Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12351
Peer-review started: March 27, 2015
First decision: April 24, 2015
Revised: May 14, 2015
Accepted: July 18, 2015
Article in press: July 18, 2015
Published online: November 21, 2015
Processing time: 238 Days and 11.7 Hours
AIM: To evaluate the antioxidant effect of N-acetylcysteine (NAC) on the stomach of rats with portal hypertension.
METHODS: Twenty-four male Wistar rats weighing ± 250 g were divided into four experimental groups (n = 6 each): Sham-operated (SO), SO + NAC, partial portal vein ligation (PPVL), and PPVL + NAC. Treatment with NAC in a dose of 10 mg/kg (i.p.) diluted in 0.6 mL of saline solution was administered daily for 7 d starting 8 d after the surgery. Animals from the PPVL and SO group received saline solution (0.6 mL) for the same period of time as the PPVL + NAC and SO + NAC group. On the 15th day the animals were anesthetized and we evaluated portal pressure by cannulating mesenteric artery. After, we removed the stomach for further analysis. We performed immunohistochemical analysis for endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF), and nitrotirosine (NTT) proteins in stomach. We also evaluated eNOS and VEGF by Western blot analysis and assessed DNA damage in blood samples by the comet assay.
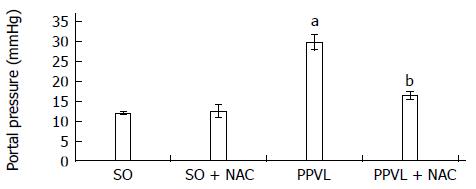
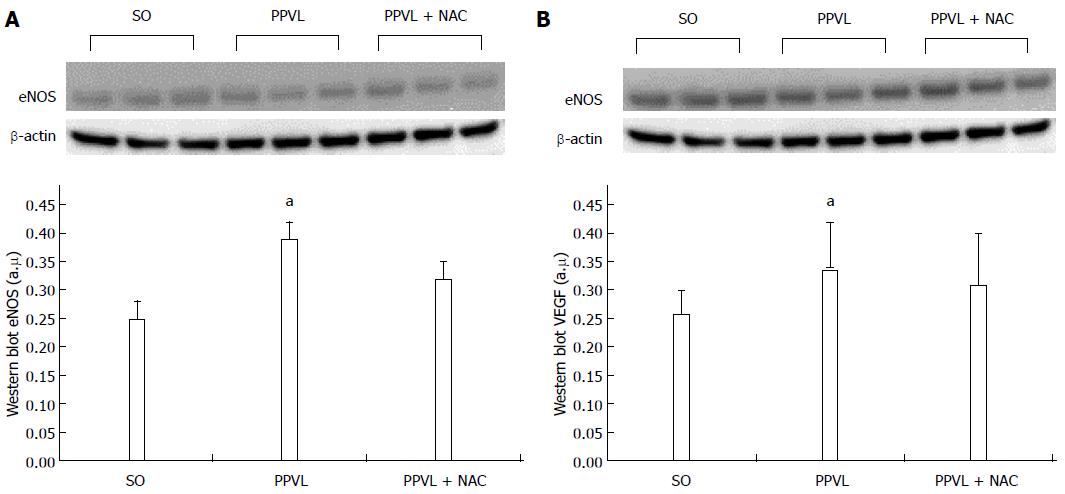
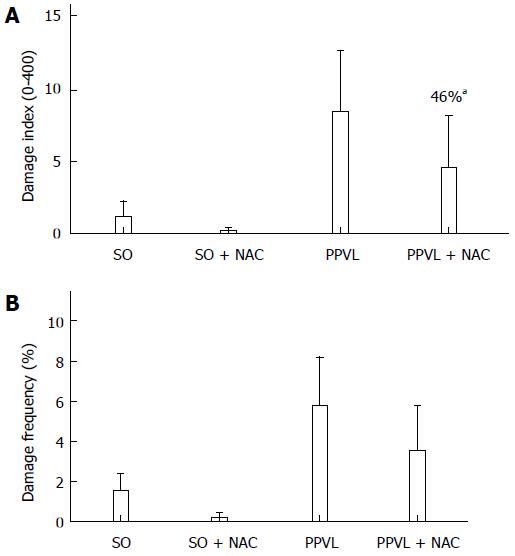
RESULTS: The portal hypertension group exhibited increases in portal pressure when compared to SO group (29.8 ± 1.8 vs 12.0 ± 0.3 mmHg) (P < 0.001). The same was observed when we compared the eNOS (56.8 ± 3.7 vs 13.46 ± 2.8 pixels) (P < 0.001), VEGF (34.9 ± 4.7 vs 17.46 ± 2.6 pixels) (P < 0.05), and NTT (39.01 ± 4.0 vs 12.77 ± 2.3 pixels) (P < 0.05) expression by immunohistochemistry of the PPVL animals with the SO group. The expression of eNOS (0.39 ± 0.03 vs 0.25 ± 0.03 a.μ) (P < 0.01) and VEGF (0.38 ± 0.04 vs 0.26 ± 0.04 a.μ) (P < 0.01) were also evaluated by Western blot analysis, and we observed an increase of both proteins on PPVL animals. We also evaluated the DNA damage by comet assay, and observed an increase on damage index and damage frequency on those animals. NAC decreased portal pressure values in PPVL + NAC animals (16.46 ± 2 vs 29.8 ± 1.8 mmHg) (P < 0.001) when compared to PPVL. The expression of eNOS (14.60 ± 4.1 vs 56.8 ± 3.7 pixels) (P < 0.001), VEGF (19.53 ± 3.2 vs 34.9 ± 4.7 pixels) (P < 0.05) and NTT (21.84 ± 0.7 vs 39.01 ± 4.0 pixels) (P < 0.05) evaluated by immunohistochemistry were also reduced in PPVL + NAC animals. Also, when evaluated by Western blot eNOS expression (0.32 ± 0.03 vs 0.39 ± 0.03 a.μ) (P < 0.05) and VEGF expression (0.31 ± 0.09 vs 0.38 ± 0.04 a.μ) (P < 0.01). Furthermore, NAC modulated DNA damage in PPVL + NAC animals.
CONCLUSION: In view of these results, we believe NAC is able to protect the stomach from the alterations induced by the PPVL procedure.
Core tip: Portal hypertension (PH) is a syndrome with serious manifestations as ascites, hepatic encephalopathy and development of collateral circulation, characterized by vasodilation and angiogenesis. This mechanism, intended to divert blood from the site of obstruction, is the leading cause of death among these patients, since it leads to upper gastrointestinal bleeding. Therapies that may contribute to control the development of collateral circulation are been investigated in an attempt to improve the quality of life of PH patients. This paper proposes a novel therapy, using an antioxidant effective in reducing this collateral circulation in an animal model of portal hypertension.
- Citation: Licks F, Hartmann RM, Marques C, Schemitt E, Colares JR, Soares MDC, Reys J, Fisher C, da Silva J, Marroni NP. N-acetylcysteine modulates angiogenesis and vasodilation in stomach such as DNA damage in blood of portal hypertensive rats. World J Gastroenterol 2015; 21(43): 12351-12360
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12351
Portal hypertension (PH) is characterized by a progressive increase in pressure within the hepatic portal system, with development of hyperdynamic circulation and a pressure gradient in the portal system (the difference between the pressure in the portal vein and the hepatic veins) exceeding 5 mmHg[1].
Conditions in which portal blood flow is reduced or obstructed by an anatomic obstacle are the determinants of PH pathogenesis. When an anatomic obstacle arises, vascular resistance to flow increases, contributing to the development of PH[2]. Depending on the location of this obstacle to blood flow, PH may be classified as pre-hepatic (portal or splenic vein thrombosis), intra-hepatic (cirrhosis), or post-hepatic (inferior vena cava thrombosis), among other examples[3].
The result of increased vascular resistance in the splanchnic circulation is increased pressure within the portal system. This leads to the development of a collateral circulation in an attempt to decompress the system, shunting blood directly into the systemic circulation, and increasing local venous blood flow through vasodilation[4]. Vasodilation in the splanchnic territory leads to the development of varices, particularly in the stomach. Gastric varices can be located from the fundus to the upper third of the stomach, and are caused by exacerbated dilation of blood vessels secondary to PH[5].
Among the various vasoactive substances implicated in the development of a splanchnic collateral circulation, nitric oxide is the key mediator involved in this process. Vasodilation and the formation of portosystemic collaterals contribute to increased blood flow and worsen PH, increasing the risk of upper gastrointestinal bleeding[6]. Nitric oxide derived from endothelial nitric oxide synthase (eNOS) plays a major role in the pathophysiology of PH. Nitric oxide is a potent vasodilator, and acts not only on the splanchnic circulation but also on the arterial circulation. It diffuses into smooth muscle cells and activates guanylate cyclase, producing cyclic guanosine monophosphate and thus contributing to the excessive vasodilation observed in PH[7].
The pathways of eNOS activation include endothelial stimuli such as shear stress, proinflammatory cytokines, and vascular endothelial growth factor (VEGF)[8,9].
The angiogenesis process, characterized by the formation of new blood vessels from preexisting ones, also contributes to the development and persistence of the collateral circulation. The role of VEGF in this process has been established by experimental studies in which it was shown to be the main angiogenic mediator in rats with PH. VEGF appears to contribute to the deterioration of PH by stimulating the development of portosystemic collaterals and increasing the permeability of the mesenteric microvasculature[10,11].
The role of oxidative stress in the vascular dysfunction of PH has been well established in the literature[12,13]. The overproduction of nitric oxide caused by the vascular abnormalities present in portal hypertension facilitates the reaction of NO to superoxide anion radical (O2-º) and forms peroxynitrite (ONOO-) contributing to an increase in oxidative phenomena[14]. The reactive oxygen species that characterize oxidative stress have the potential to bind to proteins, break DNA, and induce cell damage by interactions with various cell components. This phenomenon is associated with a series of disorders, including PH[15].
N-Acetylcysteine (NAC) is a compound that has been widely used in clinical practice for decades: as a mucolytic agent; in the management of ischemia-reperfusion injury; in acute respiratory distress syndrome and bronchitis; in the treatment of paracetamol toxicity and heavy metal poisoning; in HIV; and in psychiatric disorders. Its broad indications are due to its extensive antioxidant effects, which are attributable to its ability to react rapidly with •OH, •NO2, CO•−, and thiyl radicals, as well as to replenish vital cell components depleted by injury[16].
Within this context, the aim of the present study was to assess the effects of the antioxidant NAC in a rat model of PH, while evaluating the involvement of oxidative stress, vascular damage, and DNA damage in PH.
All animal-related procedures were performed in compliance with the guidelines of the Research Ethics Committee of Hospital de Clínicas de Porto Alegre (HCPA), state of Rio Grande do Sul, Brazil, and with the United States National Academy of Sciences Principles for Research Involving Animals[17].
We used 24 male Wistar rats, weighing 250 g each, obtained from the HCPA vivarium. All were kept in plastic bin cages, measuring 47 cm × 34 cm × 18 cm and lined with wood chips, under a 12-h dark/light cycle (lights on from 7 a.m. to 7 p.m.), at a controlled temperature of 22 ± 4 °C. The rats were fed commercially available chow (Purina® - Nutripal, Porto Alegre, RS, Brazil), 16 g/d, and had access to water ad libitum.
Animals were divided into four experimental groups (n = 6 each): sham-operated (SO), SO + NAC, partial portal vein ligation (PPVL) and PPVL + NAC. NAC (Sigma Chemical Co., St. Louis, MO, United States; CAS registry number 616-91-1) was administrated at a dose of 10 mg/kg, intraperitoneally, dissolved in 0.6 mL of normal saline solution (0.9% NaCl). This dose was based on previous studies performed by our research group[18]. Treatment was administered once daily for 7 d, starting on day 8 after surgery. Animals in the PPVL and SO groups received the same volume of saline solution, for the same period, instead of NAC.
The animals were initially anesthetized with ketamine hydrochloride (100 mg/kg i.p.) and xylazine hydrochloride (10 mg/kg i.p.). After induction of anesthesia, a midline laparotomy was performed and the bowels were gently retracted with a gauze pad soaked in saline. Briefly, the portal vein was isolated using 3-0 silk and a 20G needle was placed in front of it to establish PPVL. Both the portal vein and the needle were tied using the silk suture and the needle was withdrawn. The SO group underwent a sham version of the same procedure, in which the portal vein was not ligated[19].
On day 15 after surgery, animals were anaesthetized with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/kg i.p.). Blood was collected from the retro-orbital plexus using a heparinized capillary tube and stored in heparinized Eppendorf microtubes for later assessment of DNA damage[20,21]. After blood sampling, the abdomen was shaved and a laparotomy performed. Portal pressure was measured by cannulation of the mesenteric vein with a catheter coupled to a polygraph (Poligraph 2006, Letica Scientific Instruments, Barcelona, Spain)[19]. The animals were then killed by exsanguination under deep anesthesia[22] and we collected the stomach for the posterior analyzes. A piece of the stomach sample was frozen and stored at -80 °C and another piece was cut and fixed in 10% buffered formalin for 24 h. Paraffin blocks were cut with a rotatory microtome to create 3-mm sections.
Expression of eNOS, VEGF, and nitrotyrosine (NTT) antibody in stomach tissue was determined by immunohistochemical analysis. Antigen retrieval was performed using buffer at 100 °C, and endogenous peroxidase activity was blocked by incubation with absolute methanol. Slides were incubated with rabbit polyclonal antibody (NOS3 C-20 (sc-654), 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, United States), mouse monoclonal antibody (VEGF C-1 (sc-7269), 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, United States), and rabbit polyclonal antibody (Nitro-Tyrosine Antibody (#9691), 1:200, Cell Signaling Technology) overnight at 4 °C, then washed with buffer and incubated with secondary antibodies for eNOS (goat anti-rabbit IgG-HRP 2004), VEGF (goat anti-mouse IgG-HRP 2005), and NTT (anti-rabbit IgG-HRP 7074) for 30 min at room temperature. The slides were analyzed by a pathologist without prior knowledge of group allocation using a microscope coupled to a digital camera. Images were captured using Image-Plus software (Media Cybernetics, Bethesda, MD, United States). Quantification of eNOS, VEGF, and NTT antibody expression was performed via digital analysis in Adobe Photoshop® CS3 Extended 10.0 and involved counting pixels of areas stained by the immunohistochemical reagents. The level of expression was determined by multiplying the average density of the image by the percentage of positively stained areas (areas of brown staining)[23].
Western blot analysis was performed in cytosolic extract prepared from stomach homogenates. The supernatant fraction was collected and stored at 80 °C in aliquots until use. Protein concentration was measured as described by Bradford (1976). Lysate proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes[24,25]. The membranes were then blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TTBS) for 1 h at room temperature and probed overnight at 4 °C with rabbit polyclonal antibody [NOS3 C-20 (sc-654), Santa Cruz Biotechnology, Santa Cruz, CA, United States], mouse monoclonal antibody [VEGF C-1 (sc-7269), Santa Cruz Biotechnology, Santa Cruz, CA, United States], at 1:200-1000 dilution with TTBS in 5% nonfat dry milk, and anti-β-actin (42 kDA) antibody (Sigma Aldrich, St. Louis, MO, United States) at 1:1000 dilution with TTBS in 5% nonfat dry milk. After washing with TTBS, the membranes were incubated for 1 h at room temperature with secondary HRP-conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA, United States, 1:4.000). Protein detection was performed via chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Little Chalfont, Great Britain)[26]. The density of the specific bands was quantified with imaging densitometry software (Scion Image, Maryland, MA).
The alkaline comet assay was carried out as described elsewhere[20], with minor modifications[21]. Blood samples (50 μL) were placed in 5 μL of anticoagulant (heparin sodium, 25000 IU, Liquaemin®). Blood cell suspensions (5 μL) were embedded in 95 μL of 0.75% low melting point agarose (Gibco BRL) and spread on agarose-precoated microscope slides. After solidification, slides were placed in lysis buffer (2.5 mol/L NaCl, 100 mmol/L EDTA, and 10 mmol/L Tris, pH 10.0), with freshly added 1% Triton X-100 (Sigma) and 10% DMSO for 48 h at 4 °C. The slides were subsequently incubated in freshly prepared alkaline buffer (300 mmol/L NaOH and 1 mmol/L EDTA, pH > 13) for 20 min at 4 °C. An electric current of 300 mA and 25 V (0.90 V/cm) was applied for 15 min to perform DNA electrophoresis. The slides were then neutralized (0.4 mol/L Tris, pH 7.5), stained with silver, and viewed under a microscope. Images of 100 randomly selected cells (50 cells from each of two replicate slides) from each animal were analyzed. Cells were also visually scored according to tail size into five classes, ranging from undamaged (0) to maximally damaged (4), resulting in a single DNA damage score for each animal and, consequently, for each studied group. The damage index (DI) could thus range from 0 (completely undamaged, 100 cells × 0) to 400 (maximum damaged, 100 cells × 4). The damage frequency (%) was calculated on the basis of the number of tailed versus tailless cells.
All data are presented as mean ± SE. Statistical significance was calculated using GraphPad InStat, version 3.0 for Windows. Analysis of variance and the Student-Newman-Keuls method were used for multiple analysis. For the comet assay, the normality of variables was evaluated using the Kolmogorov-Smirnov test, and statistical differences between groups were analyzed using the nonparametric two-tailed Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Student’s t-test was used to compare damage between negative and positive controls. The critical level for rejection of the null hypothesis was considered to be a P value of < 0.05 (i.e., a significance level of 5%).
We observed a statistically significant increase in portal pressure values in the PPVL group as compared to SO animals (P < 0.001). In the NAC-treated group, these values were significantly decreased (P < 0.001) (Figure 1).
Immunohistochemical analyses for eNOS (Figure 2A; P < 0.001), VEGF (Figure 2B; P < 0.05) and NTT (Figure 2C; P < 0.05) showed that animals in the PPVL group had samples markedly positive for all three proteins. NAC treatment reduced expression of eNOS, VEGF, and NTT in stomach tissue.
Expression of eNOS (Figure 3A; P < 0.05) and VEGF (Figure 3B; P < 0.01) was increased in PPVL animals as compared with the SO group. Conversely, expression of these proteins was reduced in the PPVL + NAC group when compared with the PPVL group.
The comet assay revealed an increased DNA damage index (Figure 4A) and increased frequency of damage in blood samples (Figure 4B) in PPVL group animals. NAC treatment reduced both of these parameters, thus demonstrating the ability of NAC to modulate DNA damage in this experimental model.
PHis the main complication of cirrhosis, and is one of the leading causes of mortality in patients with chronic liver disease. The increased vascular resistance and blood flow present in PH are determining causes that elevate the portal pressure gradient, shunting blood from the liver into the systemic circulation. The subsequent formation of collateral vessels due to the NO overproduction is implicated in gastroesophageal bleeding, hepatic encephalopathy, and sepsis[27].
The search for medications that can prevent or mitigate this increase in portal pressure is extremely relevant. In the present study, we sought an alternative based on the close relationship between PH and oxidative stress and on the involvement of nitric oxide in this setting.
NAC is an antioxidant with a well-established mechanism of action, based on its ability to restore glutathione levels and act as a free radical scavenger[28]. Furthermore, NAC modulates nitric oxide through interactions of its thiol component with NO to form nitrosothiol[29].
The increased portal pressure observed in rats subjected to PPVL corroborates the findings of previous studies[30,31] and demonstrates the efficacy of the experimental model used in this study. NAC treatment effectively reduced pressure in the portal system in PPVL + NAC animals (Figure 1). Similar results were obtained in a prior study by our research group, in which NAC was found to reduce portal system pressures as a result of its ability to modulate nitric oxide and its antioxidant properties[32].
The ability of NAC to modulate nitric oxide bioavailability led to a reduction in eNOS expression in the gastric mucosa of portal hypertensive rats treated with NAC (PPVL + NAC), as assessed by immunohistochemistry (Figures 2A-C) and Western blot analysis (Figures 3A and B).
NO synthases are enzymes that play an essential role in the control of nitric oxide biosynthesis. The three main isoforms are inducible NO (iNOS) and two constitutive forms, nNOS and eNOS[33]. Nitric oxide produced by endothelial cells (eNOS) plays a major role in vascular smooth muscle relaxation. Under physiologic conditions, NO release is stimulated by acetylcholine, bradykinin, and adenosine triphosphate, among other mediators. Furthermore, the friction of circulating blood cells against vessel walls triggers eNOS-mediated nitric oxide synthesis via a shear stress mechanism to increase nitric oxide[34].
In PH, development of a hyperdynamic circulation is associated with nitric oxide overproduction, and portal hypertensive gastropathy is associated with the hemodynamic changes caused by NO triggering[35]. In the present study, the PPVL model caused an increase in portal system pressure and, consequently, increased eNOS immunoreactivity and expression in the gastric mucosa of rats in the PPVL group (Figures 2A and 3A). In a previous study, NAC reduced splanchnic vasodilation by reducing NO levels in an experimental model of cirrhosis induced by common bile duct ligation[36]. Our research group found a similar effect of NAC in rats with hepatopulmonary syndrome[18].
Nitric oxide toxicity is particularly prevalent in oxidative stress settings. The high reactivity of NO with molecules such as the superoxide anion (O2-º) produces highly injurious compounds, such as peroxynitrite (ONOO-), which may trigger nitrosative stress[37]. As PH is directly associated with oxidative stress and nitric oxide overproduction, it is to be expected that synthesis of reactive metabolites will occur, potentiating the pathophysiological changes observed in this syndrome.
This finding was proven by analysis of immunoreactivity to NTT in the gastric mucosa of study animals. We observed increased NTT reactivity in PPVL group animals (Figure 2C). The peroxynitrite generated during interactions of nitric oxide with the superoxide anion reacts with tyrosine residues and free tyrosine to produce NTT. In addition, the tyrosyl radical generated by ROS-mediated activation of tyrosine oxidizes nitric oxide, generating NTT[37-39]. The importance of NTT as a marker is based on the hypothesis that nitric oxide production will have been great enough to yield observable products such as NTT, as in the experimental model used in the present study[40].
NAC treatment effectively reduced NTT immunoreactivity in PPVL animals as compared with controls (Figure 2C), which demonstrates not only the antioxidant effect of NAC as a free radical scavenger but also its effects on NO production. Fernando et al[41] found that NAC administration was effective in reducing oxidative stress in PPVL animals as assessed by measurement of F2-isoprostanes and NO levels. In the present study, reductions in NO bioavailability and free radical generation may have enabled reduction of the peroxynitrite levels observed in the gastric mucosa of experimental animals, and may explain the protection afforded by NAC therapy.
The collateral vasculature characteristic of hyperdynamic circulation is formed by dilatation of preexisting vessels and by the angiogenesis process, which is modulated by vascular growth factors such as VEGF[42]. This process is also an alternative pathway of nitric oxide production, mediated by VEGF activation[38].
We assessed VEGF production in this experimental model by means of immunohistochemistry techniques and Western blot analysis. Rats in the PPVL group exhibited increased VEGF expression and immunoreactivity (Figures 2B and 3B) as compared with control animals. This demonstrates the presence of a stimulus for development of a collateral circulation in rats subjected to PPVL. NAC effectively reduced VEGF expression in the PPVL + NAC group, as demonstrated by both techniques employed.
Inhibition of VEGF receptor-2 has been shown to reduce hyperdynamic circulation and development of collaterals in rats with PH[10], and NAC has been shown to effectively reduce VEGF and p-VEGFR2 expression in the mesenteric vasculature of cirrhotic rats[36]. The results of the present study corroborate these findings.
Oxidative stress damages membrane lipids, proteins, and DNA[43]. The present study demonstrated an increase in DNA damage index (Figure 4A) and damage frequency (Figure 4B), as assessed by the comet assay, in PPVL animals as compared with controls. NAC reduced DNA damage when administered to PPVL animals.
NAC has antigenotoxic effects and detoxifies free radicals, which cause cellular DNA damage and has been reported to reduce cyclophosphamide-induced genotoxicity as assessed by the micronucleus assay[44]. The present study demonstrated a DNA-protective effect of NAC as shown by the comet assay. A previous investigation conducted by our research group evaluated the actions of NAC in rats with CCl4-induced cirrhosis and found antigenotoxic effects associated with its antioxidant properties[18].
In view of these findings, we conclude that NAC protects the gastric mucosa from the oxidative damage and hemodynamic changes related to nitric oxide overproduction in rats subjected to PPVL. NAC was able to reduce nitric oxide and thus decrease portal system pressure. This effect, added to its antioxidant and antiangiogenic properties, led to a reduction of the hyperdynamic collateral circulation and had a protective action on the gastric mucosa.
Portal hypertension is the leading cause of bleeding and death in cirrhotic patients due to recurrent cases of upper gastrointestinal bleeding. This high rate of bleeding is due to the development of collateral circulation, which despite being a physiological mechanism for decompressing the system, leads to progressive dilatation of the vessels and their likely breakdown especially in stomach. Therefore, is very large number of studies trying to seek a way to reduce this vasodilation. In the case of this study, we elected the antioxidant N-acetylcysteine (NAC).
NAC is a drug already used in the clinic, easily accessed, inexpensive and well - tolerated. The importance of the NAC is due to its direct and indirect antioxidant action, so important in this disease.
Previous work published by the group showed that NAC was effective in reducing oxidative stress and portal pressure in an experimental model of partial ligation of portal vein. In this present study, the authors aimed for better understanding the pathways involved in this effectiveness, pointing out the mechanisms used by this medicine to improve the gastropathy of portal hypertension.
The elucidation of the mechanisms of NAC action is of paramount importance for understanding the pathophysiology of portal hypertension. Therefore, this is indispensable to study a future alternative treatment for this disease. NAC is effective in reducing gastric damage and oxidative stress in an experimental model of portal hypertensive gastropathy that demonstrates all the physiological alterations present in patients affected by this disease.
Gastropathy is the non-inflammatory macroscopic lesions present in patients with portal hypertension. The portal hypertension is characterized by progressive increase of pressure in the portal system, which leads to a risk of vascular rupture and upper gastrointestinal bleeding.
This is a well-performed study in which the authors analyzed the protective effect of NAC on portal hypertensive gastropathy in rats. The results are interesting and suggest that this antioxidant is a potential therapeutic substance that could be used for preventing the upper gastrointestinal bleeding of portal hypertension patients.
P- Reviewer: Haddad LBD, Schwabl P, Tallis S S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Miñano C, Garcia-Tsao G. Clinical pharmacology of portal hypertension. Gastroenterol Clin North Am. 2010;39:681-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 4. | Sikuler E, Groszmann RJ. Interaction of flow and resistance in maintenance of portal hypertension in a rat model. Am J Physiol. 1986;250:G205-G212. [PubMed] |
| 5. | Triantafyllou M, Stanley AJ. Update on gastric varices. World J Gastrointest Endosc. 2014;6:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Iwakiri Y. The Molecules: Abnormal Vasculatures in the Splanchnic and Systemic Circulation in Portal Hypertension. In: Portal Hypertension - Causes and Complications. InTech 2012; . |
| 8. | Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Hori N, Wiest R, Groszmann RJ. Enhanced release of nitric oxide in response to changes in flow and shear stress in the superior mesenteric arteries of portal hypertensive rats. Hepatology. 1998;28:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodés J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Geerts AM, De Vriese AS, Vanheule E, Van Vlierberghe H, Mortier S, Cheung KJ, Demetter P, Lameire N, De Vos M, Colle I. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int. 2006;26:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther. 2010;28:e20-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Montezano AC, Touyz RM. Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012;28:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Rodríguez-Vilarrupla A, Bosch J, García-Pagán JC. Potential role of antioxidants in the treatment of portal hypertension. J Hepatol. 2007;46:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999;31:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 618] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830:4117-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 17. | Goldim JR, Raymundo MM. Pesquisa em saúde e direitos dos animais. HCPA. 1997;. |
| 18. | Vercelino R, Tieppo J, Dias AS, Marroni CA, Garcia E, Meurer L, Picada JN, Marroni NP. N-acetylcysteine effects on genotoxic and oxidative stress parameters in cirrhotic rats with hepatopulmonary syndrome. Basic Clin Pharmacol Toxicol. 2008;102:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Sikuler E, Kravetz D, Groszmann RJ. Evolution of portal hypertension and mechanisms involved in its maintenance in a rat model. Am J Physiol. 1985;248:G618-G625. [PubMed] |
| 20. | Speit G, Hartmann A. The comet assay: A sensitive genotoxicity test for the detection of DNA damage and repair. Methods in Molecular Biology: DNA Repair Protocola. Mammalian Systems: Second Edition 2006; 275-286. |
| 21. | Picada JN, Flores DG, Zettler CG, Marroni NP, Roesler R, Henriques JA. DNA damage in brain cells of mice treated with an oxidized form of apomorphine. Brain Res Mol Brain Res. 2003;114:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Association AVM. Guidelines on Euthanasia. Formerly Report of the AVMA Panel on Euthanasia. 2007;. |
| 23. | Gaffey MJ, Mills SE, Swanson PE, Zarbo RJ, Shah AR, Wick MR. Immunoreactivity for BER-EP4 in adenocarcinomas, adenomatoid tumors, and malignant mesotheliomas. Am J Surg Pathol. 1992;16:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | San-Miguel B, Alvarez M, Culebras JM, González-Gallego J, Tuñón MJ. N-acetyl-cysteine protects liver from apoptotic death in an animal model of fulminant hepatic failure. Apoptosis. 2006;11:1945-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Mauriz JL, Molpeceres V, García-Mediavilla MV, González P, Barrio JP, González-Gallego J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J Pineal Res. 2007;42:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Tuñón MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Álvarez M, Prieto J, González-Gallego J. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013;55:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Failli P, Palmieri L, D’Alfonso C, Giovannelli L, Generini S, Rosso AD, Pignone A, Stanflin N, Orsi S, Zilletti L. Effect of N-acetyl-L-cysteine on peroxynitrite and superoxide anion production of lung alveolar macrophages in systemic sclerosis. Nitric Oxide. 2002;7:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician. 2009;80:265-269. [PubMed] |
| 30. | Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004;68:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Marques C, Mauriz JL, Simonetto D, Marroni CA, Tuñon MJ, González-Gallego J, Marrón NP. Glutamine prevents gastric oxidative stress in an animal model of portal hypertension gastropathy. Ann Hepatol. 2011;10:531-539. [PubMed] |
| 32. | Licks F, Marques C, Zetler C, Martins MI, Marroni CA, Marroni NP. Antioxidant effect of N-acetylcysteine on prehepatic portal hypertensive gastropathy in rats. Ann Hepatol. 2014;13:370-377. [PubMed] |
| 33. | Shah V, Lyford G, Gores G, Farrugia G. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Busconi L, Michel T. Endothelial nitric oxide synthase membrane targeting. Evidence against involvement of a specific myristate receptor. J Biol Chem. 1994;269:25016-25020. [PubMed] |
| 35. | Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Lee PC, Yang YY, Huang CS, Hsieh SL, Lee KC, Hsieh YC, Lee TY, Lin HC. Concomitant inhibition of oxidative stress and angiogenesis by chronic hydrogen-rich saline and N-acetylcysteine treatments improves systemic, splanchnic and hepatic hemodynamics of cirrhotic rats. Hepatol Res. 2015;45:578-588. [PubMed] |
| 37. | Halliwell B, Gutteridge J. Free radical and biology and medicine. New York: Oxford 2007; . |
| 38. | Eiserich JP, Butler J, van der Vliet A, Cross CE, Halliwell B. Nitric oxide rapidly scavenges tyrosine and tryptophan radicals. Biochem J. 1995;310:745-749. [PubMed] |
| 39. | Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 379] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 40. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4458] [Article Influence: 247.7] [Reference Citation Analysis (0)] |
| 41. | Fernando B, Marley R, Holt S, Anand R, Harry D, Sanderson P, Smith R, Hamilton G, Moore K. N-acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology. 1998;28:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006;44:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453-R462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3416] [Cited by in RCA: 4592] [Article Influence: 459.2] [Reference Citation Analysis (0)] |
| 44. | Malins DC, Hellstrom KE, Anderson KM, Johnson PM, Vinson MA. Antioxidant-induced changes in oxidized DNA. Proc Natl Acad Sci USA. 2002;99:5937-5941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |