interdependence of the portal vein and hepatic artery[30]. In theory, the extra blocking of the blood supply from the hepatic artery (such as TAE/TACE) aggravates liver damage after TIPS, due to ischemia[18]. However, the impact on the clinical curative effect is still not clear. Kuo et al[18] reported that the rate of complete response was significantly greater in non-TIPS patients compared with TIPS patients (74% vs 30%, P = 0.03) after TACE. Objective response rate (complete and partial response) tended to be greater in the non-TIPS group (83% vs 50%, P = 0.09). TACE was less effective in achieving a complete or partial response in TIPS patients compared with those without TIPS. Kohi et al[21] reported that the incidence of severe hepatobiliary adverse events after TACE was nearly two times higher in patients with TIPS (70%) than in those without TIPS (36%, P = 0.046). Thus, patients with HCC and patent TIPS were more likely to develop significant hepatotoxicity after TACE than comparable patients without TIPS. Conversely, Kang et al[26] reported that after TACE, 14 of 20 (70%) patients showed a tumor response, with only one (5%) experiencing a TACE-related major complication. Selective TACE may be safe and effective for the palliative treatment of HCC in patients with TIPS. In patients with HCC and TIPS receiving locoregional tumor therapy, Padia et al[23] showed that ablation procedures resulted in low rates of hepatotoxicity. However, the above studies lacked long-term clinical observation and had a small numbers of cases. In this study, we reviewed 209 patients with HCC and portal hypertension who underwent successful TIPS. Among them, 96 cases were treated in combination with TACE/TAE; 89 in combination with TAE/TACE and RFA; and 24 in combination with RFA. Hepatic arterio-portal fistulas embolization was also used in 22 cases, and splenic artery embolization in 14. There were no procedure-related deaths and serious complications (e.g., abdominal bleeding, hepatic failure, and distant metastasis). Thus, TIPS combined with other interventional treatments, such as RFA and TACE, are technically feasible and safe for patients with HCC and portal hypertension.
Regarding efficacy, some studies have shown that TIPS combined with TACE/TAE is effective[22-24,26]. However, reports of TIPS combined with RFA and other interventional treatments are rare. Padia et al[23] reported the outcomes of locoregional tumor therapy in 48 cases of HCC treated with TIPS. Twenty-nine of 48 (60%) patients were assigned to the local treatment group, which had received RFA 39 times, chemoembolization 17 times, and yttrium-90 radioembolization 10 times. Nineteen of 48 (40%) patients received best supportive care (i.e., symptomatic management only). Follow-up imaging response showed an objective response for all ablation procedures, 67% of radioembolization procedures, and 50% of chemoembolization procedures (P = 0.001). When censored for orthotopic liver transplantation, patients undergoing treatment survived longer than those receiving supportive care (2273 d vs 439 d, P = 0.001). It was concluded that ablation appears to be safe and efficacious for patients with HCC treated with TIPS. Moreover, the survival rates at 1, 2, 3, 4, and 5 years in our study were 91.4% (191/209), 84.7% (177/209), 71.8% (150/209), 51.2% (107/209), and 38.8% (81/209), respectively. The 5 year survival rate seems better than that for treating by TACE alone, which was reported as 26%[31]. It also seems better than the survival rates at 1, 2, and 3 years for RFA of 60%, 39%, and 35%, respectively[32]. We compared the clinical characteristics of patients living ≥ 5 and < 5 years, and Child-Pugh score, PVTT, lesion diameter, hepatic arterio-portal fistulas, HCC diagnosed before or after TIPS, stent type, hepatic encephalopathy, and type of other interventional treatments had significant differences between the two groups. A prospective randomized controlled trial may be needed to confirm that the above factors affect 5 year survival. In addition, with TIPS using bare stents, it is easy to develop shunt stenosis or occlusion[16]. An increase in symptom recurrence rate and the incidence of hepatic encephalopathy after TIPS, which may adversely affect quality of life and accelerate liver function deterioration, is the main cause of death. In the study, the cumulative rates of distributary channel restenosis tended to be similar to that of expanded polytetrafluoroethylene covered stent[33]. TACE was used alone mainly for patients that were not suitable for combination with RFA or in whom the lesions had a large diameter, which made it difficult to achieve necrotic collapse of the lesions. Therefore, the curative effects were inferior to those in patients treated by TACE combined with RFA. In normal follow-up, early detection of recurrent liver lesions and timely interventional treatment are crucial. Thus, the curative effect for patients with HCC diagnosed after TIPS was better than that for patients with HCC diagnosed before TIPS.
In conclusion, TIPS combined with other interventional treatments seems a safe and efficacious choice for patients with HCC and portal hypertension.
Patients with hepatocellular carcinoma (HCC) accompanied with portal hypertension often have no opportunity to receive radical surgery or liver transplantation. For some interventional treatments, it is important to manage portal hypertension urgently in patients HCC. Transjugular intrahepatic portosystemic shunt (TIPS) is widely used as a treatment for portal hypertension. However, the safety and efficacy of TIPS combined with other interventional treatments for patients with HCC and portal hypertension are still not clear.
There are few English language studies concerning combination of TIPS with other interventional treatments for patients with HCC and portal hypertension. The small number of studies has yielded conflicting results for safety and efficacy, and they were conducted in small patient populations. Kuo et al reported that transarterial chemoembolization (TACE) was less effective in achieving complete or partial response, in TIPS patients compared with those without TIPS. Kohi et al reported that patients with HCC and patent TIPS were more likely to develop significant hepatotoxicity after TACE than comparable patients without TIPS. However, Kang et al reported that TACE may be safe and effective for the palliative treatment of HCC in patients with TIPS. Outcomes of locoregional tumor therapy for patients with HCC and TIPS by Padia et al showed that ablation procedures resulted in low rates of hepatotoxicity.
Most of the studies reporting the safety and efficacy of TIPS combined with other interventional treatments for patients with HCC and portal hypertension had no more than 20 patients, which could have affected the clinical observations and conclusions. However, the authors reviewed and analyzed complete 5 year clinical data of 209 HCC patients with portal hypertension who were treated with TIPS and other interventional treatments. The authors also analyzed the characteristics of patients living ≥ 5 and < 5 years. Moreover, the results were encouraging.
This results suggested that TIPS combined with other interventional treatments seemed a safe and efficacious choice for patients with HCC and portal hypertension. There were no procedure-related deaths and serious complications (e.g., abdominal bleeding, hepatic failure, and distant metastasis). The survival rates seem better than those reported for TACE or radiofrequency ablation alone.
TIPS is an expandable metal stent inserted via the jugular vein that creates a shunt from the portal vein to the systemic circulation via an artificial communication through the liver.
In this study, the authors evaluated the long-term clinical safety and efficacy of TIPS combined with other interventional treatments for patients with HCC and portal hypertension. The main concept is interesting because there is limited data in this patient population.
P- Reviewer: Blonski W, Grossi L, Siramolpiwat S S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Liu XM
| 1. | Lee SC, Tan HT, Chung MC. Prognostic biomarkers for prediction of recurrence of hepatocellular carcinoma: current status and future prospects. World J Gastroenterol. 2014;20:3112-3124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 2. | Li Y, Zhang Z, Shi J, Jin L, Wang L, Xu D, Wang FS. Risk factors for naturally-occurring early-onset hepatocellular carcinoma in patients with HBV-associated liver cirrhosis in China. Int J Clin Exp Med. 2015;8:1205-1212. [PubMed] |
| 3. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21351] [Article Influence: 2135.1] [Reference Citation Analysis (3)] |
| 4. | Zhu Q, Li N, Zeng X, Han Q, Li F, Yang C, Lv Y, Zhou Z, Liu Z. Hepatocellular carcinoma in a large medical center of China over a 10-year period: evolving therapeutic option and improving survival. Oncotarget. 2015;6:4440-4450. [PubMed] |
| 5. | Prajapati HJ, Xing M, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, Chen Z, Kim HS. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203:W706-W714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Sato Y, Watanabe H, Sone M, Onaya H, Sakamoto N, Osuga K, Takahashi M, Arai Y. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118:16-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | de la Serna S, Vilana R, Sánchez-Cabús S, Calatayud D, Ferrer J, Molina V, Fondevila C, Bruix J, Fuster J, García-Valdecasas JC. Results of laparoscopic radiofrequency ablation for HCC. Could the location of the tumour influence a complete response to treatment? A single European centre experience. HPB (Oxford). 2015;17:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Zhao JB, Feng C, Zhu QH, He XF, Li YH, Chen Y. Transjugular intrahepatic portosystemic shunt with covered stents for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2014;20:1602-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Jiang ZB, Shan H, Shen XY, Huang MS, Li ZR, Zhu KS, Guan SH. Transjugular intrahepatic portosystemic shunt for palliative treatment of portal hypertension secondary to portal vein tumor thrombosis. World J Gastroenterol. 2004;10:1881-1884. [PubMed] |
| 10. | Liu L, Zhao Y, Qi X, Cai G, He C, Guo W, Yin Z, Chen H, Chen X, Fan D. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res. 2014;44:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Parker R. Role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Clin Liver Dis. 2014;18:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 837] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 13. | Jothimani D, Cross TJ. Early use of TIPS for cirrhosis and variceal bleeding. N Engl J Med. 2010;363:1375; author reply 1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Qi X, Liu L, Bai M, Chen H, Wang J, Yang Z, Han G, Fan D. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol. 2014;29:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | He FL, Wang L, Zhao HW, Fan ZH, Zhao MF, Dai S, Yue ZD, Liu FQ. Transjugular intrahepatic portosystemic shunt for severe jaundice in patients with acute Budd-Chiari syndrome. World J Gastroenterol. 2015;21:2413-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Qi XS, Bai M, Yang ZP, Fan DM. Selection of a TIPS stent for management of portal hypertension in liver cirrhosis: an evidence-based review. World J Gastroenterol. 2014;20:6470-6480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | He FL, Wang L, Yue ZD, Zhao HW, Liu FQ. Parallel transjugular intrahepatic portosystemic shunt for controlling portal hypertension complications in cirrhotic patients. World J Gastroenterol. 2014;20:11835-11839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kuo YC, Kohi MP, Naeger DM, Tong RT, Kolli KP, Taylor AG, Laberge JM, Kerlan RK, Fidelman N. Efficacy of TACE in TIPS patients: comparison of treatment response to chemoembolization for hepatocellular carcinoma in patients with and without a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2013;36:1336-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Sakaguchi H, Uchida H, Maeda M, Matsuo N, Kichikawa K, Ohishi H, Nishida H, Ueno K, Nishimine K, Rösch J. Combined transjugular intrahepatic portosystemic shunt and segmental Lipiodol hepatic artery embolization for the treatment of esophagogastric varices and hepatocellular carcinoma in patients with cirrhosis: preliminary report. Cardiovasc Intervent Radiol. 1995;18:9-15. [PubMed] |
| 20. | Morse SS, Sniderman KW, Galloway S, Rapoport S, Ross GR, Glickman MG. Hepatoma, arterioportal shunting, and hyperkinetic portal hypertension: therapeutic embolization. Radiology. 1985;155:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kohi MP, Fidelman N, Naeger DM, LaBerge JM, Gordon RL, Kerlan RK. Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Tazawa J, Sakai Y, Yamane M, Kakinuma S, Maeda M, Suzuki K, Miyasaka Y, Nagayama K, Kusano F, Sato C. Long-term observation after transjugular intrahepatic portosystemic stent-shunt in two patients with hepatocellular carcinoma. J Clin Gastroenterol. 2000;31:262-267. [PubMed] |
| 23. | Padia SA, Chewning RH, Kogut MJ, Ingraham CR, Johnson GE, Bhattacharya R, Kwan SW, Monsky WL, Vaidya S, Hippe DS. Outcomes of Locoregional Tumor Therapy for Patients with Hepatocellular Carcinoma and Transjugular Intrahepatic Portosystemic Shunts. Cardiovasc Intervent Radiol. 2015;38:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Tesdal IK, Wikström M, Flechtenmacher C, Filser T, Dueber C. Percutaneous treatment of hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2006;29:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wang Z, Zhang H, Zhao H, Wang X, Tsauo J, Luo X, Li X. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol. 2014;20:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 26. | Kang JW, Kim JH, Ko GY, Gwon DI, Yoon HK, Sung KB. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012;53:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Galati G, De Vincentis A, Ripetti V, La Vaccara V, Vespasiani-Gentilucci U, Mazzarelli C, Gallo P, Luppi G, Grasso RF, Picardi A. Haemorrhoidal disease in severe portal hypertension: a combined approach with transjugular intrahepatic portosystemic shunt (TIPS) and transanal haemorrhoidal dearterialization (THD). Arch Med Sci. 2014;10:195-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Stankovic Z, Rössle M, Euringer W, Schultheiss M, Salem R, Barker A, Carr J, Langer M, Markl M, Collins JD. Effect of TIPS placement on portal and splanchnic arterial blood flow in 4-dimensional flow MRI. Eur Radiol. 2015;25:2634-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Keussen I, Song HY, Bajc M, Cwikiel W. Changes in the distribution of hepatic arterial blood flow following TIPS with uncovered stent and stent-graft: an experimental study. Cardiovasc Intervent Radiol. 2002;25:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Lautt WW, Greenway CV. Conceptual review of the hepatic vascular bed. Hepatology. 1987;7:952-963. [PubMed] |
| 31. | Matsui O, Miyayama S, Sanada J, Kobayashi S, Khoda W, Minami T, Kozaka K, Gabata T. Interventional oncology: new options for interstitial treatments and intravascular approaches: superselective TACE using iodized oil for HCC: rationale, technique and outcome. J Hepatobiliary Pancreat Sci. 2010;17:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Chuan W, Li C, Wen TF, Yan LN, Li B, Liang GL, Li KW. Short-term and long-term outcomes of surgical treatment for HCC within Milan criteria with cirrhotic portal hypertension. Hepatogastroenterology. 2014;61:2185-2190. [PubMed] |
| 33. | Weber CN, Nadolski GJ, White SB, Clark TW, Mondschein JI, Stavropoulos SW, Shlansky-Goldberg RD, Trerotola SO, Soulen MC. Long-Term Patency and Clinical Analysis of Expanded Polytetrafluoroethylene-Covered Transjugular Intrahepatic Portosystemic Shunt Stent Grafts. J Vasc Interv Radiol. 2015;26:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |